The Effect of T Regulatory Cell Infiltration on Survival Outcomes in Metastatic Pancreatic Cancer Patients with a Review of Immunobiology, Prognostic Value and Future Therapeutic Options
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
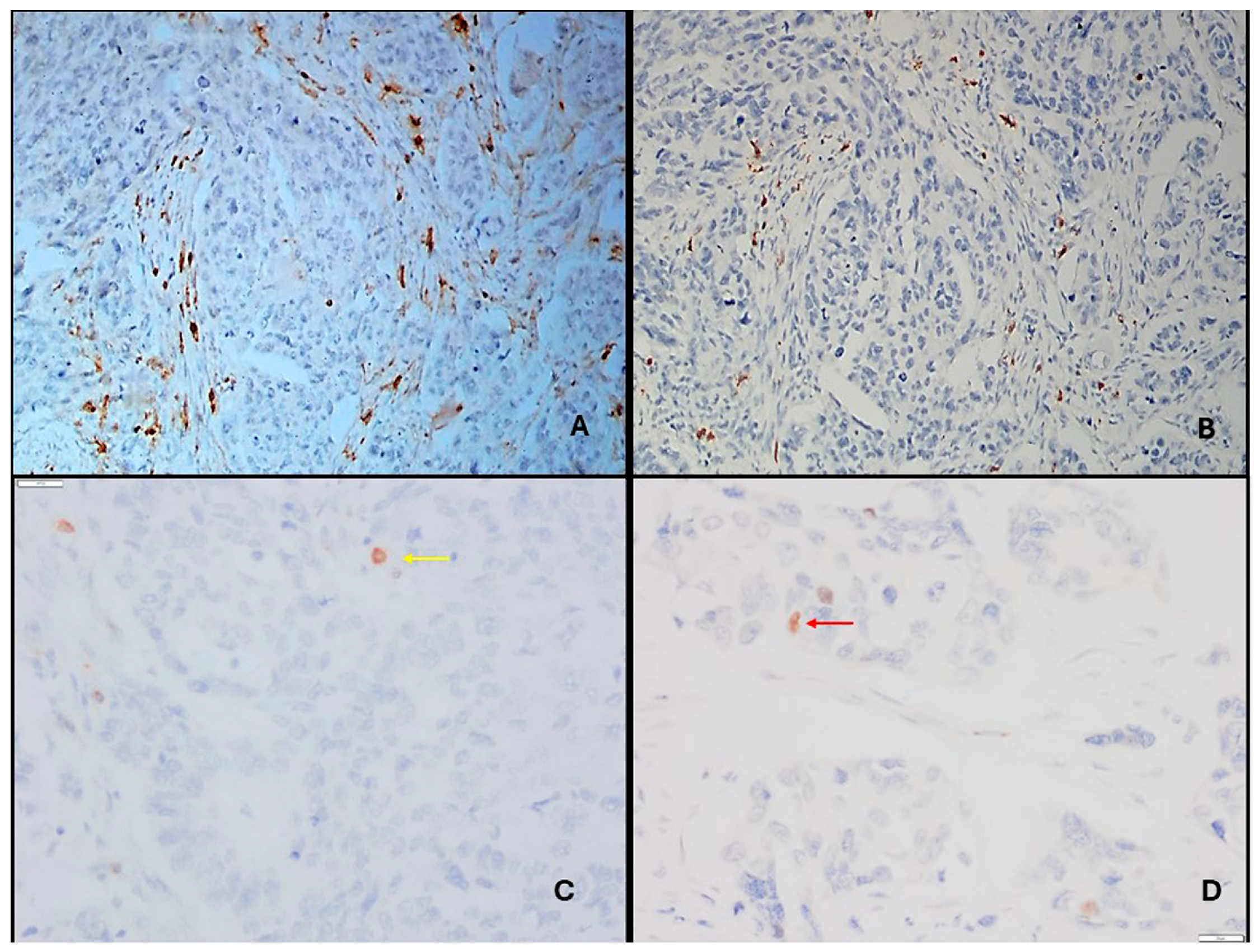
2.2. Immunohistochemical Staining
- We picked matching anatomical landmarks (tumor glands, ducts, vessels, nerves, fibrosis) to align the three slides.
