In Vitro Evaluation of Multifocal Intraocular Lenses Based on the Point Spread Function: Optical Performance and Halo Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. MIOLs Description
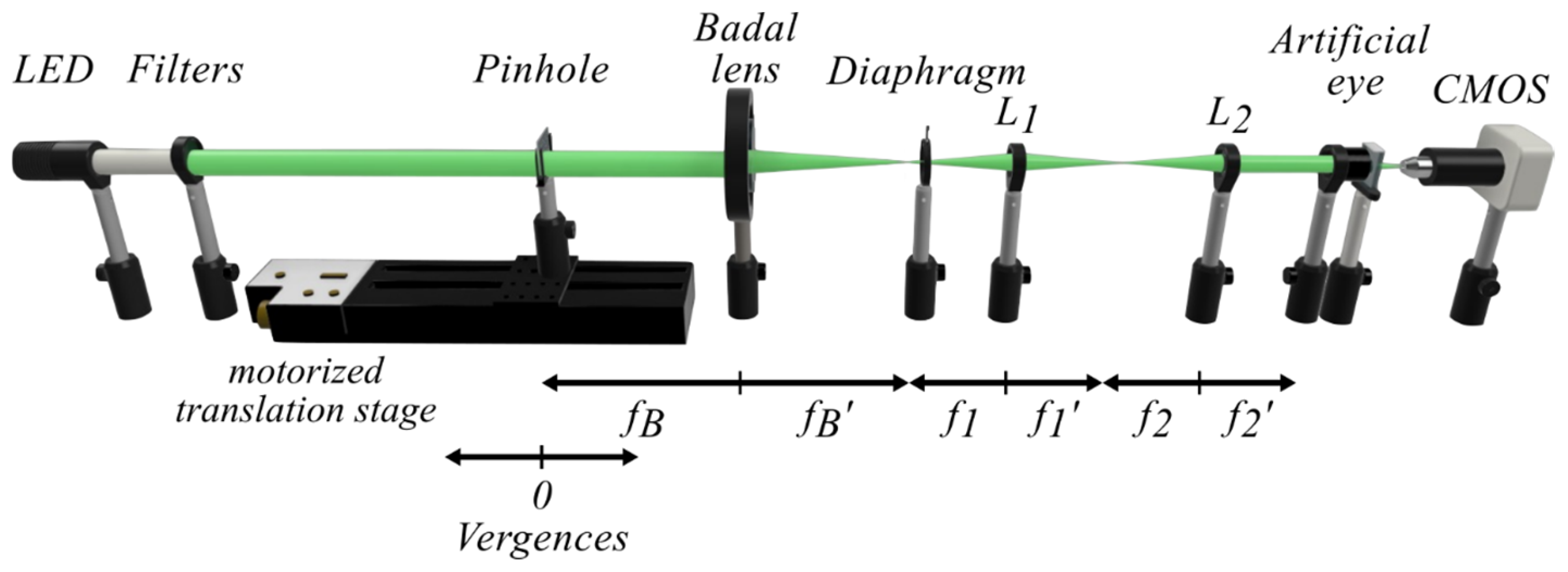
2.2. Experimental Setup
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rampat, R.; Gatinel, D. Multifocal and Extended Depth-of-Focus Intraocular Lenses in 2020. Ophthalmology 2021, 128, e164–e185. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, K.; Yu, X.; Liu, X.; Yao, K. Comparison of Trifocal or Hybrid Multifocal-Extended Depth of Focus Intraocular Lenses: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 6699. [Google Scholar] [CrossRef] [PubMed]
- Alba-Bueno, F.; Vega, F.; Millán, M.S. Halos and Multifocal Intraocular Lenses: Origin and Interpretation. Arch. Soc. Española Oftalmol. Engl. Ed. 2014, 89, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Alves-de-Carvalho, R.S.; Macedo-de-Araújo, R.J.; González-Méijome, J.M. Light Disturbance Analysis and Applications. Photonics 2024, 11, 905. [Google Scholar] [CrossRef]
- Buckhurst, P.J.; Naroo, S.A.; Davies, L.N.; Shah, S.; Drew, T.; Wolffsohn, J.S. Assessment of dysphotopsia in pseudophakic subjects with multifocal intraocular lenses. BMJ Open Ophthalmol. 2017, 1, e000064. [Google Scholar] [CrossRef]
- ISO 11979-2:2014; Ophthalmic Implants-Intraocular Lenses-Part 2: Optical Properties and Test Methods. International Organization for Standardization: London, UK, 2014.
