The Effect of Statins on Bleeding in Isolated Coronary Artery Bypass Grafting Statins in CABG
Abstract
1. Introduction
2. Methods
3. Statistics
4. Results
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salenger, R.; Arora, R.C.; Bracey, A.; D’Oria, M.; Engelman, D.T.; Evans, C.; Grant, M.C.; Gunaydin, S.; Morton, V.; Ozawa, S.; et al. Cardiac surgical bleeding, transfusion, and quality metrics: Joint consensus statement by the Enhanced Recovery After Surgery Cardiac Society and Society for the Advancement of Patient Blood Management. Ann. Thorac. Surg. 2025, 119, 280–295. [Google Scholar] [CrossRef]
- LaPar, D.J.; Crosby, I.K.; Ailawadi, G.; Ad, N.; Choi, E.; Spiess, B.D.; Rich, J.B.; Kasirajan, V.; Fonner, E.; Kron, I.L.; et al. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2013, 145, 796–804. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 55–161. [Google Scholar]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Banach, M.; Penson, P.E.; Jamialahmadi, T.; Sahebkar, A. Statin-induced nitric oxide signaling: Mechanisms and therapeutic implications. J. Clin. Med. 2019, 8, 2051. [Google Scholar] [CrossRef]
- Casani, L.; Sanchez-Gomez, S.; Vilahur, G.; Badimon, L. Pravastatin reduces thrombogenicity by mechanisms beyond plasma cholesterol lowering. Thromb. Haemost. 2005, 94, 1035–1041. [Google Scholar] [CrossRef]
- Moscardó, A.; Vallés, J.; Latorre, A.; Madrid, I.; Santos, M.T. Reduction of platelet cytosolic phospholipase A2 activity by atorvastatin and simvastatin: Biochemical regulatory mechanisms. Thromb. Res. 2013, 131, e154–e159. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Nappi, F.; Lusini, M.; Satriano, U.M.; Schilirò, D.; Spadaccio, C.; Chello, M. Effect of statins on platelet activation and function: From molecular pathways to clinical effects. BioMed Res. Int. 2021, 2021, 6661847. [Google Scholar] [CrossRef]
- Bruni, F.; Pasqui, A.L.; Pastorelli, M.; Bova, G.; Cercignani, M.; Palazzuoli, A.; Sawamura, T.; Auteri, A.; Puccetti, L. Different Effect of Statins on Platelet Oxidized-LDLReceptor (CD36 and LOX-1) Expressionin Hypercholesterolemic Subjects. Clin. Appl. Thromb. 2005, 11, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Markle, R.A.; Han, J.; Summers, B.D.; Yokoyama, T.; Hajjar, K.A.; Hajjar, D.P.; Gotto, A.M.; Nicholson, A.C. Pitavastatin alters the expression of thrombotic and fibrinolytic proteins in human vascular cells. J. Cell. Biochem. 2003, 90, 23–32. [Google Scholar] [CrossRef]
