Patient with Inflammatory Bowel Disease in a Dental Office—Which Antibiotic to Choose?—Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Bias Minimization
3. Pathophysiology of IBD Relevant to Dental Practice
3.1. Immune Dysregulation and Microbiome Alterations
3.2. Malabsorption and Nutritional Deficiencies
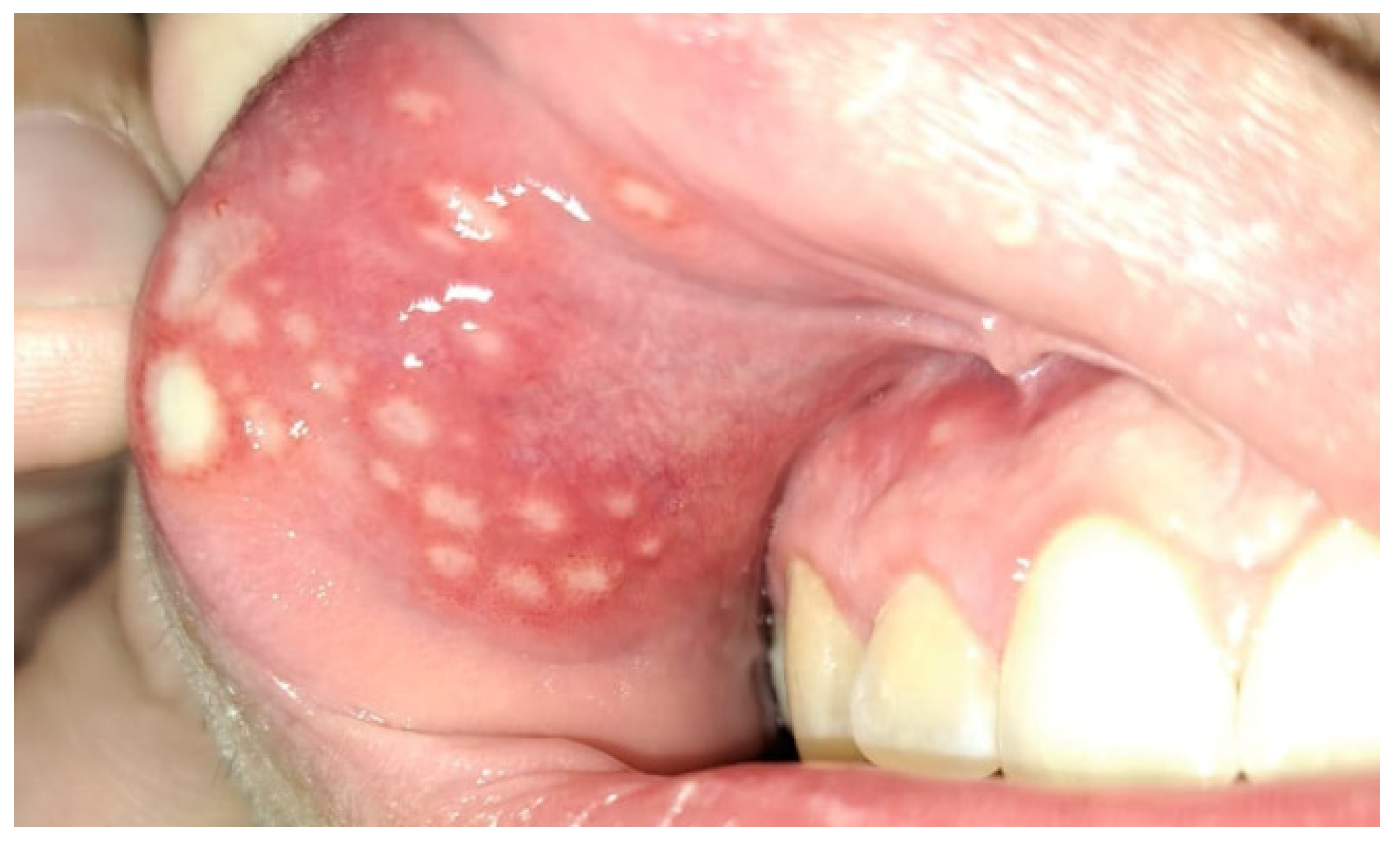
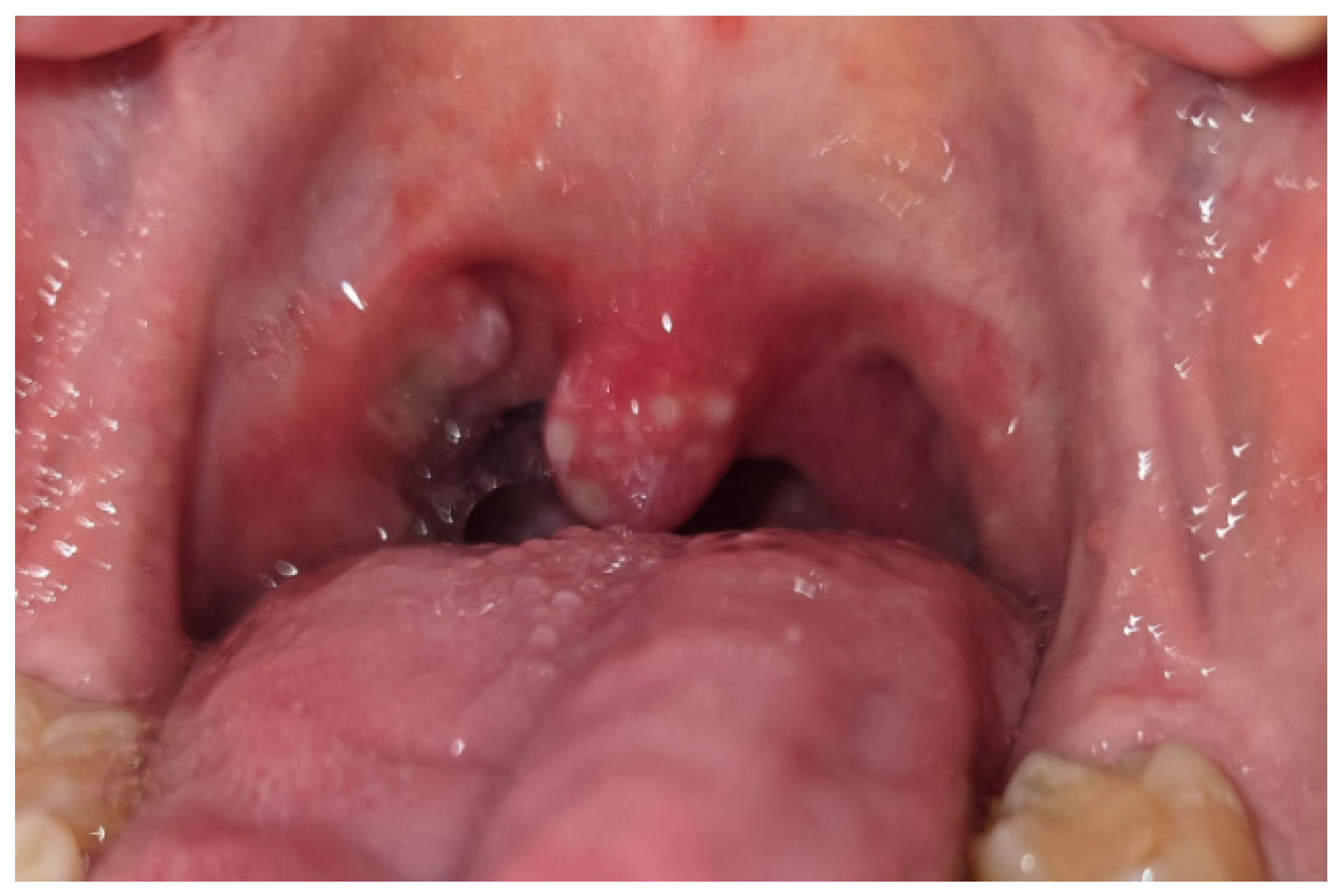
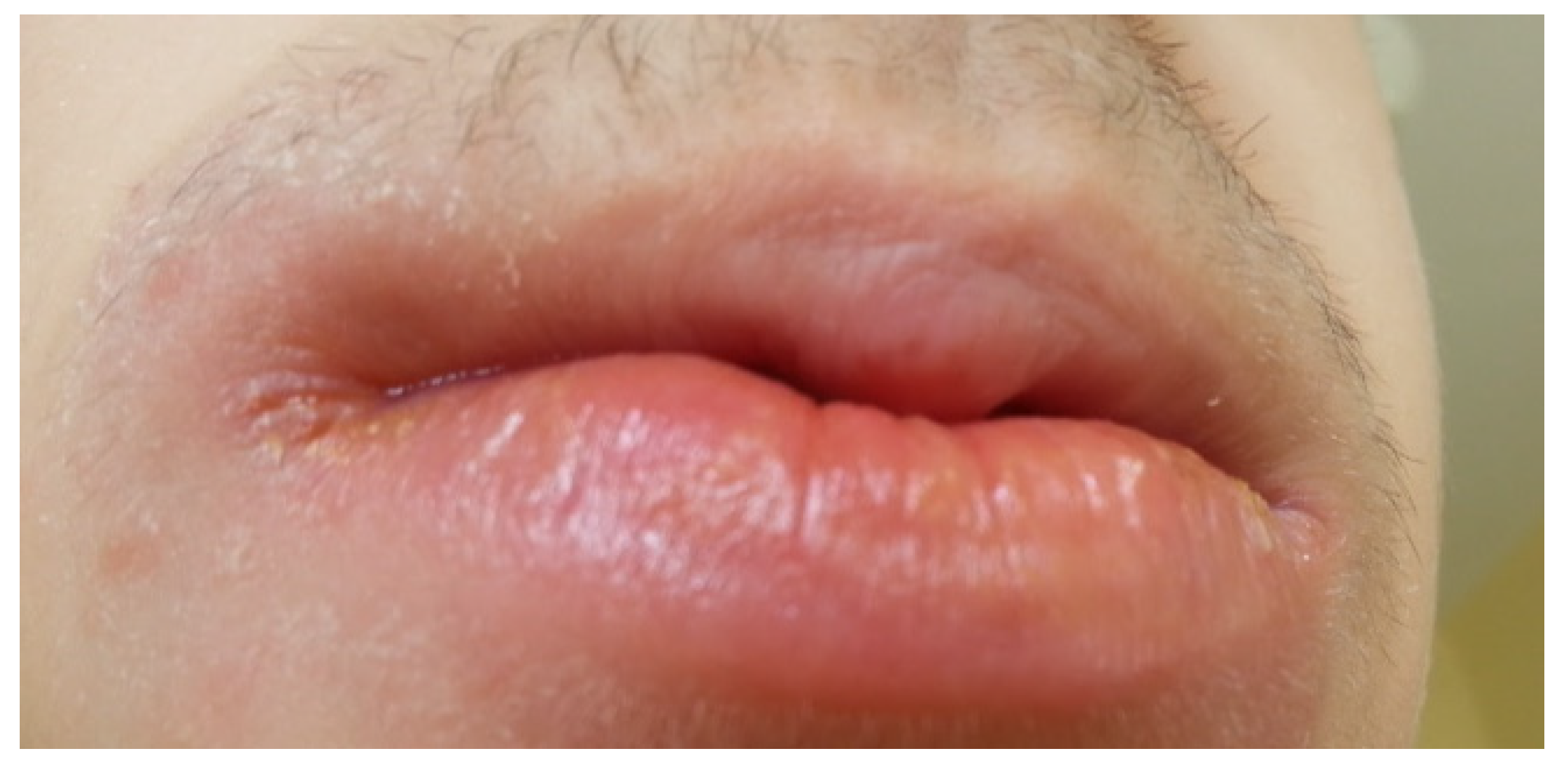
4. Oral Manifestations of IBD and Treatment
4.1. IBD Group Diseases and the Condition of the Oral Cavity
4.2. Treatment of the Oral Lesions
5. Dental Infection Susceptibility in IBD
6. Clostridioides Difficile Infection in IBD Patients
6.1. Why Are Patients with IBD a High-Risk Group?
6.2. Consequences of CDI in This Population
6.3. Antibiotic Risks
7. Management Strategies for Safe Antibiotic Use in Dentistry
7.1. Guidelines of Global Associations
7.2. Metronidazole
7.3. Local Administration of Antibiotic Carriers
7.4. Microbiome-Supportive Adjuncts
8. Discussion
8.1. General Interpretation
8.2. Strengths
8.3. Limitations
8.4. Future Directions
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Elzayat, H.; Mesto, G.; Al-Marzooq, F. Unraveling the Impact of Gut and Oral Microbiome on Gut Health in Inflammatory Bowel Diseases. Nutrients 2023, 15, 3377. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Jess, T. Implications of the Changing Epidemiology of Inflammatory Bowel Disease in a Changing World. UEG J. 2022, 10, 1113–1120. [Google Scholar] [CrossRef]
- Mosli, M.; Alawadhi, S.; Hasan, F.; Abou Rached, A.; Sanai, F.; Danese, S. Incidence, Prevalence, and Clinical Epidemiology of Inflammatory Bowel Disease in the Arab World: A Systematic Review and Meta-Analysis. Inflamm. Intest. Dis. 2021, 6, 123–131. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The Four Epidemiological Stages in the Global Evolution of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Lewis, J.D.; Parlett, L.E.; Jonsson Funk, M.L.; Brensinger, C.; Pate, V.; Wu, Q.; Dawwas, G.K.; Weiss, A.; Constant, B.D.; McCauley, M.; et al. Incidence, Prevalence, and Racial and Ethnic Distribution of Inflammatory Bowel Disease in the United States. Gastroenterology 2023, 165, 1197–1205.e2. [Google Scholar] [CrossRef]
- Park, J.; Cheon, J.H. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med. J. 2021, 62, 99. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Xie, Y.; Zhang, Y.; Jiang, S.; Wang, J.; Luo, X.; Chen, Q. Oral Manifestations Serve as Potential Signs of Ulcerative Colitis: A Review. Front. Immunol. 2022, 13, 1013900. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, D.; Chen, R.; Zhu, F.; Gong, J.; Yan, F. The Association between Periodontitis and Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. BioMed Res. Int. 2021, 2021, 6692420. [Google Scholar] [CrossRef]
- Del Vecchio, L.E.; Fiorani, M.; Tohumcu, E.; Bibbò, S.; Porcari, S.; Mele, M.C.; Pizzoferrato, M.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. Risk Factors, Diagnosis, and Management of Clostridioides Difficile Infection in Patients with Inflammatory Bowel Disease. Microorganisms 2022, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, S.K.; Oliva-Hemker, M.; Hutfless, S. The Prevalence of Clostridium Difficile Infection in Pediatric and Adult Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2014, 59, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.M.; Kelly, C.P.; Farraye, F.A. Clostridium Difficile Infection in the Inflammatory Bowel Disease Patient. Inflamm. Bowel Dis. 2013, 19, 194–204. [Google Scholar] [CrossRef]