- Łabuz, G.; Papadatou, E.; Khoramnia, R.; Auffarth, G.U. Longitudinal Chromatic Aberration and Polychro-matic Image Quality Metrics of Intraocular Lenses. J. Refract. Surg. 2018, 34, 832–838. [Google Scholar] [CrossRef]
- Chae, S.H.; Son, H.S.; Khoramnia, R.; Lee, K.H.; Choi, C.Y. Laboratory Evaluation of the Optical Properties of Two Extended-Depth-of-Focus Intraocular Lenses. BMC Ophthalmol. 2020, 20, 53. [Google Scholar] [CrossRef]
- García, S.; Salvá, L.; García-Delpech, S.; Martínez-Espert, A.; Ferrando, V.; Montagud-Martínez, D. Poly-chromatic Assessment of a Refractive Segmented EDOF Intraocular Lens. J. Clin. Med. 2022, 11, 1480. [Google Scholar] [CrossRef]
- Montagud-Martínez, D.; Ferrando, V.; Martínez-Espert, A.; Garcia-Delpech, S.; Monsoriu, J.A.; Furlan, W.D. In Vitro Chromatic Performance of Three Presbyopia-Correcting Intraocular Lenses with Different Optical Designs. J. Clin. Med. 2022, 11, 1212. [Google Scholar] [CrossRef]
- Gatinel, D.; Pagnoulle, C.; Houbrechts, Y.; Gobin, L. Design and Qualification of a Diffractive Trifocal Optical Profile for Intraocular Lenses. J. Cataract. Refract. Surg. 2011, 37, 2060–2067. [Google Scholar] [CrossRef]
- Łabuz, G.; Yan, W.; Baur, I.D.; Khoramnia, R.; Auffarth, G.U. Chromatic Aberration and Spectral Dependency of Extended-Range-of-Vision Intraocular Lens Technology. Sci. Rep. 2023, 13, 14781. [Google Scholar] [CrossRef]
- Łabuz, G.; Yan, W.; Baur, I.D.; Khoramnia, R.; Auffarth, G.U. Comparison of Five Presbyopia-Correcting Intraocular Lenses: Optical-Bench Assessment with Visual-Quality Simulation. J. Clin. Med. 2023, 12, 2523. [Google Scholar] [CrossRef]
- AT LARA Family: The Next Generation EDoF IOLs with Wider Range of Focus. Available online: https://www.zeiss.com/meditec/en/products/iols/edof-iols/at-lara-family.html?utm_source=chatgpt.com (accessed on 23 October 2025).
- Acriva Trinova Pro C Pupil Adaptive®|VSY Biotechnology GmbH. Available online: https://www.vsybiotechnology.com/detail/ophthalmology/healthcare-professionals/our-products/our-inspiring-portfolio/trifocal-iol-s/acriva-trinova-pro-c-pupil-adaptive- (accessed on 23 October 2025).
