Optilume Drug-Coated Balloon Dilation for Male Sphincteric (Membranous) Urethral Strictures: 53 Consecutive Cases
Abstract
1. Introduction
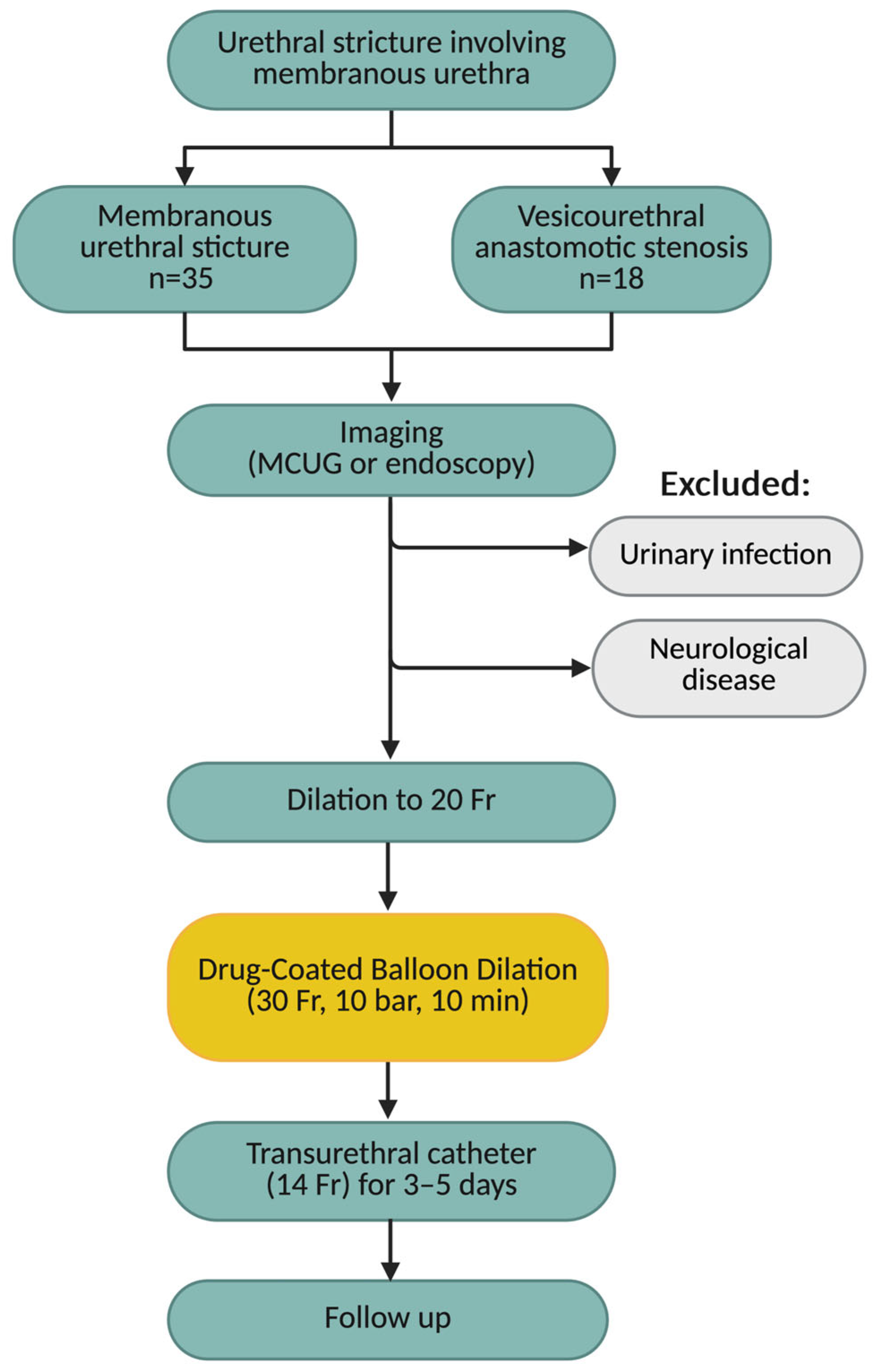
2. Materials and Methods
2.1. Study Population
2.2. Surgical Procedure and Follow-Up
2.3. Study Endpoint and Statistical Analysis
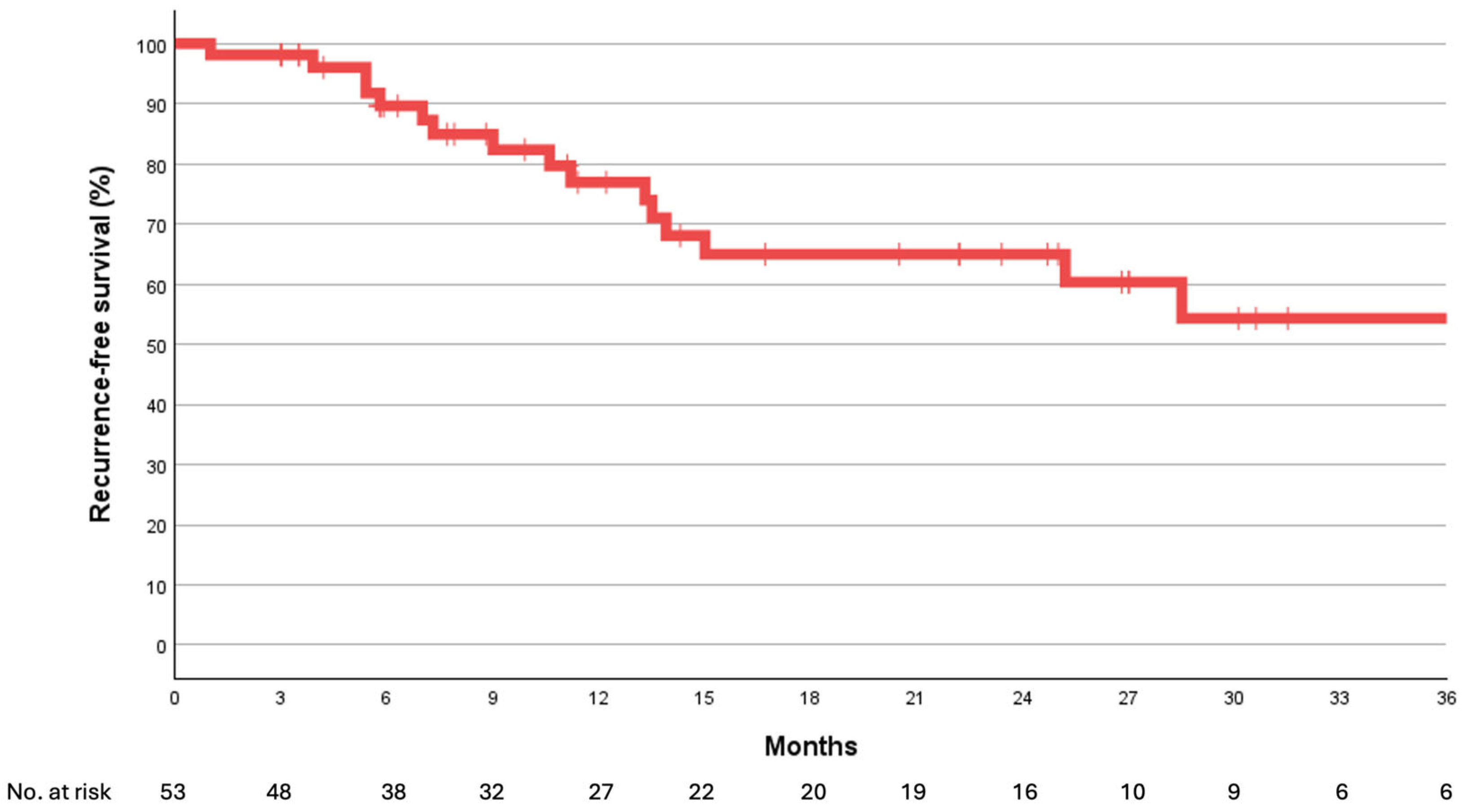
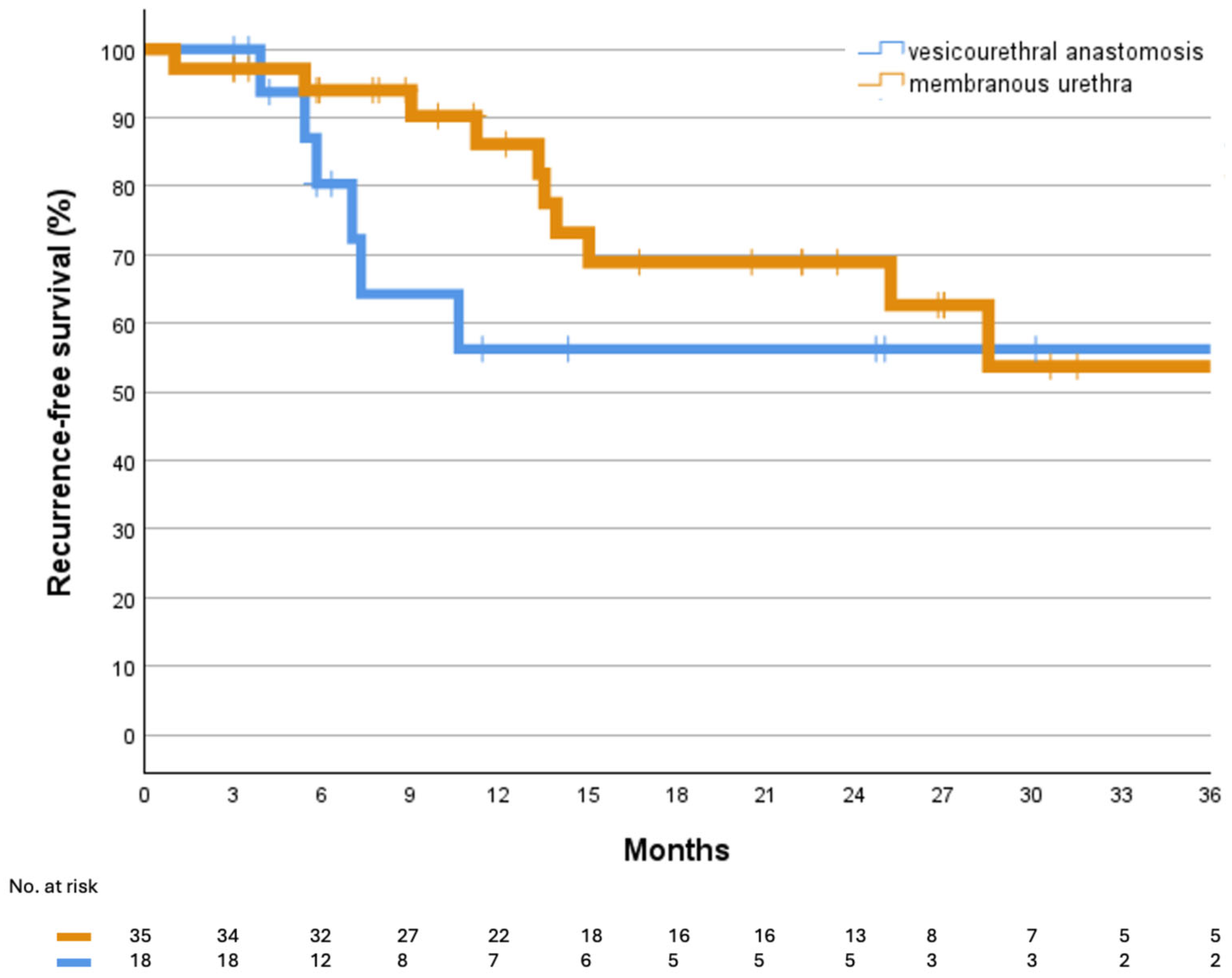
3. Results
4. Discussion
4.1. Current Treatment Strategies for Membranous Urethral Strictures and VUAS
4.2. Outcomes and Predictors of Recurrence and Continence
4.3. Clinical Role and Advantages of DCBD
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| AUS | Artificial urinary sphincter |
| BMG | Buccal mucosa graft |
| BMI | Body mass index |
| DCB | Drug-coated balloon |
| DCBD | Drug-coated balloon dilation |
| DVIU | Direct vision internal urethrotomy |
| IQR | Interquartile range |
| MCUG | Micturating Cystourethrogram |
| PGI-I | Patient Global Impression of Improvement |
| PVR | Post-void residual |
| TURP | Transurethral resection of the prostate |
| VUA | Vesicourethral anastomosis |
| VUAS | Vesicourethral anastomotic stenosis |
References
- Barbagli, G.; Montorsi, F.; Guazzoni, G.; Larcher, A.; Fossati, N.; Sansalone, S.; Romano, G.; Buffi, N.; Lazzeri, M. Ventral Oral Mucosal Onlay Graft Urethroplasty in Nontraumatic Bulbar Urethral Strictures: Surgical Technique and Multivariable Analysis of Results in 214 Patients. Eur. Urol. 2013, 64, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Favre, G.A.; Alfieri, A.G.; Gil Villa, S.A.; Tobia, I.; Giudice, C.R. Bulbomembranous Urethral Strictures Repair After Surgical Treatment of Benign Prostatic Hyperplasia. Experience From a Latin American Referral Centre. Urology 2021, 147, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.C.; Gómez, R.G.; Nikolavsky, D. Reconstruction of Membranous Urethral Strictures. Curr. Urol. Rep. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, G.N.; Pence, S.T.; Findlay, B.L.; Lee, Y.S.; Viers, B.R.; Anderson, K.T.; Warner, J.N. Endoscopic buccal urethroplasty for membranous stricture disease. Transl. Androl. Urol. 2025, 14, 2383–2390. [Google Scholar] [CrossRef]
- Hofer, M.D.; Zhao, L.C.; Morey, A.F.; Scott, J.F.; Chang, A.J.; Brandes, S.B.; Gonzalez, C.M. Outcomes after Urethroplasty for Radiotherapy Induced Bulbomembranous Urethral Stricture Disease. J. Urol. 2014, 191, 1307–1312. [Google Scholar] [CrossRef]
- Schorn, I.; Malinoff, H.; Anderson, S.; Lecy, C.; Wang, J.; Giorgianni, J.; Papandreou, G. The Lutonix® drug-coated balloon: A novel drug delivery technology for the treatment of vascular disease. Adv. Drug Deliv. Rev. 2017, 112, 78–87. [Google Scholar] [CrossRef]
- Nordanstig, J.; James, S.; Andersson, M.; Andersson, M.; Danielsson, P.; Gillgren, P.; Delle, M.; Engström, J.; Fransson, T.; Hamoud, M.; et al. Mortality with Paclitaxel-Coated Devices in Peripheral Artery Disease. N. Engl. J. Med. 2020, 383, 2538–2546. [Google Scholar] [CrossRef]
- Srikanth, P.; De Long, J.; Virasoro, R.; Elliott, S.P. A Drug-Coated Balloon Treatment for Urethral Stricture Disease: Three-Year Results from the ROBUST III Study. J. Endourol. 2025, 39, 968–974. [Google Scholar] [CrossRef]
- Jelisejevas, L.A.; Rehder, P.; Wassermann, J.; Kink, P.; Tulchiner, G. Optilume Drug-Coated Balloon for Acute Urinary Retention After Failed Treatment for Complex Recurrent Urethral Stricture Disease. Medicina 2025, 61, 700. [Google Scholar] [CrossRef]