- We identified the areas with dense CD4+ T cell infiltrates (peritumoral stroma and tumor nests).
- In those same foci on the FOXP3 slide, we counted nuclear FOXP3+ lymphocytes.
- In the corresponding area on the CD25 slide, we checked that many of those lymphocytes showed clear membranous CD25.
2.3. Immune Scoring
2.4. Statistical Analysis
3. Results
3.1. Clinical and Immunobiological Findings
3.1.1. Patient Demographics and Cellular Distribution
3.1.2. Survival and Risk Factors Affecting Survival
4. Discussion
4.1. Th and Tc Cell Infiltration: Alignment with Existing Evidence
4.2. Non-Prognostic Role of Tregs: Discrepancy and Interpretation
4.3. Integrating Translational Mechanisms with Clinical Observations
4.4. Biological and Methodological Considerations
4.5. Clinical and Translational Implications
4.6. Strength of Positive Prognostic Role of Intratumoral Effector T Cells
4.7. Future Therapeutic Directions
- a.
- Enhancing T cell recruitment into tumor nests (e.g., CXCR4/CXCL12 inhibitors, CD40 agonists).
- b.
- Reprogramming suppressive stromal and myeloid networks (CAF-dominated stroma) that limit effector T cell access.
- c.
- Selective, not global, Treg modulation, such as through CCR4 or CXCR4 antagonism, adenosine pathway blockade, or bispecific antibodies (e.g., PD-L1/TGF-β inhibitors).
- d.
- Developing spatially informed biomarkers based on digital pathology, multiplex IHC, and single-cell technologies.
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAFs | cancer-associated fibroblasts |
| CIs | confidence intervals |
| CTL | cytotoxic T lymphocyte |
| FOXP3 | forkhead box P3 |
| H&E | hematoxylin and eosin |
| HR | hazard ratio |
| IHC | immunohistochemistry |
| IT | intratumoral |
| MDSCs | myeloid-derived suppressor cells |
| mo | months |
| OR | odds ratio |
| OS | overall survival |
| PFS | progression-free survival |
| PT | peritumoral |
| PDAC | pancreatic ductal adenocarcinoma |
| TAMs | tumor-associated macrophages |
| Tc | cytotoxic T cells |
| TGF-β | transforming growth factor beta |
| Th | T helper cells |
| TME | tumor microenvironment |