- Undas, A.; Celinska-Löwenhoff, M.; Brummel-Ziedins, K.E.; Brozek, J.; Szczeklik, A.; Mann, K.G. Simvastatin given for 3 days can inhibit thrombin generation and activation of factor V and enhance factor Va inactivation in hypercholesterolemic patients. Arter. Thromb. Vasc. Biol. 2005, 25, 1524–1525. [Google Scholar] [CrossRef] [PubMed]
- Atalar, E.; Coskun, S.; Haznedaroglu, I.C.; Yücel, N.; Ozer, N.; Sivri, B.; Aksoyek, S.; Ovunc, K.; Ozmen, F. Immediate effects of fluvastain on circulating soluble endothelial protein C and free tissue factor pathway inhibitor in acute coronary syndromes. Cardiovasc. Drugs Ther. 2005, 19, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Bosinakou, E.; Kotsopoulou, M.; Antoniades, C.; Katsi, V.; Stefanadis, C. Effects of early administration of atorvastatin treatment on thrombotic process in normocholesterolemic patients with unstable angina. Int. J. Cardiol. 2006, 106, 333–337. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Lin, F.; Hsieh, L.; Wang, S.; Lin, C.; Wang, Y.; Ku, H.; Chen, J.; Chen, Y. Pravastatin induces thrombomodulin expression in TNFα-treated human aortic endothelial cells by inhibiting Rac1 and Cdc42 translocation and activity. J. Cell. Biochem. 2007, 101, 642–653. [Google Scholar] [CrossRef]
- Lin, Z.; Kumar, A.; SenBanerjee, S.; Staniszewski, K.; Parmar, K.; Vaughan, D.E.; Gimbrone, M.A., Jr.; Balasubramanian, V.; García-Cardeña, G.; Jain, M.K. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 2005, 96, e48–e57. [Google Scholar] [CrossRef]
- Antoniades, C.; Demosthenous, M.; Reilly, S.; Margaritis, M.; Zhang, M.H.; Antonopoulos, A.; Marinou, K.; Nahar, K.; Jayaram, R.; Tousoulis, D.; et al. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery: Effects of short-term pre-operative statin treatment. J. Am. Coll. Cardiol. 2012, 59, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, M.; Channon, K.M.; Antoniades, C. Statins and vein graft failure in coronary bypass surgery. Curr. Opin. Pharmacol. 2012, 12, 172–180. [Google Scholar] [CrossRef]
- Beyazal, O.F.; Yanartas, M. Association between statins and deep vein thrombosis. Turk. J. Vasc. Surg. 2025, 34, 139–146. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Amarenco, P.; Szarek, M.; Callahan, A., III; Hennerici, M.; Sillesen, H.; Zivin, J.A.; Welch, K.M.A. Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology 2008, 70, 2364–2370. [Google Scholar] [CrossRef]
- Martinez, A.I.; Freeman, P.R.; Moga, D.C. Statin use and gastrointestinal hemorrhage: A large retrospective cohort study. Am. J. Cardiovasc. Drugs 2019, 19, 65–74. [Google Scholar] [CrossRef]
