Development and Internal Validation of a Predictive Model for Operative Management in Blunt Abdominal Trauma Using Admission Physiological and Biochemical Parameters
Abstract
1. Introduction
2. Materials and Methods
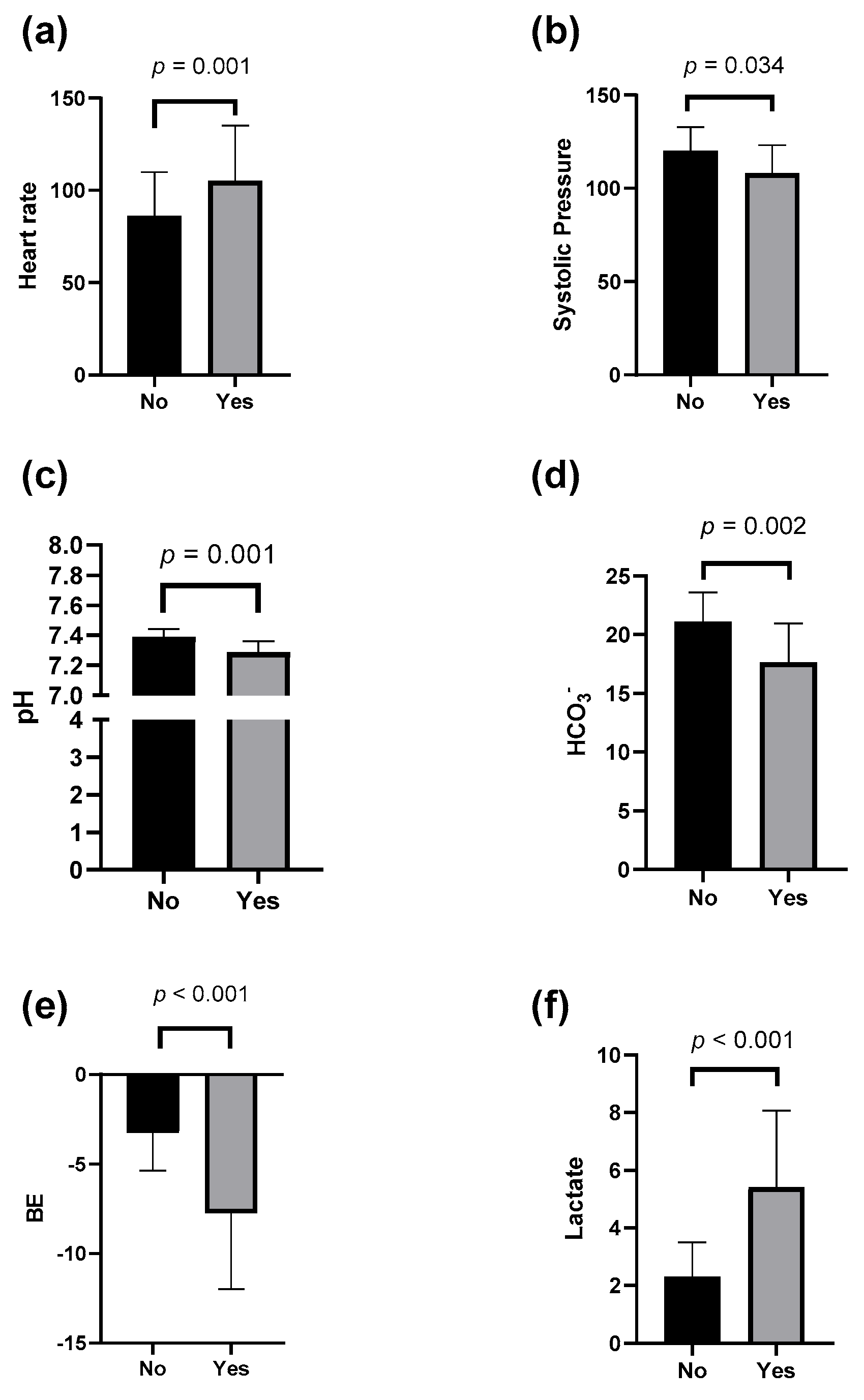
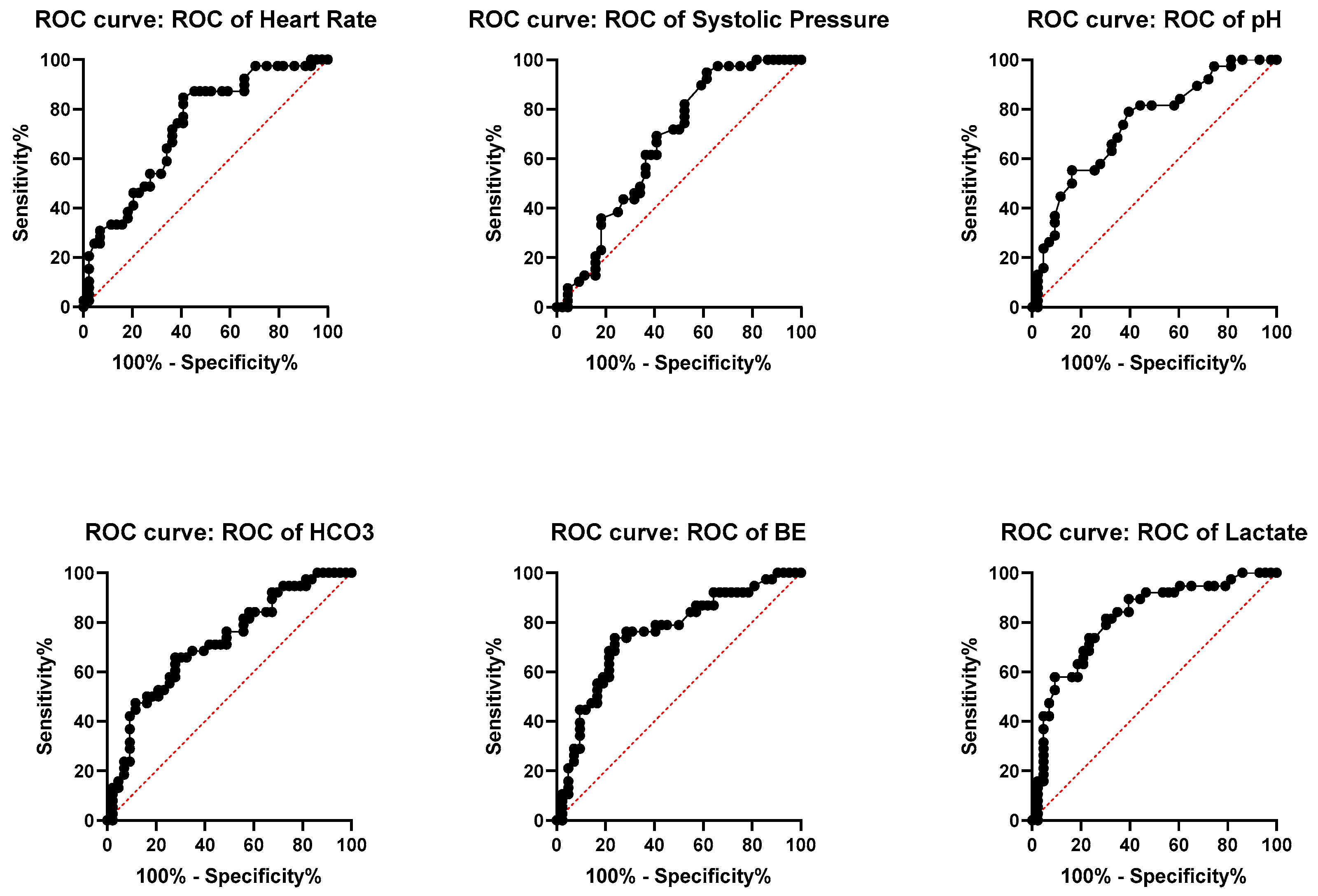
3. Results
4. Discussion
5. Limitations
6. Future Perspectives
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajitimbay, T.N.P.; Pérez, L.F.P.; Unaucho, G.S.V.; Llumiluisa, J.M.Q. Trauma abdominal cerrado. Manejo inicial en urgencias. RECIMUNDO Sci. J. Res. Knowl. 2022, 6, 112–119. [Google Scholar] [CrossRef]
- Parra-Romero, G.; Contreras-Cantero, G.; Orozco-Guibaldo, D.; Domínguez-Estrada, A.; Bravo-Cuellar, L. Trauma abdominal: Experiencia de 4961 casos en el occidente de México [Abdominal trauma: Experience of 4961 cases in Western Mexico]. Cirugía Y Cir. 2019, 87, 183–189. [Google Scholar]
- National Institute of Statistics and Geography (INEGI). Mortalidad. Available online: https://www.inegi.org.mx/temas/mortalidad (accessed on 17 June 2025).