| Tregs | regulatory T cells |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Michl, P.; Frese, K.K.; Feig, C.; Cook, N.; Jacobetz, M.A.; Lolkema, M.P.; Buchholz, M.; Olive, K.P.; Gress, T.M.; et al. Stromal biology and therapy in pancreatic cancer. Gut 2011, 60, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Mota Reyes, C.; Demir, E.; Çifcibaşı, K.; Istvanffy, R.; Friess, H.; Demir, I.E. Regulatory T Cells in Pancreatic Cancer: Of Mice and Men. Cancers 2022, 14, 4582. [Google Scholar] [CrossRef] [PubMed]
- Chouari, T.; La Costa, F.S.; Merali, N.; Jessel, M.-D.; Sivakumar, S.; Annels, N.; Frampton, A.E. Advances in Immunotherapeutics in Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 4265. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Xu, D.; Liao, M.M.; Sun, Y.; Bao, W.-D.; Yao, F.; Ma, L. Barriers and opportunities in pancreatic cancer immunotherapy. npj Precis. Oncol. 2024, 8, 199. [Google Scholar] [CrossRef]
- Yousuf, S.; Qiu, M.; Voith von Voithenberg, L.; Hulkkonen, J.; Macinkovic, I.; Schulz, A.R.; Hartmann, D.; Mueller, F.; Mijatovic, M.; Ibberson, D.; et al. Spatially Resolved Multi-Omics Single-Cell Analyses Inform Mechanisms of Immune Dysfunction in Pancreatic Cancer. Gastroenterology 2023, 165, 891–908.e14. [Google Scholar] [CrossRef]
- Chen, M.M.; Gao, Q.; Ning, H.; Chen, K.; Gao, Y.; Yu, M.; Liu, C.-Q.; Zhou, W.; Pan, J.; Wei, L.; et al. Integrated single-cell and spatial transcriptomics uncover distinct cellular subtypes involved in neural invasion in pancreatic cancer. Cancer Cell 2025, 43, 1656–1676.e10. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yanagimoto, H.; Satoi, S.; Toyokawa, H.; Hirooka, S.; Yamaki, S.; Yui, R.; Yamao, J.; Kim, S.; Kwon, A.-H. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 2012, 41, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, M.; Zlobec, I.; Perren, A.; Koelzer, V.H.; Gloor, B.; Lugli, A.; Karamitopoulou, E. Accumulation of FOXP3+ T-cells in the tumor microenvironment is associated with an epithelial-mesenchymal-transition-type tumor budding phenotype and is an independent prognostic factor in surgically resected pancreatic ductal adenocarcinoma. Oncotarget 2015, 6, 4190–4201. [Google Scholar] [CrossRef]
- Carstens, J.L.; Correa de Sampaio, P.; Yang, D.; Barua, S.; Wang, H.; Rao, A.; Allison, J.P.; LeBleu, V.S.; Kalluri, R. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 2017, 8, 15095. [Google Scholar] [CrossRef]