- Nenna, A.; Lusini, M.; Spadaccio, C.; Nappi, F.; Prestipino, F.; Barbato, R.; Casacalenda, A.; Pugliese, G.; Barberi, F.; Giacinto, O.; et al. Preoperative atorvastatin reduces bleeding and blood products use in patients undergoing on-pump coronary artery bypass grafting. J. Cardiovasc. Med. 2017, 18, 976–982. [Google Scholar] [CrossRef]
- Wu, H.-H.; Chang, S.-H.; Lee, T.-H.; Tu, H.-T.; Liu, C.-H.; Chang, T.-Y. Concurrent use of statins decreases major bleeding and intracerebral hemorrhage in non-valvular atrial fibrillation patients taking direct oral anticoagulants—A nationwide cohort study. Front. Cardiovasc. Med. 2022, 9, 969259. [Google Scholar] [CrossRef]
- Powell, B.D.; Bybee, K.A.; Valeti, U.; Thomas, R.J.; Kopecky, S.L.; Mullany, C.J.; Wright, R.S. Influence of preoperative lipid-lowering therapy on postoperative outcome in patients undergoing coronary artery bypass grafting. Am. J. Cardiol. 2007, 99, 785–789. [Google Scholar] [CrossRef]
- Antoniou, T.; Macdonald, E.M.; Yao, Z.; Hollands, S.; Gomes, T.; Tadrous, M.; Mamdani, M.M.; Juurlink, D.N. Association between statin use and ischemic stroke or major hemorrhage in patients taking dabigatran for atrial fibrillation. Can. Med. Assoc. J. 2017, 189, E4–E10. [Google Scholar] [CrossRef]
- Putzu, A.; Capelli, B.; Belletti, A.; Cassina, T.; Ferrari, E.; Gallo, M.; Casso, G.; Landoni, G. Perioperative statin therapy in cardiac surgery: A meta-analysis of randomized controlled trials. Crit. Care 2016, 20, 395. [Google Scholar] [CrossRef]
- Servadei, F.; Scimeca, M.; Palumbo, V.; Oddi, F.M.; Bonfiglio, R.; Giacobbi, E.; Menghini, R.; Casagrande, V.; Cardellini, M.; Martelli, E.; et al. Aging and Sex Modify the Risk of Carotid Plaque Thrombosis Related to Dyslipidemic Profile. Stroke 2025, 56, 2879–2887. [Google Scholar] [CrossRef] [PubMed]
- Layton, J.B.; Hansen, M.K.; Jakobsen, C.-J.; Kshirsagar, A.V.; Andreasen, J.J.; Hjortdal, V.E.; Rasmussen, B.S.; Simpson, R.J.; Brookhart, M.A.; Christiansen, C.F. Statin initiation and acute kidney injury following elective cardiovascular surgery: A population cohort study in Denmark. Eur. J. Cardio Thoracic Surg. 2016, 49, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-C.; Shen, P.; Liu, K.-X. Perioperative statins do not prevent acute kidney injury after cardiac surgery: A meta-analysis of randomized controlled trials. J. Cardiothorac. Vasc. Anesth. 2017, 31, 2086–2092. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Stone, N.J.; Lee, R.; McGee, E.C., Jr.; Malaisrie, S.C.; Silverberg, R.A.; McCarthy, P.M. Perioperative statin therapy reduces mortality in normolipidemic patients undergoing cardiac surgery. J. Thorac. Cardiovasc. Surg. 2010, 140, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.W.; Slottosch, I.; Wahlers, T.; Liakopoulos, O.J. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database Syst. Rev. 2015, 8, CD008493. [Google Scholar]