- Kapriniotis, K.; Loufopoulos, I.; Apostolopoulou, A.; Anderson, P.C.B.; Papaefstathiou, E. Drug-Coated Balloon Treatment for Urethral Strictures: Is This the Future? A Review of the Current Literature. J. Clin. Med. 2025, 14, 2854. [Google Scholar] [CrossRef]
- Oszczudłowski, M.; Białek, Ł.; Vetterlein, M.W. Paclitaxel-coated Balloon Dilation for Urethral Stricture Disease: 5 Years of Clinical Insights and Future Directions for Optilume. Eur. Urol. Focus 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pansadoro, V.; Emiliozzi, P. Iatrogenic prostatic urethral strictures: Classification and endoscopic treatment. Urology 1999, 53, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Sharma, A.; Shaikh, I.; Andankar, M.; Pathak, H. Safety and Efficacy of Trans-Perineal Urethroplasty for Management of Post-Traumatic Urethral Strictures in Pediatric Age-Group. Urol. Int. 2021, 105, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.B.; Joglekar, O.; Alkandari, M.; Joshi, P.M. Management of post TURP strictures. World J. Urol. 2019, 37, 589–594. [Google Scholar] [CrossRef]
- Sterling, J.; Simhan, J.; Flynn, B.J.; Rusilko, P.; França, W.A.; Ramirez, E.A.; Angulo, J.C.; Martins, F.E.; Patel, H.V.; Higgins, M.; et al. Multi-Institutional Outcomes of Dorsal Onlay Buccal Mucosal Graft Urethroplasty in Patients With Postprostatectomy, Postradiation Anastomotic Stenosis. J. Urol. 2024, 211, 596–604. [Google Scholar] [CrossRef]
- Britton, C.J.; Sharma, V.; Fadel, A.E.; Bearrick, E.; Findlay, B.L.; Frank, I.; Tollefson, M.K.; Karnes, R.J.; Viers, B.R. Vesicourethral Anastomotic Stenosis Following Radical Prostatectomy: Risk Factors, Natural History, and Treatment Outcomes. J. Urol. 2023, 210, 312–322. [Google Scholar] [CrossRef]
- Naser-Tavakolian, A.; Lee, Z. A review of management options for vesicourethral anastomotic stenosis and the emergence of robotic reconstruction. Transl. Androl. Urol. 2025, 14, 2405–2418. [Google Scholar] [CrossRef]