- Daniel, P.R.O.; Reyna, G.M.D.; Valencia, B.S.S.; Lopez, C.A.A.; Correa, T.K.A.; Belen, A.C.A.; Josue, R.D.A.; Franco, C.L.R. Revisión Bibliográfica: Manejo del trauma penetrante de abdomen. Braz. J. Health Rev. 2023, 6, 1956–1968. [Google Scholar] [CrossRef]
- Shojaee, M.; Sabzghabaei, A.; Heidari, A. Efficacy of new scoring system for diagnosis of abdominal injury after blunt abdominal trauma in patients referred to emergency department. Chin. J. Traumatol. 2020, 23, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.A.; Abdul Raheem, O.A.; Abdallah, H.A.; Yousef, M.A. Epidemiological, evaluation and outcome of pure abdominal trauma victims who underwent surgical exploratory laparotomy. Al Azhar Assiut Med. J. 2016, 14, 24–28. [Google Scholar] [CrossRef]
- Brenner, M.; Hicks, C. Major Abdominal Trauma, Critical Decisions and New Frontiers in Management. Emerg. Med. Clin. N. Am. 2018, 36, 149–160. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Menéndez, P.; Asensio, J.A. Lesiones vasculares abdominales. Cirugía Española 2012, 90, 215–221. [Google Scholar] [CrossRef]
- Yogananda, N.; Pintar, F.A.; Maltese, M.R. Biomechanics of abdominal injuries. Crit. Rev. Biomed. Eng. 2001, 29, 173–246. [Google Scholar] [CrossRef]
- Pacheco, F.A. Trauma de abdomen. Rev. Médica Clínica 2011, 22, 623–630. [Google Scholar] [CrossRef]
- Diercks, D.; Clarke, S.; Moreira, M. Initial Evaluation and Management of Blunt Abdominal Trauma in Adults. UpToDate. 2023. Available online: https://www.uptodate.com/contents/initial-evaluation-and-management-of-blunt-thoracic-trauma-in-adults (accessed on 20 May 2025).
- Bége, T.; Brunet, C.; Berdah, S.V. Hollow viscus injury due to blunt trauma: A review. J. Visc. Surg. 2016, 153, 61–68. [Google Scholar] [CrossRef]
- Coccolini, F.; Coimbra, R.; Ordóñez, C.; Kluger, Y.; Vega, F.; Moore, E.E.; Biffl, W.; Peitzman, A.; Horer, T.; Abu-Zidan, F.M.; et al. Panel de expertos de WSES. Traumatismo hepático: Directrices WSES 2020. Rev. Mund. Cirugía Emerg. WJES 2020, 15, 24. [Google Scholar] [CrossRef]
- Coccolini, F.; Fugazzola, P.; Morganti, L.; Ceresoli, M.; Magnone, S.; Montori, G.; Tomasoni, M.; Maccatrozzo, S.; Allievi, N.; Occhionorelli, S.; et al. The World Society of Emergency Surgery (WSES) spleen trauma classification: A useful tool in the management of splenic trauma. World J. Emerg. Surg. 2019, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Moore, E.E.; Kluger, Y.; Biffl, W.; Leppaniemi, A.; Matsumura, Y.; Kim, F.; Peitzman, A.B.; Fraga, G.P.; Sartelli, M.; et al. WSES-AAST Expert Panel. Kidney and uro-trauma: WSES-AAST guidelines. World J. Emerg. Surg. 2019, 14, 54. [Google Scholar] [CrossRef]
- Serna, C.; Serna, J.J.; Caicedo, Y.; Padilla, N.; Gallego, L.M.; Salcedo, A.; Rodríguez-Holguín, F.; González Hadad, A.; García, A.; Herrera, M.A.; et al. Control de daños en trauma esplénico: “Preserva un órgano, preserva la vida”. Colomb. Méd. 2021, 52, e4084794. [Google Scholar] [CrossRef]
- Silvio, L.; Madrazo, Z.; Ramos, E. Actualización del tratamiento de los traumatismos hepáticos. Cirugía Española 2008, 83, 227–234. [Google Scholar] [CrossRef]