- D’Aoust, J.; Battat, R.; Bessissow, T. Management of Inflammatory Bowel Disease with Clostridium Difficile Infection. World J. Gastroenterol. 2017, 23, 4986. [Google Scholar] [CrossRef]
- Sehgal, K.; Yadav, D.; Khanna, S. The Interplay of Clostridioides Difficile Infection and Inflammatory Bowel Disease. Ther. Adv. Gastroenterol. 2021, 14, 17562848211020285. [Google Scholar] [CrossRef]
- Martirosian, G.; Hryniewicz, W.; Ozorowski, T.; Pawlik, K.; Deptuła, A. Zakażenia Clostridioides (Clostridium) Difficile: Epidemiologia, Diagnostyka, Terapia, Profilaktyka; Wydanie Drugie; Narodowy Instytut Leków: Warszawa, Poland, 2018; ISBN 978-83-949636-0-6. [Google Scholar]
- Kaczmarzyk, T.; Babiuch, K.; Bołtacz-Rzepkowska, E.; Dominiak, M.; Konopka, T.; Lipski, M.; Olczak-Kowalczyk, D.; Szeląg, A.; Szuta, M.; Hryniewicz, W. Polish Dental Association and National Programme to Protect Antibiotics Working Group Recommendations for Administration of Antibiotics in Dentistry. J. Stomatol. 2018, 71, 457–465. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Pokatayev, V.; Lefkovith, A.; Carter, G.T.; Creasey, E.A.; Krishna, C.; Subramanian, S.; Kochar, B.; Ashenberg, O.; Lau, H.; et al. The Landscape of Immune Dysregulation in Crohn’s Disease Revealed through Single-Cell Transcriptomic Profiling in the Ileum and Colon. Immunity 2023, 56, 444–458.e5. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A. Alteration of the Gut Microbiome in Inflammatory Bowel Disease. Digestion 2023, 104, 16–23. [Google Scholar] [CrossRef]
- Xu, X.; Ocansey, D.K.W.; Hang, S.; Wang, B.; Amoah, S.; Yi, C.; Zhang, X.; Liu, L.; Mao, F. The Gut Metagenomics and Metabolomics Signature in Patients with Inflammatory Bowel Disease. Gut Pathog. 2022, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Mustafi, S.; Sinha, R.; Sarkar, S.; Giri, D.; Saha, A.P.; Yadav, P. Comparative Estimation of Serum Levels of Vitamin A, Vitamin B12, Vitamin D and Vitamin E in Patients with Recurrent Aphthous Stomatitis and Normal Individuals—A Case-Control Study. J. Indian. Acad. Oral Med. Radiol. 2021, 33, 442–446. [Google Scholar] [CrossRef]
- Valvano, M.; Capannolo, A.; Cesaro, N.; Stefanelli, G.; Fabiani, S.; Frassino, S.; Monaco, S.; Magistroni, M.; Viscido, A.; Latella, G. Nutrition, Nutritional Status, Micronutrients Deficiency, and Disease Course of Inflammatory Bowel Disease. Nutrients 2023, 15, 3824. [Google Scholar] [CrossRef]
- Valvano, M.; Faenza, S.; Cortellini, F.; Vinci, A.; Ingravalle, F.; Calabrò, M.; Scurti, L.; Di Nezza, M.; Valerio, S.; Viscido, A.; et al. The Relationship Between Nutritional Status, Micronutrient Deficiency, and Disease Activity in IBD Patients: A Multicenter Cross-Sectional Study. Nutrients 2025, 17, 2690. [Google Scholar] [CrossRef]
- Muhvić-Urek, M.; Tomac-Stojmenović, M.; Mijandrušić-Sinčić, B. Oral Pathology in Inflammatory Bowel Disease. World J. Gastroenterol. 2016, 22, 5655. [Google Scholar] [CrossRef]
- Alkhouri, R.H.; Hashmi, H.; Baker, R.D.; Gelfond, D.; Baker, S.S. Vitamin and Mineral Status in Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 89–92. [Google Scholar] [CrossRef]
- Kim, S.Y.; Mun, E.C.; Chung, J.-W.; Ha, M.; Ahn, S.-M.; Han, M.-D.; Han, S.-H.; Yun, S.-C.; Kim, J.H.; Kim, K.O.; et al. Increased Genomic Damage and Vitamin B Status in Inflammatory Bowel Disease Patients: A Case-Control, Prospective, Pilot Study. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 837, 42–47. [Google Scholar] [CrossRef]