- Angelin, A.; Gil-de-Gómez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J.; Kopinski, P.K.; Wang, L.; et al. FOXP3 reprograms T-cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017, 25, 1282–1293.e7. [Google Scholar] [CrossRef]
- de Reuver, P.R.; Mehta, S.; Gill, P.; Andrici, J.; D’Urso, L.; Clarkson, A.; Mittal, A.; Hugh, T.J.; Samra, J.S.; Gill, A.J. Immunoregulatory Forkhead Box Protein p3-Positive Lymphocytes Are Associated with Overall Survival in Patients with Pancreatic Neuroendocrine Tumors. J. Am. Coll. Surg. 2016, 222, 281–287. [Google Scholar] [CrossRef]
- Seo, Y.D.; Pillarisetty, V.G. T-cell programming in pancreatic adenocarcinoma: A review. Cancer Gene Ther. 2017, 24, 106–113. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.-C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef]
- Sun, D.; Hu, Y.; Peng, J.; Wang, S. Construction of T-Cell-Related Prognostic Risk Models and Prediction of Tumor Immune Microenvironment Regulation in Pancreatic Adenocarcinoma via Integrated Analysis of Single-Cell RNA-Seq and Bulk RNA-Seq. Int. J. Mol. Sci. 2025, 26, 2384. [Google Scholar] [CrossRef]
- Fukunaga, A.; Miyamoto, M.; Cho, Y.; Murakami, S.; Kawarada, Y.; Oshikiri, T.; Kato, K.; Kurokawa, T.; Suzuoki, M.; Nakakubo, Y.; et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004, 28, e26–e31. [Google Scholar] [CrossRef]
- Lang, X.; Wang, X.; Han, M.; Guo, Y. Nanoparticle-Mediated Synergistic Chemoimmunotherapy for Cancer Treatment. Int. J. Nanomed. 2024, 19, 4533–4568. [Google Scholar] [CrossRef]
- Kim, M.H.; Jang, M.; Kim, H.; Lee, W.J.; Kang, C.M.; Choi, H.J. Distinct immunological properties of the two histological subtypes of adenocarcinoma of the ampulla of Vater. Cancer Immunol. Immunother. 2019, 68, 443–454. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Bajor, D.L.; Winograd, R.; Evans, R.A.; Bayne, L.J.; Beatty, G.L. CD40 immunotherapy for pancreatic cancer. Cancer Immunol. Immunother. 2013, 62, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Richmond, A.; Yan, C. Harnessing the potential of CD40 agonism in cancer therapy. Cytokine Growth Factor. Rev. 2024, 75, 40–56. [Google Scholar] [CrossRef]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, K.; Zhao, R.; Ji, T.; Wang, X.; Yang, X.; Zhang, Y.; Cheng, K.; Liu, S.; Hao, J.; et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials 2016, 102, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, X.; Guo, S.; Zhang, C.; Tang, Y.; Tian, Y.; Ni, B.; Lu, B.; Wang, H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS ONE 2014, 9, e91551. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Chougnet, C.; Hildeman, D. Helios-controller of Treg stability and function. Transl. Cancer Res. 2016, 5 (Suppl. S2), S338–S341. [Google Scholar] [CrossRef] [PubMed]
- Leao, I.C.; Ganesan, P.; Armstrong, T.D.; Jaffee, E.M. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8+ T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin. Transl. Sci. 2008, 1, 228–239. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, Z.; Yan, S.; Chen, B.; Wu, H.; Cao, H.; Lin, C.; Liu, Z. Metabolic and immune crosstalk between cancer-associated fibroblasts and pancreatic cancer cells. J. Transl. Med. 2025, 23, 1118. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.H.; Li, N. Immune evasion in cancer: Mechanisms and cutting-edge therapeutic approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef]
- Shan, F.; Somasundaram, A.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer 2022, 8, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, H.; Liang, Z. Cellular crosstalk of regulatory T cells in pancreatic ductal adenocarcinoma. J. Pancreatol. 2024, 7, 131–140. [Google Scholar] [CrossRef]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, J.; Li, A.; Niu, M.; Yan, Y.; Jiao, Y.; Luo, S.; Zhou, P.; Wu, K. The construction, expression, and enhanced anti-tumor activity of YM101: A bispecific antibody simultaneously targeting TGF-β and PD-L1. J. Hematol. Oncol. 2021, 14, 27. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, J.; Chen, Q.; Xu, X.; Gao, J.; Li, X.; Shao, Q.; Zhou, B.; Zhou, H.; Wei, S.; et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. 2022, 39, 110986. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, Y.; Zhao, C.; Ma, P.; Zeng, S.; Ju, D.; Zhao, M.; Yu, M.; Shi, Y. Regulatory T cells in homeostasis and disease: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2025, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J.; Zhao, H.; Liu, Z.; Zhang, Y.; Liu, X.; Wang, F. Infiltrating CD4/CD8 high T cells shows good prognostic impact in pancreatic cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 8820–8828. [Google Scholar] [PubMed]