- Ferrando, V.; Montagud-Martínez, D.; Martínez-Espert, A.; Furlan, W.D. Profile of a New Extended Range-of-Vision IOL: Comments on the Laboratory Study by Tognetto et Al. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 4021–4022. [Google Scholar] [CrossRef] [PubMed]
- Tognetto, D.; Giglio, R.; De Giacinto, C.; Pastore, M.R.; Cirigliano, G.; Piñero, D.P.; Turco, G. Profile of a New Extended Range-of-Vision IOL: A Laboratory Study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, T. Nondiffractive Wavefront-Shaping Extended Range-of-Vision Intraocular Lens. J. Cataract. Refract. Surg. 2020, 46, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.W. Introduction to Fourier Optics, 3rd ed.; Roberts & Company Publishers: Greenwood Village, CO, USA, 2005. [Google Scholar]
- Calatayud, A.; Remón, L.; Martos, J.; Furlan, W.D.; Monsoriu, J.A. Imaging Quality of Multifocal Intraocular Lenses: Automated Assessment Setup. Ophthalmic Physiol. Opt. 2013, 33, 420–426. [Google Scholar] [CrossRef]
- Watson, A.B.; Yellott, J.I. A unified formula for light-adapted pupil size. J. Vis. 2012, 12, 12. [Google Scholar] [CrossRef]
- Loewenfeld, E. Pupillary changes related to age. In Topics in Neuro-Opthalmology; Williams & Wilkins: Baltimore, MA, USA, 1979; pp. 124–150. [Google Scholar]
- Fernández, J.; Rodríguez-Vallejo, M.; Martínez, J.; Burguera, N.; Piñero, D.P. Pupil diameter in patients with multifocal intraocular lenses. J. Refract. Surg. 2020, 36, 750–756. [Google Scholar] [CrossRef]