- Le, T. Micronutrient Deficiencies in Patients with Inflammatory Bowel Disease. Cutis 2024, 113, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Cianconi, G.; De Angelis, R.; Pujia, A.M.; Arcuri, C. Hypovitaminosis and Its Association with Recurrent Aphthous Stomatitis: A Comprehensive Review of Clinical Correlations and Diagnostic Considerations. Front. Oral Health 2025, 6, 1520067. [Google Scholar] [CrossRef]
- Esquivel-Pedraza, L.; Fernández-Cuevas, L.; Delgado-Martínez, I.; Cicero-Casarrubias, A.; Milke-García, M.D.P.; Chang-Bool, E.M.; Barragán-Heredia, L.M.; Maldonado-Molina, J.; Rivera-Flores, R.L.; Yamamoto-Furusho, J.K.; et al. Oral Mucosal Findings in Ambulatory Patients with Inflammatory Bowel Disease. Braz. Oral Res. 2025, 39, e095. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.L.; Kurian, D.; Elliott, M.S.; Han, Z. Vitamin D Deficiency in Patients with Intestinal Malabsorption Syndromes—Think in and Outside the Gut. J. Dig. Dis. 2015, 16, 617–633. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, H.; Schmidt, M.; Huth, A.; Reiner, J.; Glass, Ä.; Lamprecht, G. Clinical Factors Are Associated with Vitamin D Levels in IBD Patients: A Retrospective Analysis. J. Dig. Dis. 2018, 19, 24–32. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Al-Qadhi, G.; Halboub, E.; Alaizari, N.; Almeslet, A.; Ali, K.; Osman, S.A.A. Vitamin D Deficiency and Risk of Recurrent Aphthous Stomatitis: Updated Meta-Analysis with Trial Sequential Analysis. Front. Nutr. 2023, 10, 1132191. [Google Scholar] [CrossRef]
- Lauritano, D.; Boccalari, E.; Stasio, D.; Vella, D.F.; Carinci, F.; Lucchese, A.; Petruzzi, M. Prevalence of Oral Lesions and Correlation with Intestinal Symptoms of Inflammatory Bowel Disease: A Systematic Review. Diagnostics 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Saeid Seyedian, S.; Nokhostin, F.; Dargahi Malamir, M. A Review of the Diagnosis, Prevention, and Treatment Methods of Inflammatory Bowel Disease. JMedLife 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, S.; Niemczyk, W.; Prokurat, M.; Grudnik, K.; Kuleszyński, M.; Niciejewska, E.; Lau, K.; Kasperczyk, J. Impact of E-Cigarettes on the Oral Health-Literature Review. Polski Merkur. Lek. 2023, 51, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.W.; Brand, H.S.; Iqbal, S.; De Boer, N.K.H.; Forouzanfar, T.; De Visscher, J.G.A.M. A Self-Reported Survey on Oral Health Problems in Patients with Inflammatory Bowel Disease with a Stoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, e80–e86. [Google Scholar] [CrossRef]
- Agossa, K.; Roman, L.; Gosset, M.; Yzet, C.; Fumery, M. Periodontal and Dental Health in Inflammatory Bowel Diseases: A Systematic Review. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 1–15. [Google Scholar] [CrossRef]
- Khozeimeh, F.; Shakerin, H.; Daghaghzadeh, H.; Najarzadegan, F.; Golestannejad, Z.; Adibi, P. Oral Manifestations in Inflammatory Bowel Disease: A Cross-Sectional Study in Isfahan. Dent. Res. J. 2021, 18, 4. [Google Scholar] [CrossRef]