- Iacobellis, F.; Di Serafino, M.; Caruso, M.; Dell’Aversano Orabona, G.; Rinaldo, C.; Grimaldi, D.; Verde, F.; Sabatino, V.; Schillirò, M.L.; Giacobbe, G.; et al. Non operative Management of Politraumatized Patients: Body Imaging Beyond CT. Diagnostics 2023, 13, 1347. [Google Scholar] [CrossRef]
- Sarquis, L.M.; Collaço, I.A.; Toderke, E.L.; Fontes, H.S.; Nassif, A.T.; Freitas, A.C.T.D. Epidemiological profile of patients undergoing non-operative management of solid organ injury and associated factors with mortality. Rev. Col. Bras. Cir. 2024, 51, e20243734. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, L.; Coimbra, R.; Goes, A.M., Jr.; Reva, V.; Santorelli, J.; Moore, E.E.; Galante, J.M.; Abu-Zidan, F.; Peitzman, A.B.; Ordonez, C.A. American Association for the Surgery of Trauma-World Society of Emergency Surgery guidelines on diagnosis and management of abdominal vascular injuries. J. Trauma Acute Care Surg. 2020, 89, 1197–1211. [Google Scholar] [CrossRef]
- Mahawar, R.; Shinde, R.; Jogdand, S. Successful non-operative managmenment of multiple intra-abdmoinal solid organ injiry after blunt abdominal trauma: A case report. Pan Afr. Med. J. 2022, 43, 54. [Google Scholar] [CrossRef]
- Podda, M.; De Simone, B.; Ceresoli, M.; Virdis, F.; Favi, F.; Wiik Larsen, J.; Coccolini, F.; Sartelli, M.; Pararas, N.; Beka, S.G.; et al. Follow-up strategies for patients with splenic trauma managed non-operatively: The 2022 World Society of Emergency Surgery consensus document. World J. Emerg. Surg. 2022, 17, 52. [Google Scholar] [CrossRef]
- Cunha, S.C.; De-Oliveira Filho, A.G.; Miranda, M.L.; Silva, M.A.C.P.; Pegolo, P.T.C.; Lopes, L.R.; Bustorff-Silva, J.M. Analysis of the efficacy and safety of conservative treatment of blunt abdominal trauma in children: Retrospective study. Conservative treatment of blunt abdominal trauma in children. Rev. Col. Bras. Cir. 2023, 50, e20233429. [Google Scholar] [CrossRef]
- Malhotra, A.K.; Ivatury, R.R.; Latifi, R. Blunt abdominal trauma: Evaluation and indications for laparotomy. Scand. J. Surg. 2002, 91, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, O.J.A.; Guevara, T.L.; Sánchez, A.J.M. Trauma abdominal penetrante. Cir. Ciruj. 2006, 74, 431–442. [Google Scholar]
- Isenhour, J.L.; Marx, J. Advanced in abdominal trauma. Emergency Medicine Clin. N. Am. 2007, 25, 713–733. [Google Scholar] [CrossRef] [PubMed]
- Basilio, O.A.; Olguín, L.A.; Delgadillo, G.S.; López, C.O. Reintervención en trauma abdominal. Trauma 2005, 8, 5–9. [Google Scholar]
- Moura, F. Ruling out ubtra-abdmoinal injuries in blunt trauma patientes using clinical criterio and abdominal iltrasound. Rev. Col. Bras. Cir. 2017, 44, 623–626. [Google Scholar] [CrossRef]
- Poletti, P.A.; Mirvis, S.E.; Shanmuganathan, K.; Takada, T.; Killeen, K.L.; Perlmutter, D.; Hahn, J.; Mermillod, B. Blunt abdominal trauma patients: Can organ injury be excluded without performins computer tomography? J. Trauma Injuty Infect. Crit. Care 2004, 57, 1072–1081. [Google Scholar] [CrossRef]
