Dyslipidemia Patterns in Adults with Congenital Heart Disease: Focus on HDL Cholesterol
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Clinical Variables
2.3. CHD Classification
2.4. Laboratory Methods and Lipid Profile Definitions
2.5. Clinical Follow-Up
2.6. Statistical Analysis
3. Results
4. Discussion
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, J. The global burden of congenital heart disease. Cardiovasc. J. Afr. 2013, 24, 141–145. [Google Scholar] [CrossRef]
- Tyagi, A.; Sontakke, T. The Transition of Children Living With Congenital Heart Disease to Adult Care. Cureus 2023, 15, e50179. [Google Scholar] [CrossRef]
- Wu, F.M.; Mendelson, M.E.; Huang, Y.; Palfrey, H.; Valente, A.M.; Drucker, N.A.; Moran, A.M.; Yeager, S.B.; de Ferranti, S.D.; New England Congenital Cardiology Association (NECCA). Dyslipidemia Among Adults With Congenital Heart Disease. JACC Adv. 2022, 1, 100081. [Google Scholar] [CrossRef]
- Arrobas Velilla, T.; Guijarro, C.; Campuzano Ruiz, R.; Rodríguez Piñero, M.; Valderrama Marcos, J.F.; Pérez Pérez, A.; Botana López, M.A.; Morais López, A.; García Donaire, J.A.; Obaya, J.C.; et al. Consensus document for lipid profile determination and reporting in Spanish clinical laboratories. What parameters should be included in a basic lipid profile? Clin. Investig. Arterioscler. 2023, 35, 91–100. [Google Scholar]
- Bigras, J.L. Cardiovascular Risk Factors in Patients With Congenital Heart Disease. Can. J. Cardiol. 2020, 36, 1458–1466. [Google Scholar] [CrossRef]
- Awerbach, J.D.; Krasuski, R.A.; Camitta, M.G.W. Coronary Disease and Modifying Cardiovascular Risk in Adult Congenital Heart Disease Patients: Should General Guidelines Apply? Prog. Cardiovasc. Dis. 2018, 61, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.R.; Bhatt, A.B. Cardiovascular Prevention Among Young Adults with Congenital Heart Disease. Curr. Atheroscler. Rep. 2022, 24, 509–514. [Google Scholar] [CrossRef]
- Webb, G.; Williams, R. 32nd Bethesda Conference: “Care of the adult with congenital heart disease”. J. Am. Coll. Cardiol. 2001, 37, 1162–1165. [Google Scholar] [CrossRef][Green Version]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EASGuidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Asghari, S.; Aref-Eshghi, E.; Godwin, M.; Duke, P.; Williamson, T.; Mahdavian, M. Single and mixed dyslipidaemia in Canadian primary care settings: Findings from the Canadian primary care sentinel surveillance network database. BMJ Open 2015, 5, e007954. [Google Scholar] [CrossRef][Green Version]
- Hicks, K.A.; Tcheng, J.E.; Bozkurt, B.; Chaitman, B.R.; Cutlip, D.E.; Farb, A.; Fonarow, G.C.; Jacobs, J.P.; Jaff, M.R.; Lichtman, J.H.; et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology /American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J. Am. Coll. Cardiol. 2015, 66, 403–469. [Google Scholar]
- Ma, F.; Li, P.; Zhang, S.; Shi, W.; Wang, J.; Ma, Q.; Zhao, M.; Nie, Z.; Xiao, H.; Chen, X.; et al. Decreased lipid levels in adult with congenital heart disease: A systematic review and Meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 523. [Google Scholar] [CrossRef]
- Masson, W.; Barbagelata, L.; Lobo, M.; Corral, P.; Nogueira, J.P.; Lucas, L. Dyslipidemia in adults with congenital heart disease: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Hertiš Petek, T.; Petek, T.; Močnik, M.; Marčun Varda, N. Systemic Inflammation, Oxidative Stress and Cardiovascular Health in Children and Adolescents: A Systematic Review. Antioxidants 2022, 11, 894. [Google Scholar] [CrossRef]
- Lewis, G.F.; Rader, D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. Effect of inflammation on HDL structure and function. Curr. Opin. Lipidol. 2016, 27, 521–530. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Nègre-Salvayre, A.; Salvayre, R.; Dousset, N.; Curatola, G. Structural modifications of HDL and functional consequences. Atherosclerosis 2006, 184, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vanreusel, I.; Taeymans, J.; Van Craenenbroeck, E.; Segers, V.F.M.; Van Berendoncks, A.; Briedé, J.J.; Hens, W. Elevated oxidative stress in patients with congenital heart disease and the effect of cyanosis: A meta-analysis. Free Radic. Res. 2023, 57, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Bhale, A.S.; Meilhac, O.; d’Hellencourt, C.L.; Vijayalakshmi, M.A.; Venkataraman, K. Cholesterol transport and beyond: Illuminating the versatile functions of HDL apolipoproteins through structural insights and functional implications. Biofactors 2024, 50, 922–956. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2023, 44, 1394–1407. [Google Scholar] [CrossRef]