- Tan, C.X.W.; Brand, H.S.; De Boer, N.K.H.; Forouzanfar, T. Gastrointestinal Diseases and Their Oro-Dental Manifestations: Part 2: Ulcerative Colitis. Br. Dent. J. 2017, 222, 53–57. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Brigo, S.; Mangia, M.; Saracco, G.M.; Astegiano, M.; Pellicano, R. Oral Manifestations of Inflammatory Bowel Disease and the Role of Non-Invasive Surrogate Markers of Disease Activity. Medicines 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.H.; Torres, J.; Roda, G.; Brygo, A.; Delaporte, E.; Colombel, J.-F. Review Article: Non-malignant Oral Manifestations in Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2015, 42, 40–60. [Google Scholar] [CrossRef]
- Jundt, J.S.; Gutta, R. Characteristics and Cost Impact of Severe Odontogenic Infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 558–566. [Google Scholar] [CrossRef]
- Piekoszewska-Ziętek, P.; Turska-Szybka, A.; Olczak-Kowalczyk, D. Odontogenic Infections—Review of the Literature. Nowa Stomatol. 2016, 21, 120–134. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Kuśka-Kielbratowska, A.; Fiegler-Rudol, J.; Niemczyk, W.; Mertas, A.; Skaba, D.; Wiench, R. The Prevalence and Drug Susceptibility of Candida Species and an Analysis of Risk Factors for Oral Candidiasis—A Retrospective Study. Antibiotics 2025, 14, 876. [Google Scholar] [CrossRef]
- Fiegler-Rudol, J.; Niemczyk, W.; Janik, K.; Zawilska, A.; Kępa, M.; Tanasiewicz, M. How to Deal with Pulpitis: An Overview of New Approaches. Dent. J. 2025, 13, 25. [Google Scholar] [CrossRef]
- Marruganti, C.; Discepoli, N.; Gaeta, C.; Franciosi, G.; Ferrari, M.; Grandini, S. Dental Caries Occurrence in Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Caries Res. 2021, 55, 485–495. [Google Scholar] [CrossRef]
- Nijakowski, K.; Gruszczyński, D.; Surdacka, A. Oral Health Status in Patients with Inflammatory Bowel Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11521. [Google Scholar] [CrossRef]
- Marafini, I.; Salvatori, S.; Troncone, E.; Scarozza, P.; Fantini, E.; Monteleone, G. No Effect of a Liquid Diet in the Management of Patients with Stricturing Crohn’s Disease. Int. J. Color. Dis. 2020, 35, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Adamina, M.; Gerasimidis, K.; Sigall-Boneh, R.; Zmora, O.; De Buck Van Overstraeten, A.; Campmans-Kuijpers, M.; Ellul, P.; Katsanos, K.; Kotze, P.G.; Noor, N.; et al. Perioperative Dietary Therapy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Fiegler-Rudol, J.; Niemczyk, W.; Netkowska, M.; Demel, K.; Talaska, J.; Klimczak, T.; Hochuł, D. The Influence of Parenteral Nutrition on the Condition of the Oral Cavity: Literature Review. Wiad. Lek. 2024, 77, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium Difficile Infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, T.; Esther, L. Análisis de La Producción de Factores de Virulencia y Resistencia a Antibióticos En Biopelícula de Clostridioides (Clostridium) Difficile En Infecciones Recurrentes y No Recurrentes; Universidad Autónoma Nuevo León: San Nicolas de los Garza, Mexico, 2019. [Google Scholar]
- Smits, W.K.; Lyras, D.; Lacy, D.B.; Wilcox, M.H.; Kuijper, E.J. Clostridium Difficile Infection. Nat. Rev. Dis. Primers 2016, 2, 16020. [Google Scholar] [CrossRef]
- Clayton, E.M.; Rea, M.C.; Shanahan, F.; Quigley, E.M.M.; Kiely, B.; Hill, C.; Ross, R.P. The Vexed Relationship Between Clostridium Difficile and Inflammatory Bowel Disease: An Assessment of Carriage in an Outpatient Setting Among Patients in Remission. Am. J. Gastroenterol. 2009, 104, 1162–1169. [Google Scholar] [CrossRef]