- García, A.; Millán, M.; Burbano, D.; Ordoñez, C.A.; Parra, M.W.; Caicedo, Y.; Hadad, A.G.; Herrera, M.A.; Pino, L.F.; Rodríguez-Holguín, F.; et al. Damage control in abdominal vascular trauma. Colomb. Med. 2021, 52, 4808. [Google Scholar] [CrossRef]
- Osejos Moreira, W.D.; Villarreal Chamorro, E.I.; Morales Silva, B.L.; Espinosa de los Monteros Garrido, F.J. Lactato sérico como predictor de mortalidad: Artículo de revisión. Política Y Contexto 2023, 8, 40–51. [Google Scholar]
- Martínez González, V.; Mendoza Rodríguez, M.; López González, A.; Cortés Munguía, J.A.; Mendoza Portillo, E. Depuración de lactato como marcador de mortalidad en paciente con trauma. Med. Crítica 2019, 33, 170–175. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, F.A. American Association for the Surgery of Trauma Organ Injury Scaling: 50th Anniversary Review Article of the Journal of Trauma. J. Trauma Inj. Infect. Crit. Care 2010, 69, 1600–1601. [Google Scholar] [CrossRef] [PubMed]
- Illescas, G. Escalas e indices de severidad en trauma. Trauma 2003, 3, 88–98. [Google Scholar]
- Lefering, R. Trauma score systems for quality assessment. Eur. J. Trauma. 2002, 28, 52–63. [Google Scholar] [CrossRef]
- Yanar, H.; Ertekin, C.; Taviloglu, K.; Kabay, B.; Bakkaloglu, H.; Guloglu, R. Tratamiento no quirúrgico de lesiones de múltiples órganos sólidos intraabdominales después de un traumatismo abdominal cerrado. J. Trauma 2008, 64, 943–948. [Google Scholar] [CrossRef]
- Singh, A.; Prasad, G.; Mishra, P.; Vishkarma, K.; Shamim, R. Lessons learned from blunt trauma abdomen: Surgical experience in level I trauma centre. Turk. J. Surg. 2021, 37, 277–285. [Google Scholar] [CrossRef]
- Karachentsev, S. Blunt trauma to abdominal solid organs: An experience of non-operative management at a rural hospital in Zambia. Pan Afr. Med. J. 2021, 38, 89. [Google Scholar] [CrossRef]
- Natarajan, B.; Gupta, P.K.; Cemaj, S.; Sorensen, M.; Hatzoudis, G.I.; Forse, R.A. FAST scan: Is it worth doing in hemodynamically stable blunt trauma patients? Surgery 2010, 148, 695–701. [Google Scholar] [CrossRef]
- Stefanopol, I.A.; Danila, D.M.; Voda-Chelmu, C.; Barbu, R.E.; Moisa, H.; Busila, C.; Baroiu, L. Non-operative Management-The First Option in the Treatment of Blunt Liver and Spleen Trauma in Pediatric Patients. Chirurgia 2024, 119, 65–75. [Google Scholar] [CrossRef]
- Ruscelli, P.; Gemini, A.; Rimini, M.; Santella, S.; Candelari, R.; Rosati, M.; Paci, E.; Marconi, V.; Renzi, C.; Commissari, R.; et al. The role of grade of injury in non-operative management of blunt hepatic and splenic trauma: Case series from a multicenter experience. Medicine 2019, 98, e16746. [Google Scholar] [CrossRef]
- Giannopoulos, G.A.; Katsoulis, I.E.; Tzanakis, N.E.; Patsaouras, P.A.; Digalakis, M.K. Non-operative management of blunt abdominal trauma. Is it safe and feasible in a district general hospital? Scand. J. Trauma Resusc. Emerg. Med. 2009, 17, 22. [Google Scholar] [CrossRef]