- International Commission on Illumination (CIE). CIE Spectral Luminous Efficiency for Photopic Vision; CIEdataTableDatabase; International Commission on Illumination (CIE): Vienna, AT, USA, 2019. [Google Scholar]
- Gatinel, D.; Houbrechts, Y. Comparison of Bifocal and Trifocal Diffractive and Refractive Intraocular Lenses Using an Optical Bench. J. Cataract. Refract. Surg. 2013, 39, 1093–1099. [Google Scholar] [CrossRef]
- Ruiz-Alcocer, J.; Madrid-Costa, D.; García-Lázaro, S.; Ferrer-Blasco, T.; Montés-Micó, R. Optical Performance of Two New Trifocal Intraocular Lenses: Through-focus Modulation Transfer Function and Influence of Pupil Size. Clin. Exp. Ophthalmol. 2014, 42, 271–276. [Google Scholar] [CrossRef]
- Loicq, J.; Willet, N.; Gatinel, D. Topography and Longitudinal Chromatic Aberration Characterizations of Refractive–Diffractive Multifocal Intraocular Lenses. J. Cataract. Refract. Surg. 2019, 45, 1650–1659. [Google Scholar] [CrossRef]
- Can, E.; Senel, E.C.; Holmström, S.T.S.; Piñero, D.P. Comparison of the Optical Behaviour of Five Different Multifocal Diffractive Intraocular Lenses in a Model Eye. Sci. Rep. 2023, 13, 19646. [Google Scholar] [CrossRef]
- Vega, F.; Faria-Ribeiro, M.; Armengol, J.; Millán, M.S. Pitfalls of Using NIR-Based Clinical Instruments to Test Eyes Implanted with Diffractive Intraocular Lenses. Diagnostics 2023, 13, 1259. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-S.; Łabuz, G.; Khoramnia, R.; Yildirim, T.M.; Auffarth, G.U. Laboratory Analysis and Ray Visualization of Diffractive Optics with Enhanced Intermediate Vision. BMC Ophthalmol. 2021, 21, 197. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.J.; Kim, K.H. In Vitro Optical Performance of Multifocal and Extended Depth-of-Focus Intraocular Lenses in Spherical Aberration Conditions. J. Cataract. Refract. Surg. 2022, 48, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Millán, M.S.; Vega, F. Extended Depth of Focus Intraocular Lens Chromatic Performance. Biomed. Opt. Express 2017, 8, 4294. [Google Scholar] [CrossRef]
- Baur, I.D.; Yan, W.; Auffarth, G.U.; Khoramnia, R.; Łabuz, G. Optical Quality and Higher Order Aberrations of Refractive Extended Depth of Focus Intraocular Lenses. J. Refract. Surg. 2023, 39, 668–674. [Google Scholar] [CrossRef]
- Pieh, S.; Artmayr, C.; Pai, V.; Schartmüller, D.; Kriechbaum, K. Through-Focus Response of Extended Depth of Focus Intraocular Lenses. J. Refract. Surg. 2022, 38, 497–501. [Google Scholar] [CrossRef]
- Fernández-Vega-Cueto, L.; Madrid-Costa, D.; Alfonso-Bartolozzi, B.; Vega, F.; Millán, M.S.; Alfonso, J.F. Optical and Clinical Outcomes of an Extended Range of Vision Intraocular Lens. J. Refract. Surg. 2022, 38, 168–176. [Google Scholar] [CrossRef]
- Vinas-Pena, M.; de Castro, A.; Dorronsoro, C.; Gonzalez-Ramos, A.; Redzovic, S.; Willet, N.; Garzon, N.; Marcos, S. Understanding In Vivo Chromatic Aberrations in Pseudophakic Eyes Using on Bench and Computational Approaches. Photonics 2022, 9, 226. [Google Scholar] [CrossRef]
| Fine Vision POD F | Acriva Trinova Pro C | AT LARA 829MP | AcrySof IQ Vivity | |
|---|---|---|---|---|
| Optical design | Diffractive, aspheric | Diffractive, aspheric | Diffractive, aspheric | “X-Wave™”, aspheric |