| n = 62 | |
|---|---|
| Age, median (min–max) | 66 (47–86) |
| Gender (male/female) | 37(59.7)/25(40.3) |
| ECOG PS, median (min–max) | 1 (0–3) |
| <2 | 43 (69.4) |
| ≥2 | 19 (30.6) |
| Tumor size, median (min–max) | 3 (1–9.5) |
| LVI, n (%) | 49 (80.3) |
| PNI, n (%) | 57 (93.4) |
| Grade, n (%) | |
| 1 | 1 (1.6) |
| 2 | 54 (88.6) |
| 3 | 6 (9.8) |
| PT, median (min–max) | |
| Th PT | 41.8 (0–181) |
| Tc PT | 47.4 (0–122) |
| Treg PT | 2.9 (0–62.8) |
| IT, median (min–max) | |
| Th IT | 16.8 (0–216) |
| Tc IT | 19.6 (0–100) |
| Treg IT | 2.2 (0–27) |
| Relative abundance (density gradient) | |
| Th IT/PT | 0.39 (0.04–2.3) |
| Tc IT/PT | 0.47 (0.04–1.76) |
| Treg IT/PT | 0.57 (0–14) |
| OS | PFS | |||
|---|---|---|---|---|
| Median Survival, Months (95% CI) | p | Median Survival, Months (95% CI) | p | |
| Gender | ||||
| Female | 16 (10.839–21.161) | 0.160 | 7 (4.681–9.319) | 0.326 |
| Male | 9 (5.664–12.336) | 5 (3.082–6.918) | ||
| Tumor size | ||||
| ≤3 | 16 (12.326–19.674) | 0.124 | 5 (2.619–7.381) | 0.174 |
| >3 | 9 (7.322–10.678) | 4 (0.215–7.785) | ||
| LVI | ||||
| No | 14 (10.124–17.876) | 0.190 | 6 (1.371–10.629) | 0.351 |
| Yes | 10 (7.235–12.765) | 5 (3.028–6.972) | ||
| PNI | ||||
| No | 2 (0–12.78) | 0.248 | 5 (0–10.988) | 0.911 |
| Yes | 12 (8.266–15.734) | 5 (2.822–7.178) | ||
| Grade | ||||
| 1–2 | 12 (7.744–16.256) | 0.430 | 5 (3.081–6.919) | 0.118 |
| 3 | 8 (0–17.602) | 4 (1.737–6.263) | ||
| Liver metastases | ||||
| No | 12 (6.271–17.729) | 0.803 | 6 (3.084–8.916) | 0.265 |
| Yes | 11 (6.038–15.962) | 4 (2.576–5.424) | ||
| Lung metastases | ||||
| No | 9 (5.758–12.242) | 0.472 | 6 (2.702–9.298) | 0.364 |
| Yes | 14 (8.978–19.022) | 5 (3.749–6.251) | ||
| Bone metastases | ||||
| No | 12 (8.663–15.337) | 0.395 | 5 (3.302–6.698) | 0.898 |
| Yes | 9 (0–20.202) | 6 (NA) | ||
| Lymph node metastases | ||||
| No | 14 (11.69–16.31) | 0.334 | 8 (6.952–9.048) | 0.221 |
| Yes | 10 (6.59–13.41) | 4 (2.701–5.299) | ||
| Omental metastases | ||||
| No | 14 (8.413–19.587) | 0.262 | 6 (4.195–7.805) | 0.506 |
| Yes | 10 (6.056–13.944) | 5 (3.864–6.136) | ||