- Naser-Tavakolian, A.; Elbakry, A.; Doersch, K.; Bhalla, R.; Driscoll, C.; Slota, J.; Flynn, B.; Zhao, L.; Lee, Z. IP01-36 A Multi-Institutional Experience of Robotic Reconstruction of Vesicourethral Anastomotic Stenosis. J. Urol. 2025, 213, e94. [Google Scholar] [CrossRef]
- Barbagli, G.; Montorsi, F.; Balò, S.; Sansalone, S.; Loreto, C.; Butnaru, D.; Bini, V.; Lazzeri, M. Treatments of 1242 bulbar urethral strictures: Multivariable statistical analysis of results. World J. Urol. 2019, 37, 1165–1171. [Google Scholar] [CrossRef]
- Calvo, C.I.; Fender, K.; Hoy, N.; Rourke, K. Affirming Long-Term Outcomes After Contemporary Urethroplasty: The Adverse Impact of Increasing Stricture Length, Lichen Sclerosus, Radiation, and Infectious Strictures. J. Urol. 2024, 211, 455–464. [Google Scholar] [CrossRef]
- Bonanni, M.; Rehak, L.; Massaro, G.; Benedetto, D.; Matteucci, A.; Russo, G.; Esperto, F.; Federici, M.; Mauriello, A.; Sangiorgi, G.M. Autologous Immune Cell-Based Regenerative Therapies to Treat Vasculogenic Erectile Dysfunction: Is the Immuno-Centric Revolution Ready for the Prime Time? Biomedicines 2022, 10, 1091. [Google Scholar] [CrossRef]
| Parameter | Result |
|---|---|
| Age (years) | 68 ± 15 [IQR: 55–76] |
| Body mass index (kg/m2) | 26.2 ± 4.99 [IQR: 23.5–30.3] |
| Prior interventions | 2.5 [IQR: 1–7.25] |
| PVR (baseline) (mL) | 70 [IQR: 0–257.5] |
| PVR (postoperative) (mL) | 0 [IQR: 0–11] |
| Follow-up (months) | 13.3 ± [IQR: 7.0–27.4] |
| Stricture length (cm) | 3 [IQR: 2–4] |
| Ever smoker | |
| Yes | 13 (24.5%) |
| No | 40 (75.5%) |
| Diabetes | |
| Yes | 11 (20.75%) |
| No | 42 (79.25%) |
| Irradiation | |
| Yes | 17 (32%) |
| No | 36 (68%) |
| Etiology | |
| Iatrogenic | 37 (69.8%) |