- Singh, H.; Nugent, Z.; Yu, B.N.; Lix, L.M.; Targownik, L.E.; Bernstein, C.N. Higher Incidence of Clostridium Difficile Infection Among Individuals with Inflammatory Bowel Disease. Gastroenterology 2017, 153, 430–438.e2. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Margalit, M.; Bossuyt, P.; Maul, J.; Shapira, Y.; Bojic, D.; Chermesh, I.; Al-Rifai, A.; Schoepfer, A.; Bosani, M.; et al. Combination Immunomodulator and Antibiotic Treatment in Patients with Inflammatory Bowel Disease and Clostridium Difficile Infection. Clin. Gastroenterol. Hepatol. 2009, 7, 981–987. [Google Scholar] [CrossRef]
- Khanna, S. Management of Clostridioides Difficile Infection in Patients with Inflammatory Bowel Disease. Intest. Res. 2021, 19, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Balram, B.; Battat, R.; Al-Khoury, A.; D’Aoust, J.; Afif, W.; Bitton, A.; Lakatos, P.L.; Bessissow, T. Risk Factors Associated with Clostridium Difficile Infection in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 2019, 13, 27–38. [Google Scholar] [CrossRef]
- Burisch, J.; Munkholm, P. Inflammatory Bowel Disease Epidemiology. Curr. Opin. Gastroenterol. 2013, 29, 357–362. [Google Scholar] [CrossRef]
- Sunenshine, R.H.; McDonald, L.C. Clostridium Difficile-Associated Disease: New Challenges from an Established Pathogen. Clevel. Clin. J. Med. 2006, 73, 187–197. [Google Scholar] [CrossRef]
- Pattani, R.; Palda, V.A.; Hwang, S.W.; Shah, P.S. Probiotics for the Prevention of Antibiotic-Associated Diarrhea and Clostridium Difficile Infection among Hospitalized Patients: Systematic Review and Meta-Analysis. Open Med. 2013, 7, e56–e67. [Google Scholar] [PubMed]
- Kukla, M.; Cisek, K. Diagnosis, Prophylaxis and Treatment of Clostridium Difficile Infection According to 2017 Guidelines of Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Gastroenterol. Klin. 2019, 11, 43–54. [Google Scholar]
- Flynn, T.R. Evidence-Based Principles of Antibiotic Therapy. In Evidence-Based Oral Surgery; Ferneini, E.M., Goupil, M.T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 283–316. ISBN 978-3-319-91360-5. [Google Scholar]
- Krasuska-Sławińska, E. Zakażenia Zębopochodne, Zapalenie Tkanek Przyzębia Oraz Profilaktyka Zakażeń Odległych–Wskazania Do Antybiotykoterapii. Med. Faktów 2020, 13, 322–330. [Google Scholar] [CrossRef]
- Brown, K.A.; Khanafer, N.; Daneman, N.; Fisman, D.N. Meta-Analysis of Antibiotics and the Risk of Community-Associated Clostridium Difficile Infection. Antimicrob. Agents Chemother. 2013, 57, 2326–2332. [Google Scholar] [CrossRef]
- Tong, D.C.; Rothwell, B.R. Antibiotic Prophylaxis in Dentistry: A Review and Practice Recommendations. J. Am. Dent. Assoc. 2000, 131, 366–374. [Google Scholar] [CrossRef]
- Actis, G.; Pellicano, R.; Fadda, M.; Rosina, F. Antibiotics and Non-Steroidal Anti-Inflammatory Drugs in Outpatient Practice: Indications and Unwanted Effects in a Gastroenterological Setting. Curr. Drug Saf. 2014, 9, 133–137. [Google Scholar] [CrossRef]
- Diogenes, C.; Cha, B. AAE Guidance on Antibiotic Prophylaxis for Patients at Risk of Systemic Disease. Am. Assoc. Endodontists 2017, 43, 1409–1413. [Google Scholar]