- Ponnusamy, P.; Poovathai, S.; Ramakrishnan, R.; Sangreshi, V. Conservative management a safer option in high-grade renal injuries: Our institutional experience. Asian J. Med. Sci. 2023, 14, 197–200. [Google Scholar] [CrossRef]
- Atkins, K.; Schneider, A.; Charles, A. Negative laparotomy rates and outcomes following blunt traumatic injury in the United States. Injury 2023, 54, 110894. [Google Scholar] [CrossRef] [PubMed]
- Kanlerd, A.; Auksornchart, K.; Boonyasatid, P. Non-operative management for abdominal solidorgan injuries: A literature review. Chin. J. Traumatol. 2022, 25, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Asllanaj, H. Limitations of Ultrasound Examination in Trauma. Albanian J. Trauma Emerg. Surg. 2024, 8, 1525–1531. [Google Scholar] [CrossRef]
| Patients (n = 83) | |
|---|---|
| Age in years, median (IQR) | 35 (23–45) |
| Male sex, n (%) | 70 (84.3) |
| Operative management, n (%) | |
| Yes | 39 (47) |
| No | 44 (53) |
| Mechanisms of injury, n (%) | |
| Motorcycle incidents | 22 (26.5) |
| Assaults | 13 (15.7) |
| Car incidents | 19 (22.9) |
| Run-over incidents | 16 (19.3) |
| Falls from height | 8 (9.6) |
| Others | 5 (6) |
| FAST (+) | 64 (77.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampayo-Candia, R.; Guzmán-Martín, C.A.; Vázquez-Toledo, M.A.; Sánchez-Muñoz, F.; Berruecos-Romero, A.; Juárez-Villa, D.; García-Hernández, A.K.; Hernández-García, A.; Chávez-Alba, B.M.; Zepeda-Quiroz, I.; et al. Development and Internal Validation of a Predictive Model for Operative Management in Blunt Abdominal Trauma Using Admission Physiological and Biochemical Parameters. J. Clin. Med. 2025, 14, 8379. https://doi.org/10.3390/jcm14238379
Sampayo-Candia R, Guzmán-Martín CA, Vázquez-Toledo MA, Sánchez-Muñoz F, Berruecos-Romero A, Juárez-Villa D, García-Hernández AK, Hernández-García A, Chávez-Alba BM, Zepeda-Quiroz I, et al. Development and Internal Validation of a Predictive Model for Operative Management in Blunt Abdominal Trauma Using Admission Physiological and Biochemical Parameters. Journal of Clinical Medicine. 2025; 14(23):8379. https://doi.org/10.3390/jcm14238379
Chicago/Turabian StyleSampayo-Candia, Raúl, Carlos A. Guzmán-Martín, Miguel A. Vázquez-Toledo, Fausto Sánchez-Muñoz, Alejandro Berruecos-Romero, Daniel Juárez-Villa, Ana Karen García-Hernández, Adriana Hernández-García, Belén Marisol Chávez-Alba, Iván Zepeda-Quiroz, and et al. 2025. "Development and Internal Validation of a Predictive Model for Operative Management in Blunt Abdominal Trauma Using Admission Physiological and Biochemical Parameters" Journal of Clinical Medicine 14, no. 23: 8379. https://doi.org/10.3390/jcm14238379
APA StyleSampayo-Candia, R., Guzmán-Martín, C. A., Vázquez-Toledo, M. A., Sánchez-Muñoz, F., Berruecos-Romero, A., Juárez-Villa, D., García-Hernández, A. K., Hernández-García, A., Chávez-Alba, B. M., Zepeda-Quiroz, I., & Trueba-Lozano, D. (2025). Development and Internal Validation of a Predictive Model for Operative Management in Blunt Abdominal Trauma Using Admission Physiological and Biochemical Parameters. Journal of Clinical Medicine, 14(23), 8379. https://doi.org/10.3390/jcm14238379