| Congenital | 6 (11.3%) |
| Idiopathic | 5 (9.4%) |
| Traumatic | 4 (7.6%) |
| Lichen sclerosus | 1 (1.9%) |
| Stricture location | |
| Membranous urethra | 35 (66%) |
| Vesicourethral anastomosis | 18 (34%) |
| Complications (Clavien–Dindo) | |
| None | 51 (96.2%) |
| Grade I | 2 (3.8%) |
| ASA class | |
| ASA I | 8 (15%) |
| ASA II | 33 (62.3%) |
| ASA III | 11 (20.8%) |
| ASA IV | 1 (1.9%) |
| Preoperative incontinence status | |
| Patients with urinary incontinence | 19 (35.9%) |
| Patients without urinary incontinence | 34 (64.1%) |
| Postoperative incontinence status | |
| Patients with urinary incontinence | 21 (39.6%) |
| Patients without urinary incontinence | 32 (60.4%) |
| De novo incontinence | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelisejevas, L.A.; Tulchiner, G.; Rehder, P. Optilume Drug-Coated Balloon Dilation for Male Sphincteric (Membranous) Urethral Strictures: 53 Consecutive Cases. J. Clin. Med. 2025, 14, 8369. https://doi.org/10.3390/jcm14238369
Jelisejevas LA, Tulchiner G, Rehder P. Optilume Drug-Coated Balloon Dilation for Male Sphincteric (Membranous) Urethral Strictures: 53 Consecutive Cases. Journal of Clinical Medicine. 2025; 14(23):8369. https://doi.org/10.3390/jcm14238369
Chicago/Turabian StyleJelisejevas, Lukas Andrius, Gennadi Tulchiner, and Peter Rehder. 2025. "Optilume Drug-Coated Balloon Dilation for Male Sphincteric (Membranous) Urethral Strictures: 53 Consecutive Cases" Journal of Clinical Medicine 14, no. 23: 8369. https://doi.org/10.3390/jcm14238369
APA StyleJelisejevas, L. A., Tulchiner, G., & Rehder, P. (2025). Optilume Drug-Coated Balloon Dilation for Male Sphincteric (Membranous) Urethral Strictures: 53 Consecutive Cases. Journal of Clinical Medicine, 14(23), 8369. https://doi.org/10.3390/jcm14238369