- Segura-Egea, J.J.; Gould, K.; Hakan Şen, B.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjäderhane, L.; Dummer, P.M.H. European Society of Endodontology Position Statement: The Use of Antibiotics in Endodontics. Int. Endod. J. 2018, 51, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.H.; Dayer, M.; Lockhart, P.B.; McGurk, M.; Shanson, D.; Prendergast, B.; Chambers, J.B. A Change in the NICE Guidelines on Antibiotic Prophylaxis. Br. Dent. J. 2016, 221, 112–114. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Tampi, M.P.; Abt, E.; Aminoshariae, A.; Durkin, M.J.; Fouad, A.F.; Gopal, P.; Hatten, B.W.; Kennedy, E.; Lang, M.S.; et al. Evidence-Based Clinical Practice Guideline on Antibiotic Use for the Urgent Management of Pulpal- and Periapical-Related Dental Pain and Intraoral Swelling. J. Am. Dent. Assoc. 2019, 150, 906–921.e12. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Antibiotic pro-Phylaxis for Dental Patients at Risk for Infection. The Reference Manualof Pediatric Dentistry. Chicago, III. Am. Acad. Pediatr. Dent. 2022, 500–506. [Google Scholar]
- Thornhill, M.H.; Gibson, T.B.; Yoon, F.; Dayer, M.J.; Prendergast, B.D.; Lockhart, P.B.; O’Gara, P.T.; Baddour, L.M. Antibiotic Prophylaxis Against Infective Endocarditis Before Invasive Dental Procedures. J. Am. Coll. Cardiol. 2022, 80, 1029–1041. [Google Scholar] [CrossRef]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef]
- Bishop, E.J.; Tiruvoipati, R. Management of Clostridioides Difficile Infection in Adults and Challenges in Clinical Practice: Review and Comparison of Current IDSA/SHEA, ESCMID and ASID Guidelines. J. Antimicrob. Chemother. 2023, 78, 21–30. [Google Scholar] [CrossRef]
- Smith, A. Metronidazole Resistance: A Hidden Epidemic? Br. Dent. J. 2018, 224, 403–404. [Google Scholar] [CrossRef]
- Mitchell, D.A. Metronidazole: Its Use in Clinical Dentistry. J. Clin. Periodontol. 1984, 11, 145–158. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic Therapy in Dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Vermeire, S.; Van Assche, G.; Noman, M.; Aerden, I.; Van Olmen, G.; Rutgeerts, P. Therapy of Metronidazole with Azathioprine to Prevent Postoperative Recurrence of Crohn’s Disease: A Controlled Randomized Trial. Gastroenterology 2008, 135, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Ceruelos, A.H.; Romero-Quezada, L.C.; Ruvalcaba, J.C.; Contreras, L.L. Therapeutic Uses of Metronidazole and Its Side Effects: An Update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar]
- Ingham, H.R.; Hood, F.J.C.; Bradnum, P.; Tharagonnet, D.; Selkon, J.B. Metronidazole Compared with Penicillin in the Treatment of Acute Dental Infections. Br. J. Oral. Surg. 1977, 14, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Shah, S.; Rudagi, B.; Ticku, S.; Gaikwad, S.; Shitole, D. Metronidazole for the Prophylaxis of Alveolar Osteitis: A Systematic Review and Meta-Analysis. J. Maxillofac. Oral Surg. 2024, 24, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Robinson, P.; Rangarajan, K.; Scott, S.; Angco, L. The Role of Single-Shot Metronidazole in the Prevention of Clostridium Difficile Infection Following Ileostomy Reversal Surgery. Int. J. Color. Dis. 2017, 32, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Tulstrup, M.V.-L.; Christensen, E.G.; Carvalho, V.; Linninge, C.; Ahrné, S.; Højberg, O.; Licht, T.R.; Bahl, M.I. Antibiotic Treatment Affects Intestinal Permeability and Gut Microbial Composition in Wistar Rats Dependent on Antibiotic Class. PLoS ONE 2015, 10, e0144854. [Google Scholar] [CrossRef]
- Reymunde Duran, D.A.; Chung, S.S.; DuPont, A.W. S2118 Recurrent Clostridioides Difficile Infections Caused by Topical Clindamycin. Am. J. Gastroenterol. 2022, 117, e1439. [Google Scholar] [CrossRef]
- Niemczyk, W.; Żurek, J.; Niemczyk, S.; Kępa, M.; Zięba, N.; Misiołek, M.; Wiench, R. Antibiotic-Loaded Platelet-Rich Fibrin (AL-PRF) as a New Carrier for Antimicrobials: A Systematic Review of In Vitro Studies. Int. J. Mol. Sci. 2025, 26, 2140. [Google Scholar] [CrossRef]