- Sakamoto, H.; Watanabe, Y.; Satou, M. Do preoperative statins reduce atrial fibrillation after coronary artery bypass grafting? Ann. Thorac. Cardiovasc. Surg. 2011, 17, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Aydın, U.; Yılmaz, M.; Düzyol, Ç.; Ata, Y.; Türk, T.; Orhan, A.L.; Koçoğulları, C.U. Efficiency of postoperative statin treatment for preventing new-onset postoperative atrial fibrillation in patients undergoing isolated coronary artery bypass grafting: A prospective randomized study. Anatol. J. Cardiol. 2015, 15, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Jayaram, R.; Jiang, L.; Emberson, J.; Zhao, Y.; Li, Q.; Du, J.; Guarguagli, S.; Hill, M.; Chen, Z.; et al. Perioperative rosuvastatin in cardiac surgery. N. Engl. J. Med. 2016, 374, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
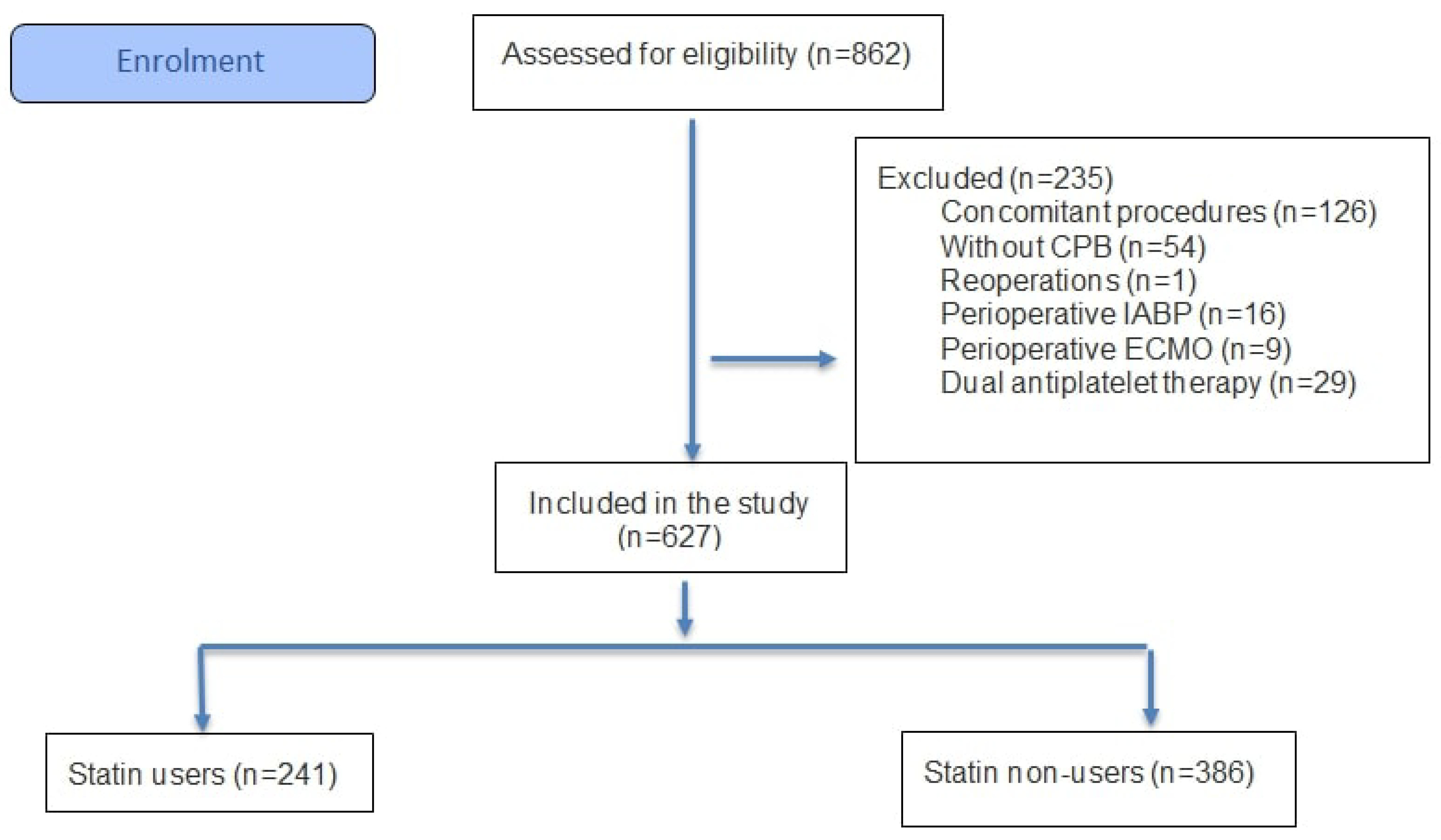
| Group A (n = 241) Statin (+) | Group B (n = 386) Statin (−) | ||||||
|---|---|---|---|---|---|---|---|
| Min-Max or n (%) | Median (Mean) | IQR | Min-Max or n (%) | Median (Mean) | IQR | p | |
| Demographic data | |||||||
| Gender female | 47 (19.5) | 72 (18.7) | 0.79 | ||||
| Age (years) | 38–84 | 60 | 10 | 34–86 | 61 | 14 | 0.34 |
| Height (cm) | 137–183 | 170 | 12 | 135–196 | 169 | 10 | 0.73 |
| Weight (kg) | 53–127 | 80 | 18 | 45–123 | 79.5 | 17 | 0.15 |
| Body surface area (kg/m2) | 1.44–2.42 | 1.91 | 0.21 | 1.26–2.42 | 1.89 | 0.21 | 0.22 |
| Body mass index (m2) | 18.7–55.6 | 28.3 | 5.7 | 19.6–42.8 | 27.7 | 5.6 | 0.14 |
| Comorbid diseases | |||||||
| Diabetes Mellitus | 140 (58.1) | 192 (49.7) | 0.04 | ||||
| Hypertension | 171 (71) | 216 (56) | <0.001 | ||||
| Chronic obstructive pulmonary disease | 20 (8.3) | 27 (7) | 0.54 | ||||
| Cerebrovascular accident | 11 (4.6) | 28 (7.3) | 0.17 | ||||
| Preoperative atrial fibrillation | 2 (0.8) | 3 (0.8) | 0.63 | ||||
| Chronic renal failure | 12 (5) | 18 (4.7) | 0.85 | ||||
| Peripheral artery disease | 14 (5.8) | 24 (6.2) | 0.83 | ||||
| Thyroid disorder | 13 (5.4) | 30 (7.8) | 0.25 | ||||
| EuroSCORE II | 1–13 | 1 (1.9) | 1 | 1–42 | 1 (2.3) | 1 | 0.17 |
| Vasoactive inotropic score (VIS) | 0–70 | 0 (3.6) | 4 | 0–440 | 0 (5.7) | 5 | 0.56 |
| Echocardiographic findings | |||||||
| Preop ejection fraction (%) | 22–65 | 60 | 10 | 20–65 | 55 | 10 | 0.17 |
| Postop ejection fraction (%) | 14–32 | 23 | 5 | 14–33 | 23 | 4 | 0.28 |
| Preop TAPSE | 20–65 | 55 | 10 | 25–65 | 55 | 10 | 0.25 |
| Postop TAPSE | 11–29 | 16 | 6 | 9–31 | 17 | 4 | 0.51 |