| First-line chemotherapy type | ||||
| Gemcitabine mono | 8 (4.329–11.671) | 0.001 | 3 (1.973–4.027) | 0.007 |
| Combination chemo | 16 (12.174–19.826) | 7 (4.009–9.991) | ||
| Second-line chemotherapy | ||||
| No | 8 (5.27–10.73) | 0.004 | 5 (3.292–6.708) | 0.722 |
| Yes | 17 (15.805–18.195) | 6 (2.636–9.364) | ||
| OS | PFS | |||
|---|---|---|---|---|
| Median Survival, Months (95% CI) | p | Median Survival, Months (95% CI) | p | |
| Th IT * | ||||
| ≤16.8 | 8 (5.372–10.628) | <0.001 | 4 (2.976–5.024) | 0.001 |
| >16.8 | 17 (12.525–21.475) | 9 (5.946–12.054) | ||
| Th PT * | ||||
| ≤41.8 | 9 (6.465–11.535) | 0.003 | 5 (3.53–6.47) | 0.011 |
| >41.8 | 17 (16.045–17.955) | 8 (3.766–12.234) | ||
| Tc IT * | ||||
| ≤19.6 | 9 (6.489–11.511) | 0.018 | 4 (2.482–5.518) | 0.066 |
| >19.6 | 16 (11.934–20.066) | 7 (4.915–9.085) | ||
| Tc PT * | ||||
| ≤47.4 | 10 (6.067–13.933) | 0.099 | 5 (3.508–6.492) | 0.160 |
| >47.4 | 16 (11.445–20.555) | 6 (2.833–9.167) | ||
| Treg IT * | ||||
| ≤2.2 | 10 (7.234–12.766) | 0.179 | 5 (2.836–7.164) | 0.321 |
| >2.2 | 14 (10.392–17.608) | 5 (1.41–8.59) | ||
| Treg PT * | ||||
| ≤2.9 | 11 (8.058–13.942) | 0.330 | 5 (2.871–7.129) | 0.734 |
| >2.9 | 14 (6.86–21.14) | 5 (2.377–7.623) | ||
| Th IT/PT ** | ||||
| ≤0.39 | 14 (7.363–20.637) | 0.531 | 5 (3.566–6.434) | 0.673 |
| >0.39 | 12 (9.03–14.97) | 6 (3.705–8.295) | ||
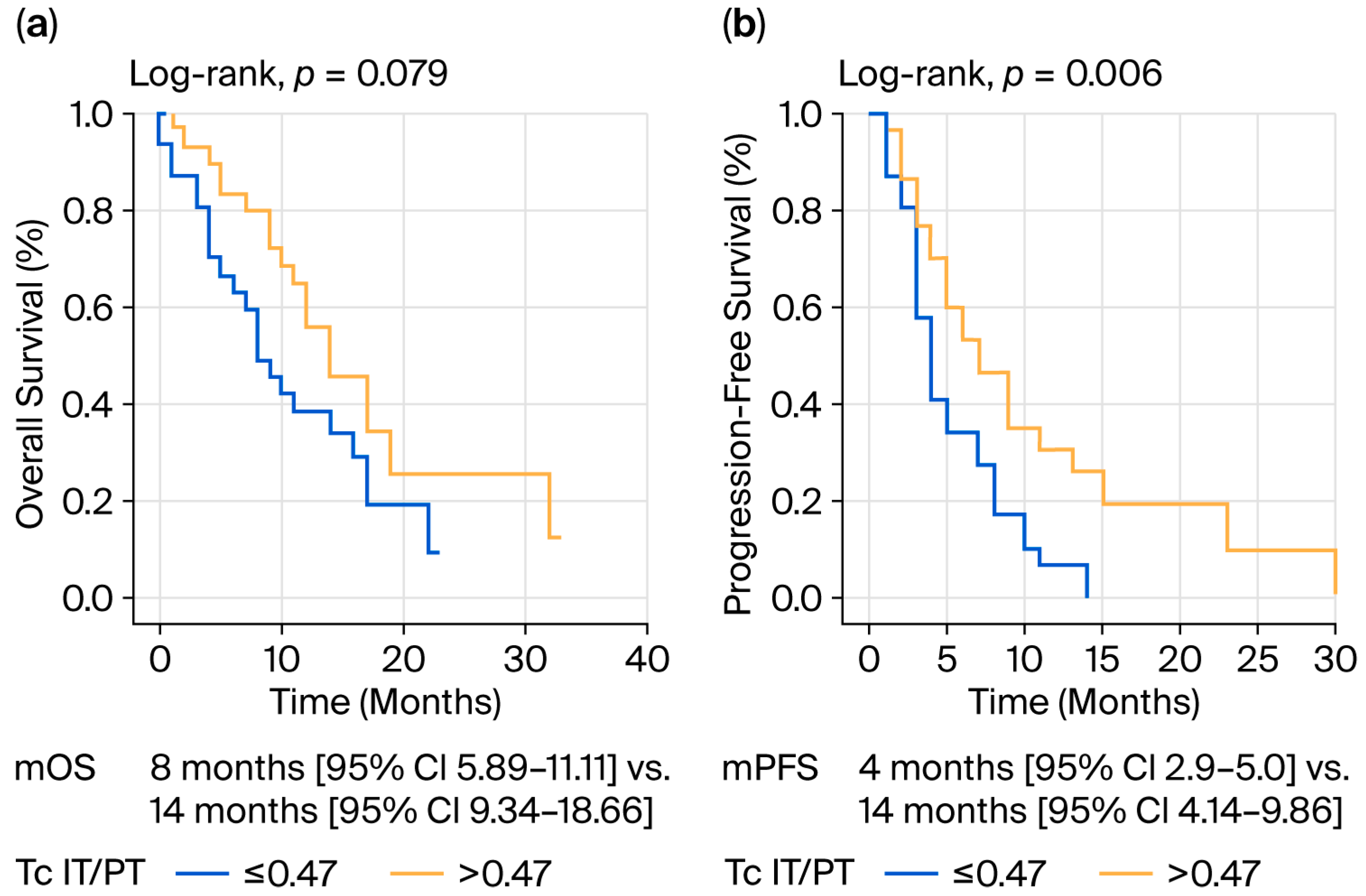
| Tc IT/PT ** | ||||
| ≤0.47 | 8 (4.89–11.11) | 0.079 | 4 (2.971–5.029) | 0.006 |
| >0.47 | 14 (9.341–18.659) | 7 (4.144–9.856) | ||
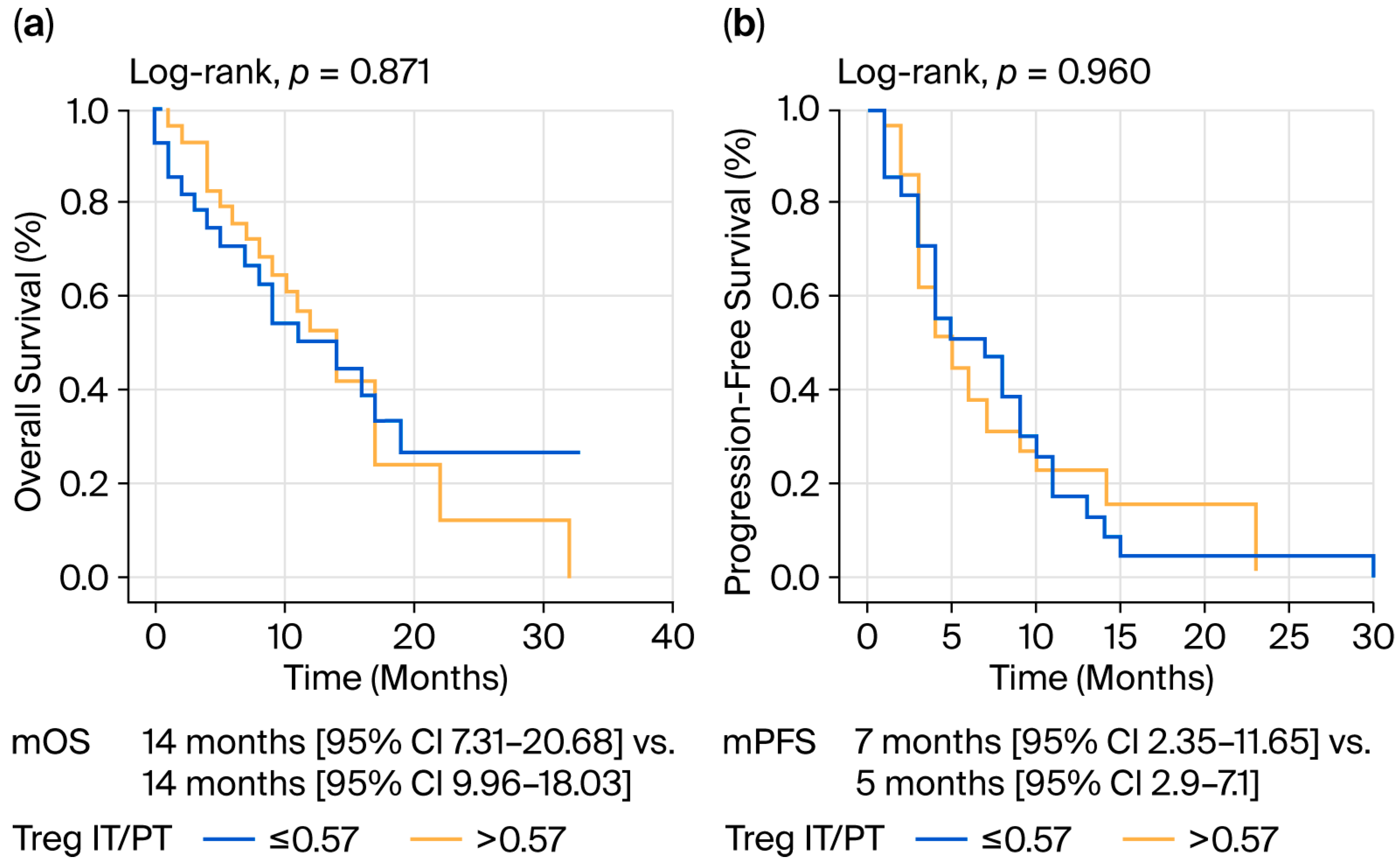
| Treg IT/PT ** | ||||
| ≤0.57 | 14 (7.318–20.682) | 0.871 | 7 (2.35–11.65) | 0.960 |
| >0.57 | 14 (9.961–18.039) | 5 (2.9–7.1) | ||
| Treg/Th IT ** | ||||
| ≤0.13 | 16 (12.001–19.999) | 0.059 | 6 (3.316–8.684) | 0.111 |
| >0.13 | 9 (4.396–13.604) | 4 (2.759–5.241) | ||
| Treg/Th PT ** | ||||
| ≤0.06 | 14 (5.912–22.088) | 0.874 | 6 (3.705–8.295) | 0.594 |
| >0.06 | 11 (6.416–15.584) | 4 (2.326–5.674) | ||
| Treg/Tc IT ** | ||||
| ≤0.11 | 12 (7.087–16.913) | 0.681 | 6 (3.853–8.147) | 0.159 |
| >0.11 | 11 (5.609–16.391) | 4 (3.112–4.888) | ||
| Treg/Tc PT ** | ||||
| ≤0.06 | 12 (8.175–15.825) | 0.565 | 5 (3.461–6.539) | 0.450 |
| >0.06 | 14 (8.881–19.119) | 7 (2.996–11.004) | ||