- Niemczyk, W.; Kępa, M.; Żurek, J.; Aboud, A.; Skaba, D.; Wiench, R. Comparative Evaluation of Platelet-Rich Fibrin (PRF) and Concentrated Growth Factor (CGF) as Carriers for Antibiotics—In Vitro Study. Int. J. Mol. Sci. 2025, 26, 4303. [Google Scholar] [CrossRef]
- Niemczyk, W.; Niemczyk, S.; Odrzywolska, O.; Doroz, P.; Hochuł, D.; Zawadzka, K. Application of I-PRF in Dentistry. Wiadomości Lek. 2024, 77, 2348–2352. [Google Scholar] [CrossRef]
- Nitzan, O. Role of Antibiotics for Treatment of Inflammatory Bowel Disease. World J. Gastroenterol. 2016, 22, 1078. [Google Scholar] [CrossRef]
- Niemczyk, W.; Kępa, M.; Żurek, J.; Aboud, A.; Skaba, D.; Wiench, R. Application of Platelet-Rich Fibrin and Concentrated Growth Factors as Carriers for Antifungal Drugs—In Vitro Study. J. Clin. Med. 2025, 14, 5111. [Google Scholar] [CrossRef]
- Ghazwani, M.; Vasudevan, R.; Kandasamy, G.; Hani, U.; Niharika, G.; Naredla, M.; Devanandan, P.; Puvvada, R.C.; Almehizia, A.A.; Hakami, A.R.; et al. Development and In Vitro Characterization of Antibiotic-Loaded Nanocarriers for Dental Delivery. Molecules 2023, 28, 2914. [Google Scholar] [CrossRef] [PubMed]
- Kida, D.; Zakrzewska, A.; Zborowski, J.; Szulc, M.; Karolewicz, B. Polymer-Based Carriers in Dental Local Healing—Review and Future Challenges. Materials 2021, 14, 3948. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Ardestani, A.K.; Hassani, Z.; Alam, M.; Abbasi, K.; Kahrizi, S.; Madihi, N.; Abbasiparashkouh, Z.; Mohammadi, A.; Shahab, P.; et al. The Current Novel Drug Delivery System (Natural and Chemical Composites) in Dental Infections for Antibiotics Resistance: A Narrative Review. Cell. Mol. Biol. 2022, 68, 141–160. [Google Scholar] [CrossRef]
- Trusek, A.; Kijak, E. Drug Carriers Based on Graphene Oxide and Hydrogel: Opportunities and Challenges in Infection Control Tested by Amoxicillin Release. Materials 2021, 14, 3182. [Google Scholar] [CrossRef] [PubMed]
- Hakim, L.K.; Yazdanian, M.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Khayatan, D.; Seifalian, A.; Ranjbar, R.; Yazdanian, A. Biocompatible and Biomaterials Application in Drug Delivery System in Oral Cavity. Evid.-Based Complement. Altern. Med. 2021, 2021, 9011226. [Google Scholar] [CrossRef]
- Lê, A.; Mantel, M.; Marchix, J.; Bodinier, M.; Jan, G.; Rolli-Derkinderen, M. Inflammatory Bowel Disease Therapeutic Strategies by Modulation of the Microbiota: How and When to Introduce Pre-, pro-, Syn-, or Postbiotics? Am. J. Physiol.-Gastrointest. Liver Physiol. 2022, 323, G523–G553. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
- Al Sharaby, A.; Abugoukh, T.M.; Ahmed, W.; Ahmed, S.; Elshaikh, A.O. Do Probiotics Prevent Clostridium Difficile-Associated Diarrhea? Cureus 2022, 14, e27624. [Google Scholar] [CrossRef]
- Heil, E.L.; Harris, A.D.; Brown, C.; Seung, H.; Thom, K.A.; Von Rosenvinge, E.; Sorongon, S.; Pineles, L.; Goodman, K.E.; Leekha, S. A Multicenter Evaluation of Probiotic Use for the Primary Prevention of Clostridioides Difficile Infection. Clin. Infect. Dis. 2021, 73, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.; Keating, G.; Georgousopoulou, E.; Hespe, C.; Levett, K. Probiotics for the Prevention of Antibiotic-Associated Diarrhoea: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e043054. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Guan, X.-X.; Tang, Y.-J.; Sun, J.-F.; Wang, X.-K.; Wang, W.-D.; Fan, J.-M. Clinical Effects and Gut Microbiota Changes of Using Probiotics, Prebiotics or Synbiotics in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef] [PubMed]