| Operative data | |||||||
| Emergency surgery | 8 (3.3) | 24 (6.2) | 0.10 | ||||
| Coronary endarterectomy | 12 (5) | 19 (4.9) | 0.97 | ||||
| LITA usage | 218 (90.5) | 336 (87) | 0.19 | ||||
| The number of grafts | 1–6 | 3 (3.1) | 1 | 1–7 (3.1) | 3 | 1 | 0.21 |
| Cross-clamp time (min) | 26–201 | 76 | 46 | 22–217 | 81 | 46 | 0.14 |
| Cardiopulmonary bypass time (min) | 52–268 | 123 | 50 | 40–407 | 130 | 54 | 0.11 |
| Group A (n = 241) Statin (+) | Group B (n = 386) Statin (−) | ||||||
|---|---|---|---|---|---|---|---|
| Min-Max or n (%) | Median (Mean) | IQR | Min-Max or n (%) | Median (Mean) | IQR | p | |
| Blood products and bleeding amounts | |||||||
| Intraop red blood cells using | 0–6 | 0 (0.78) | 1 | 0–13 | 0 (0.83) | 2 | 0.23 |
| Intraop fresh frozen plasma using | 0–4 | 0 (0.27) | 0 | 0–3 | 0 (0.21) | 0 | 0.30 |
| Intraop platelet suspensions | 0–2 | 0 (0.04) | 0 | 0–2 | 0 (0.06) | 0 | 0.33 |
| Postop red blood cells using | 0–8 | 0 (0.91) | 1 | 0–16 | 0 (0.98) | 1 | 0.96 |
| Postop fresh frozen plasma using | 0–7 | 0 (0.75) | 1 | 0–9 | 0 (0.75) | 1 | 0.99 |
| Postop platelet suspensions | 0–2 | 0 (0.07) | 0 | 0–6 | 0 (0.08) | 0 | 0.15 |
| Postop 1st day amount of bleeding (ml) | 50–2100 | 450 (512) | 300 | 50–1725 | 400 (481) | 300 | 0.16 |
| Postop total amount of bleeding (ml) | 200–2950 | 750 (827) | 350 | 100–3025 | 700 (782) | 450 | 0.09 |
| Intraoperative medications | |||||||
| Preoperative ACT | 79–256 | 126 (130) | 40 | 56–357 | 126 (132) | 38 | 0.81 |
| Postoperative ACT | 86–237 | 123 (125) | 22 | 59–240 | 125 (128) | 27 | 0.08 |
| Dose of tranexamic acid (mg) | 0–3000 | 1000 (788) | 1000 | 0–3000 | 1000 (827) | 1000 | 0.48 |
| Dose of fibrinogen concentrate (mg) | 0–4000 | 0 (414) | 500 | 0–4000 | 0 (318) | 0 | 0.11 |
| Group A (n = 241) Statin (+) | Group B (n = 386) Statin (−) | ||||
|---|---|---|---|---|---|
| Min-Max or n (%) | Median (IQR) | Min-Max or n (%) | Median (IQR) | p | |
| Preop laboratory parameters | |||||
| White blood cells (109/L) | 3.2–18.6 | 8.3 (2.8) | 2–19.4 | 8.6 (3) | 0.37 |
| Hematocrit (%) | 24–52 | 40.9 (5.6) | 20.5–52.5 | 41.3 (6.1) | 0.10 |
| Platelets (109/L) | 112–586 | 249 (77) | 63–565 | 253 (99) | 0.40 |
| Creatinine (mg/dL) | 0.27–9.02 | 0.95 (0.30) | 0.42–11.8 | 0.91 (0.29) | 0.06 |
| Sodium (mEq/L) | 127–146 | 139 (4) | 124–150 | 139 (4) | 0.64 |
| Potassium (mEq/L) | 3.4–6 | 4.37 (0.62) | 2.96–6.37 | 4.36 (0.6) | 0.69 |
| Low-density lipoprotein (mg/dL) | 31–243 | 87 (48) | 24–377 | 116.5 (57) | <0.001 |
| Postop 1st day laboratory parameters | |||||