| Power (D) | 13 | 20 | 14 | 20 |
| Material | Hydrophilic (26%) acrylic | Hydrophilic | Hydrophilic (25%) acrylic | Hydrophobic Acry-late/Methacrylate Copolymer |
| Refractive index | 1.46 | 1.46 | 1.46 | 1.55 |
| Abbe number | 58 | 58 | 56.5 | 37 |
| SA (μm) | −0.11 | −0.10 | 0.00 | −0.20 |
| Addition’s diffraction Orders | Far: m = 0; Inter.: m = +1 Near: m′ = +1 | Far: m = −1; Inter.: m = 0; Near: m = +1 | Far: m = +1; Inter.: m = +2; | - |
| 3.0 mm | Far | Intermediate | Near |
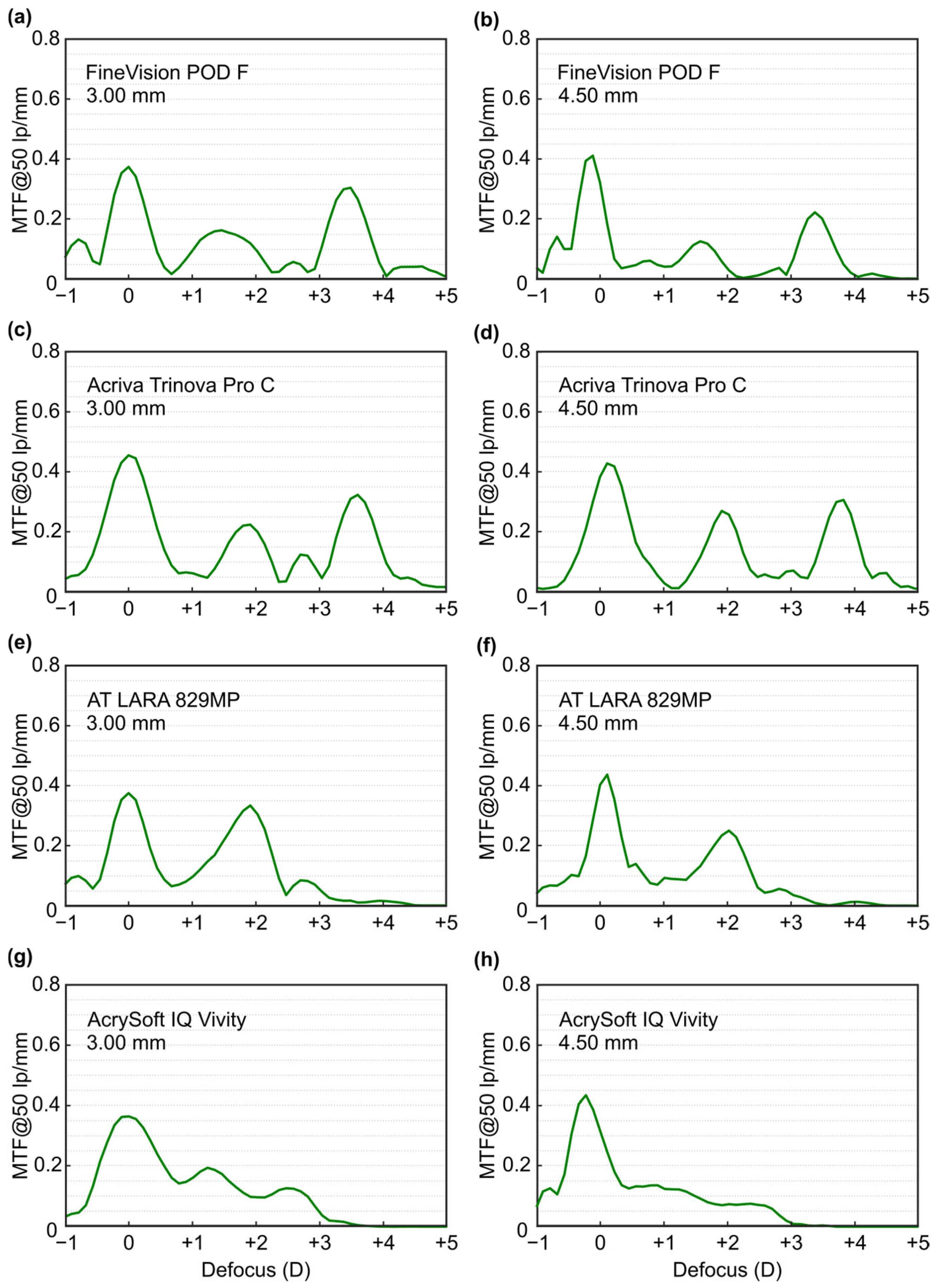
|---|---|---|---|
| Fine Vision POD F | 0.37 | 0.16 | 0.31 |
| Acriva Trinova Pro | 0.46 | 0.23 | 0.32 |
| AT LARA 829MP | 0.38 | 0.33 | - |
| AcrySof IQ Vivity | 0.37 | 0.13 | - |
| 4.5 mm | |||
| Fine Vision POD F | 0.41 | 0.12 | 0.22 |
| Acriva Trinova Pro | 0.43 | 0.27 | 0.30 |
| AT LARA 829MP | 0.44 | 0.25 | - |
| AcrySof IQ Vivity | 0.43 | 0.12 | - |
| 3.0 mm | Far | Intermediate | Near |
|---|---|---|---|
| Fine Vision POD F | 23.9 | 12.2 | 17.0 |
| Acriva Trinova Pro | 26.1 | 16.7 | 19.1 |
| AT LARA 829MP | 24.9 | 20.6 | - |
| AcrySof IQ Vivity | 29.3 | 22.8 | - |
| 4.5 mm | |||
| Fine Vision POD F | 26.1 | 7.0 | 12.1 |
| Acriva Trinova Pro | 25.5 | 18.5 | 18.0 |
| AT LARA 829MP | 28.5 | 15.4 | - |
| AcrySof IQ Vivity | 35.0 | 14.9 | - |
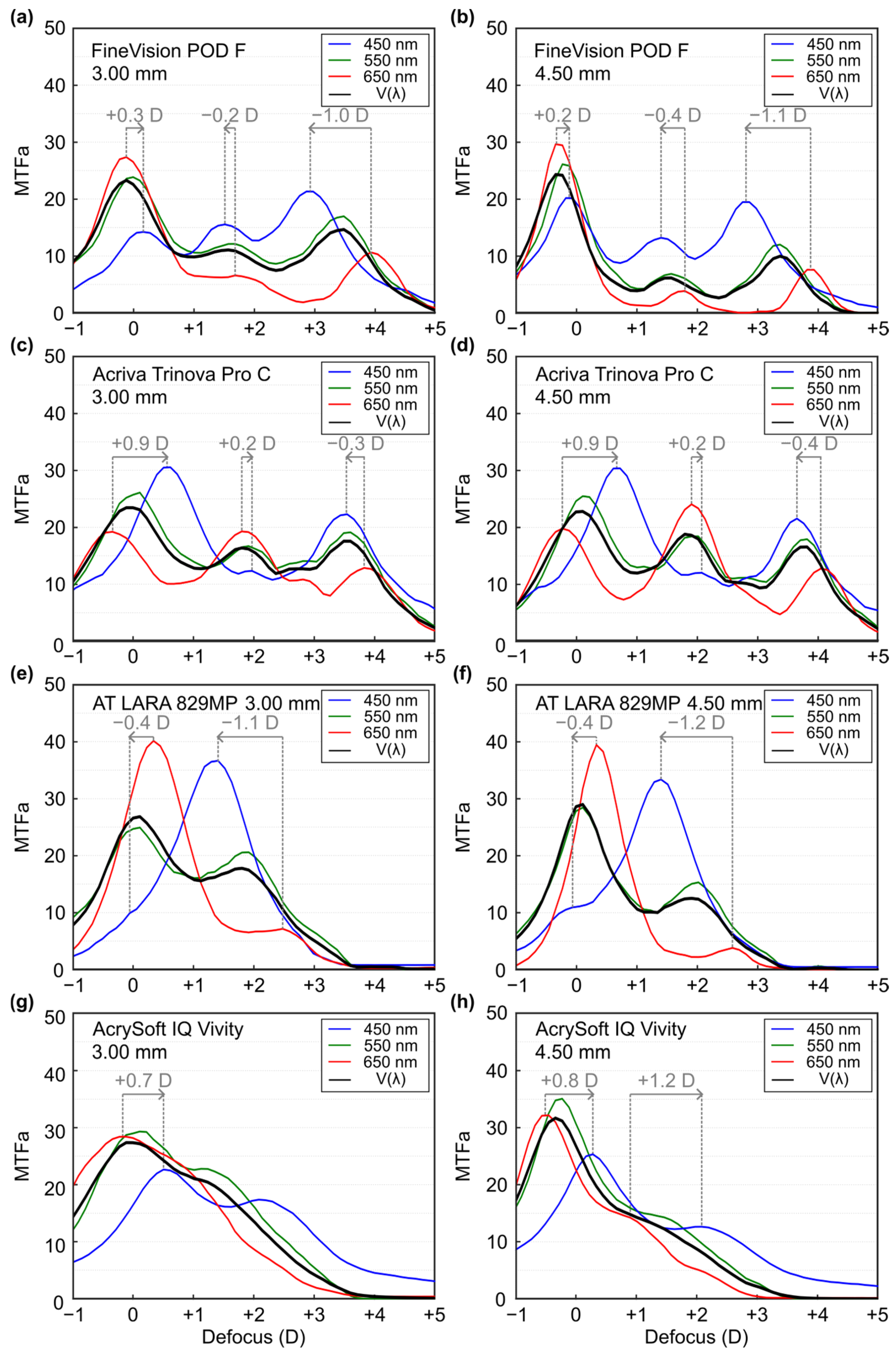
| 3.0 mm | Far | Intermediate | Near |
|---|---|---|---|
| Fine Vision POD F | +0.3 D | −0.2 D | −1.0 D |
| Acriva Trinova Pro | +0.9 D | +0.2 D | −0.3 D |
| AT LARA 829MP | −0.4 D | −1.1 D | - |
| AcrySof IQ Vivity | +0.7 D | - | - |
| 4.5 mm | |||