- Akutko, K.; Stawarski, A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2466. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Parigi, T.L.; Vieujean, S.; Paridaens, K.; Dalgaard, K.; Peyrin-Biroulet, L.; Danese, S. Efficacy, Safety, and Concerns on Microbiota Modulation, Antibiotics, Probiotics, and Fecal Microbial Transplant for Inflammatory Bowel Disease and Other Gastrointestinal Conditions: Results from an International Survey. Microorganisms 2023, 11, 2806. [Google Scholar] [CrossRef]
- Lee, N.; Park, Y.-S.; Kang, D.-K.; Paik, H.-D. Paraprobiotics: Definition, Manufacturing Methods, and Functionality. Food Sci. Biotechnol. 2023, 32, 1981–1991. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
| Database | Search Terms | Number of Results |
|---|---|---|
| PubMed | (“Dentistry” OR “Oral health” OR “Stomatology”) AND (“CDI” OR “Clostridium difficile” OR “Clostridioides difficile” OR “periprocedural antibiotic therapy” OR “antibiotic therapy”) AND (“IBD” OR “Crohn disease” OR “inflammatory bowel disease” OR “Ulcerative Colitis”). | 440 |
| Embase | (‘Dentistry’ OR ‘Oral Health’ OR ‘Stomatology’) AND (‘CDI’ OR ‘Clostridium difficile’ OR ‘Clostridioides difficile’ OR ‘Periprocedural Antibiotic Therapy’ OR ‘Antibiotic Therapy’) AND (‘IBD’ OR ‘Crohn Disease’ OR ‘Inflammatory Bowel Disease’ OR ‘Ulcerative Colitis’) | 258 |
| Scopus | (“Oral”) AND (“CDI” OR “Clostridium difficile” OR “Clostridioides difficile” OR “Periprocedural Antibiotic Therapy” OR “Antibiotic Therapy”) AND (“IBD” OR “Crohn Disease” OR “Inflammatory Bowel Disease” OR “Ulcerative Colitis”) | 587 |
| Google Scholar | “Dentistry” OR “Oral Health” OR “Stomatology” AND “CDI” OR “Clostridium difficile” OR “Clostridioides difficile” OR “Periprocedural Antibiotic Therapy” OR “Antibiotic Therapy” AND “IBD” OR “Crohn Disease” OR “Inflammatory Bowel Disease” OR “Ulcerative Colitis” | 500 most accurate results were included in the review process |
| High Risk | Moderate Risk | Low Risk |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemczyk, S.; Niemczyk, W.; Bąk-Drabik, K.; Latusek-Kotyczka, K.; Zawilska, A.; Wiench, R.; Hadzik, J.; Dominiak, M. Patient with Inflammatory Bowel Disease in a Dental Office—Which Antibiotic to Choose?—Narrative Review. J. Clin. Med. 2025, 14, 8392. https://doi.org/10.3390/jcm14238392
Niemczyk S, Niemczyk W, Bąk-Drabik K, Latusek-Kotyczka K, Zawilska A, Wiench R, Hadzik J, Dominiak M. Patient with Inflammatory Bowel Disease in a Dental Office—Which Antibiotic to Choose?—Narrative Review. Journal of Clinical Medicine. 2025; 14(23):8392. https://doi.org/10.3390/jcm14238392
Chicago/Turabian StyleNiemczyk, Stanisław, Wojciech Niemczyk, Katarzyna Bąk-Drabik, Katarzyna Latusek-Kotyczka, Anna Zawilska, Rafał Wiench, Jakub Hadzik, and Marzena Dominiak. 2025. "Patient with Inflammatory Bowel Disease in a Dental Office—Which Antibiotic to Choose?—Narrative Review" Journal of Clinical Medicine 14, no. 23: 8392. https://doi.org/10.3390/jcm14238392
APA StyleNiemczyk, S., Niemczyk, W., Bąk-Drabik, K., Latusek-Kotyczka, K., Zawilska, A., Wiench, R., Hadzik, J., & Dominiak, M. (2025). Patient with Inflammatory Bowel Disease in a Dental Office—Which Antibiotic to Choose?—Narrative Review. Journal of Clinical Medicine, 14(23), 8392. https://doi.org/10.3390/jcm14238392