| White blood cells (109/L) | 7.2–44.2 | 16.3 (8.1) | 5.3–50.3 | 16.5 (7.9) | 0.63 |
| Hematocrit (%) | 20.5–37.5 | 28 (5.4) | 19.8–60 | 28.4 (5.1) | 0.36 |
| Platelets (109/L) | 53–385 | 179 (75) | 42–496 | 190 (82) | 0.06 |
| Creatinine (mg/dL) | 0.08–5.38 | 1.2 (0.46) | 0.04–7.7 | 1.18 (0.41) | 0.29 |
| Sodium (mEq/L) | 135–155 | 143 (5) | 133–153 | 142 (4) | 0.52 |
| Potassium (mEq/L) | 2.75–5.76 | 4.22 (0.69) | 2.6–5.76 | 4.31 (0.68) | 0.14 |
| Group A (n = 241) Statin (+) | Group B (n = 386) Statin (−) | p | |
|---|---|---|---|
| Postoperative exploration | 15 (6.2) | 17 (4.4) | 0.39 |
| Cerebrovascular accident | 2 (0.8) | 9 (2.3) | 0.16 |
| Continuous renal replacement therapy | 4 (1.7) | 9 (2.3) | 0.56 |
| Postop atrial fibrillation | 21 (8.7) | 74 (19.2) | <0.001 |
| Deep sternal wound infection | 11 (4.6) | 14 (3.6) | 0.56 |
| Gastrointestinal bleeding | 2 (0.8) | 1 (0.3) | 0.57 |
| Mortality | 3 (1.2) | 14 (3.6) | 0.07 |
| Group A (n = 241) Statin (+) | Group B (n = 386) Statin (−) | ||||||
|---|---|---|---|---|---|---|---|
| Min-max or | Median (Mean) | IQR | Min-max or | Median (Mean) | IQR | p | |
| Intubation time (hours) | 1–240 | 10 | 7 | 2–672 | 9 | 6 | 0.09 |
| Intensive care unit stay (days) | 1–22 | 2 (2.6) | 1 | 1.28 | 2 (2.9) | 1 | 0.04 |
| Hospital stay (days) | 2–104 | 6 | 3 | 1–64 | 7 | 3 | 0.25 |
| Odds Ratio | CI % 95 | p | |
|---|---|---|---|
| Gender female | 0.597 | 0.311–1.147 | 0.12 |
| Age | 1.091 | 1.060–1.124 | <0.001 |
| Diabetes Mellitus | 0.779 | 0.476–1.274 | 0.31 |
| Hypertension | 1.085 | 0.650–1.813 | 0.75 |
| Statin usage | 0.418 | 0.245–0.712 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaarslan, M.; Beyazal, O.F.; Kayalar, N.; Yanartas, M. The Effect of Statins on Bleeding in Isolated Coronary Artery Bypass Grafting Statins in CABG. J. Clin. Med. 2025, 14, 8402. https://doi.org/10.3390/jcm14238402
Karaarslan M, Beyazal OF, Kayalar N, Yanartas M. The Effect of Statins on Bleeding in Isolated Coronary Artery Bypass Grafting Statins in CABG. Journal of Clinical Medicine. 2025; 14(23):8402. https://doi.org/10.3390/jcm14238402
Chicago/Turabian StyleKaraarslan, Mustafa, Osman Fehmi Beyazal, Nihan Kayalar, and Mehmed Yanartas. 2025. "The Effect of Statins on Bleeding in Isolated Coronary Artery Bypass Grafting Statins in CABG" Journal of Clinical Medicine 14, no. 23: 8402. https://doi.org/10.3390/jcm14238402
APA StyleKaraarslan, M., Beyazal, O. F., Kayalar, N., & Yanartas, M. (2025). The Effect of Statins on Bleeding in Isolated Coronary Artery Bypass Grafting Statins in CABG. Journal of Clinical Medicine, 14(23), 8402. https://doi.org/10.3390/jcm14238402