| Fine Vision POD F | +0.2 D | −0.4 D | −1.1 D |
| Acriva Trinova Pro | −0.9 D | +0.2 D | −0.4 D |
| AT LARA 829MP | −0.4 D | −1.2 D | - |
| AcrySof IQ Vivity | +0.8 D | +1.2 D | - |
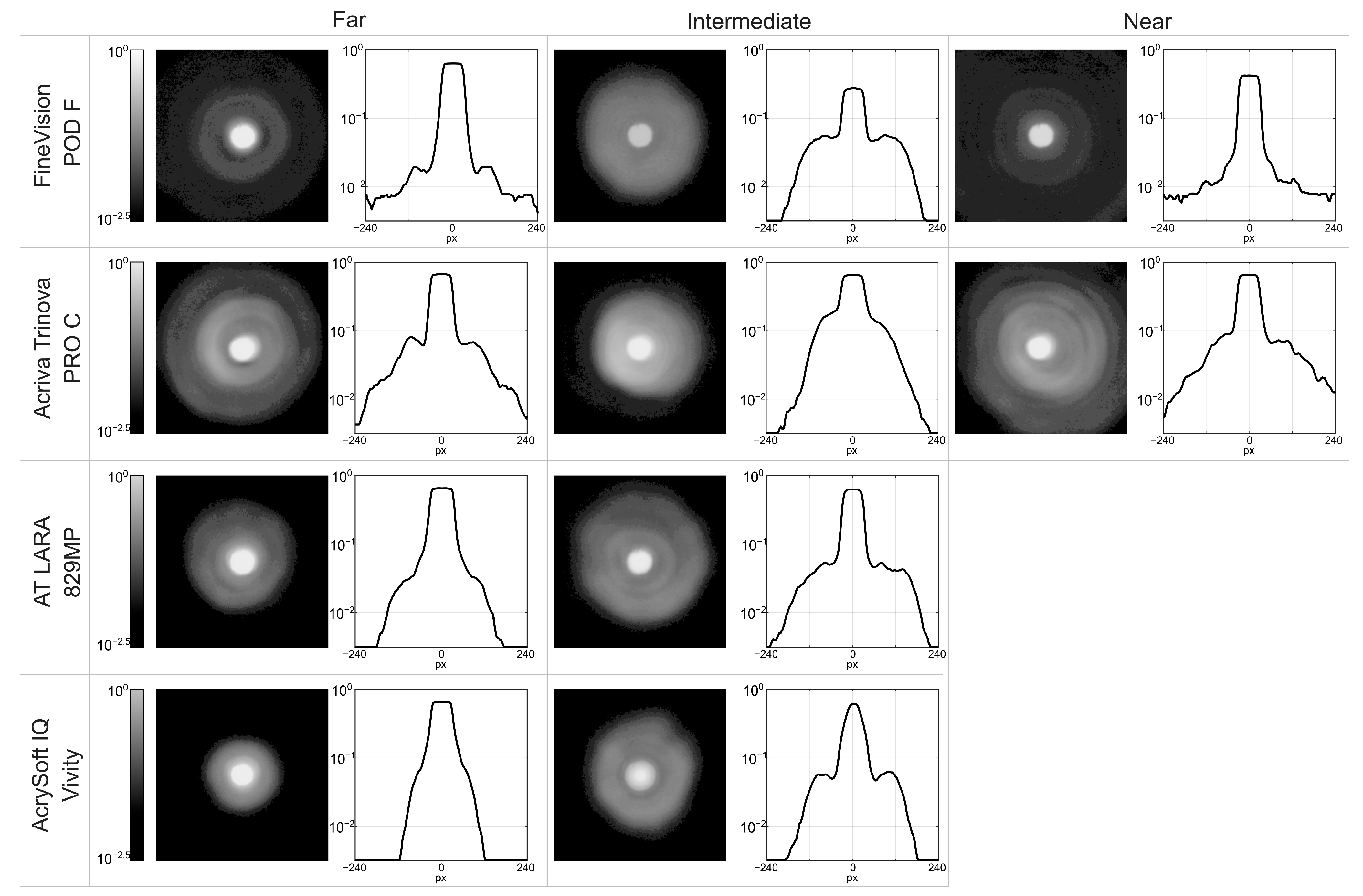
| Far | log10 Intermediate | Near | |
|---|---|---|---|
| Fine Vision POD F | −0.20 | −0.56 | −0.38 |
| Acriva Trinova Pro | −0.18 | −0.19 | −0.19 |
| AT LARA 829MP | −0.19 | −0.21 | - |
| AcrySof IQ Vivity | −0.19 | −0.22 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Espert, A.; García-Delpech, S.; Furlan, W.D. In Vitro Evaluation of Multifocal Intraocular Lenses Based on the Point Spread Function: Optical Performance and Halo Formation. J. Clin. Med. 2025, 14, 8368. https://doi.org/10.3390/jcm14238368
Martínez-Espert A, García-Delpech S, Furlan WD. In Vitro Evaluation of Multifocal Intraocular Lenses Based on the Point Spread Function: Optical Performance and Halo Formation. Journal of Clinical Medicine. 2025; 14(23):8368. https://doi.org/10.3390/jcm14238368
Chicago/Turabian StyleMartínez-Espert, Anabel, Salvador García-Delpech, and Walter D. Furlan. 2025. "In Vitro Evaluation of Multifocal Intraocular Lenses Based on the Point Spread Function: Optical Performance and Halo Formation" Journal of Clinical Medicine 14, no. 23: 8368. https://doi.org/10.3390/jcm14238368
APA StyleMartínez-Espert, A., García-Delpech, S., & Furlan, W. D. (2025). In Vitro Evaluation of Multifocal Intraocular Lenses Based on the Point Spread Function: Optical Performance and Halo Formation. Journal of Clinical Medicine, 14(23), 8368. https://doi.org/10.3390/jcm14238368