| OS | PFS | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p | HR (95% CI) | p |
| Age | ||||
| ≤66 | Reference | - | Reference | - |
| >66 | 1.873 (0.826–4.25) | 0.133 | 1.336 (0.648–2.754) | 0.432 |
| ECOG PS | ||||
| <2 | Reference | - | Reference | - |
| ≥2 | 0.81 (0.35–1.875) | 0.623 | 2.03 (0.995–4.14) | 0.052 |
| First-line chemotherapy type | ||||
| Gemcitabine mono | 1.534 (0.707–3.325) | 0.279 | 1.316 (0.667–2.597) | 0.429 |
| Combination chemo | Reference | - | Reference | - |
| Second-line chemotherapy | ||||
| No | 2.434 (1.073–5.518) | 0.033 | ||
| Yes | Reference | - | ||
| Th IT * | ||||
| ≤16.8 | 3.275 (1.171–9.153) | 0.024 | 0.891 (0.32–2.482) | 0.826 |
| >16.8 | Reference | - | Reference | |
| Th PT * | ||||
| ≤41.8 | 1.956 (0.731–5.229) | 0.181 | ||
| >41.8 | Reference | |||
| Tc IT * | ||||
| ≤19.6 | 0.795 (0.334–1.889) | 0.603 | ||
| >19.6 | Reference | - | ||
| Tc IT/PT ** | ||||
| ≤0.47 | 2.437 (1.303–4.556) | 0.005 | ||
| >0.47 | Reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kıvrak Salim, D.; Sadullahoglu, C. The Effect of T Regulatory Cell Infiltration on Survival Outcomes in Metastatic Pancreatic Cancer Patients with a Review of Immunobiology, Prognostic Value and Future Therapeutic Options. J. Clin. Med. 2025, 14, 8394. https://doi.org/10.3390/jcm14238394
Kıvrak Salim D, Sadullahoglu C. The Effect of T Regulatory Cell Infiltration on Survival Outcomes in Metastatic Pancreatic Cancer Patients with a Review of Immunobiology, Prognostic Value and Future Therapeutic Options. Journal of Clinical Medicine. 2025; 14(23):8394. https://doi.org/10.3390/jcm14238394
Chicago/Turabian StyleKıvrak Salim, Derya, and Canan Sadullahoglu. 2025. "The Effect of T Regulatory Cell Infiltration on Survival Outcomes in Metastatic Pancreatic Cancer Patients with a Review of Immunobiology, Prognostic Value and Future Therapeutic Options" Journal of Clinical Medicine 14, no. 23: 8394. https://doi.org/10.3390/jcm14238394
APA StyleKıvrak Salim, D., & Sadullahoglu, C. (2025). The Effect of T Regulatory Cell Infiltration on Survival Outcomes in Metastatic Pancreatic Cancer Patients with a Review of Immunobiology, Prognostic Value and Future Therapeutic Options. Journal of Clinical Medicine, 14(23), 8394. https://doi.org/10.3390/jcm14238394