- Parra, S.; Saballs, M.; DiNubile, M.; Feliu, M.; Iftimie, S.; Revuelta, L.; Pavón, R.; Àvila, A.; Levinson, S.; Castro, A. Low HDL-c levels at admission are associated with greater severity and worse clinical outcomes in patients with COVID-19 disease. Atheroscler. Plus 2023, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- von Eckardstein, A. High Density Lipoproteins: Is There a Comeback as a Therapeutic Target? Handb. Exp. Pharmacol. 2022, 270, 157–200. [Google Scholar] [PubMed]
- Mach, F.; Koskinas, K.C.; Roeters van Lennep, J.E.; Tokgözoğlu, L.; Badimon, L.; Baigent, C.; Benn, M.; Binder, C.J.; Catapano, A.L.; De Backer, G.G.; et al. 2025 Focused Update of the 2019 ESC/EASGuidelines for the management of dyslipidaemias. Eur. Heart J. 2025, 46, 4359–4378. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Quintana, E.; Rodríguez-Hernández, J.L.; Rodríguez-González, F.; Riaño-Ruiz, M.; Fraguela-Medina, C.; Girolimetti, A.; Jiménez-Rodríguez, S. Cardiovascular risk factors and arterial thrombotic events in congenital heart disease patients. Int. J. Clin. Pract. 2019, 73, 1–8. [Google Scholar] [CrossRef]
- Karsenty, C.; Zhao, A.; Marijon, E.; Ladouceur, M. Risk of thromboembolic complications in adult congenital heart disease: A literature review. Arch. Cardiovasc. Dis. 2018, 111, 613–620. [Google Scholar] [CrossRef]
- Karsenty, C.; Waldmann, V.; Mulder, B.; Hascoet, S.; Ladouceur, M. Thromboembolic complications in adult congenital heart disease: The knowns and the unknowns. Clin. Res. Cardiol. 2021, 110, 1380–1391. [Google Scholar] [CrossRef]
- Silvey, M.; Brandão, L.R. Risk Factors, Prophylaxis, and Treatment of Venous Thromboembolism in Congenital Heart Disease Patients. Front. Pediatr. 2017, 5, 146. [Google Scholar] [CrossRef]
- Masuda, K.; Ishizu, T.; Niwa, K.; Takechi, F.; Tateno, S.; Horigome, H.; Aonuma, K. Increased risk of thromboembolic events in adult congenital heart disease patients with atrial tachyarrhythmias. Int. J. Cardiol. 2017, 234, 69–75. [Google Scholar] [CrossRef]
| Variable | CHD (n = 521) | Control (n = 1782) | p-Value |
|---|---|---|---|
| Age, years | 34 ± 14 | 33 ± 11 | 0.061 |
| Sex (male), n (%) | 300 (57.6%) | 984 (55.2%) | 0.340 |
| Hypertension, n (%) | 73 (14%) | 176 (9.9%) | 0.008 |
| Diabetes, n (%) | 26 (5%) | 45 (2.5%) | 0.004 |
| BMI, kg/m2 | 24 ± 5 | 24 ± 6 | 0.893 |
| Smoker, n (%) | 28 (5.4%) | 290 (16.3%) | <0.001 |
| Statins, n (%) | 44 (8.7%) | 122 (6.9%) | 0.160 |
| Arterial thrombosis, n (%) | 21 (4%) | 9 (0.5%) | <0.001 |
| Dyslipidemia | |||
| Total cholesterol, mg/dL | 164.5 ± 39.2 | 180.6 ± 38.5 | <0.001 |
| Total cholesterol (≥240 mg/dL), n (%) | 22 (4.2%) | 133 (7.5%) | 0.158 |
| LDL cholesterol, mg/dL | 94.9 ± 32.1 | 107.0 ± 31.4 | <0.001 |
| LDL cholesterol (≥130 mg/dL), n (%) | 73 (14.0%) | 392 (22.0%) | 0.014 |
| HDL cholesterol, mg/dL | 49.7 ± 11.7 | 53.1 ± 11.8 | <0.001 |
| HDL (<40 mg/dL), n (%) | 104 (20.0%) | 207 (11.6%) | <0.001 |
| TG, mg/dL | 97.0 ± 53.1 | 102.4 ± 54.6 | 0.045 |
| TG (≥150 mg/dL), n (%) | 70 (13.5%) | 270 (15.2%) | 0.662 |
| Mixed LDL + TG, n (%) | 19 (3.7%) | 93 (5.2%) | 0.428 |
| Mixed LDL + HDL, n (%) | 9 (1.7%) | 22 (1.2%) | 0.808 |
| Variables | Simple CHD | Moderate CHD | Great CHD | Control Group | p-Value |
|---|---|---|---|---|---|
| Patients, n | 265 | 159 | 97 | 1782 | |
| Age, years | 34 ± 15 | 33 ± 13 | 37 ± 14 | 33 ± 11 | 0.012 |
| Sex (male), n (%) | 157 (59.5%) | 90 (56.6%) | 53 (54.6%) | 984 (55.2%) | 0.657 |
| BMI, kg/m2 | 24 ± 5 | 24 ± 5 | 24 ± 5 | 24 ± 6 | 0.897 |
| Hypertension, n (%) | 38 (14.4%) | 28 (17.6%) | 7 (7.2%) | 176 (9.9%) | 0.003 |
| Diabetes, n (%) | 16 (6.1%) | 4 (2.5%) | 6 (6.2%) | 45 (2.5%) | 0.005 |
| Dyslipidemia | |||||
| Total Cholesterol ≥ 240, n (%) | 14 (5.3%) | 4 (2.5%) | 4 (4.1%) | 133 (7.5%) | 0.047 |
| LDL ≥ 130 mg/dL, n (%) | 43 (16.3%) | 17 (10.7%) | 13 (13.4%) | 392 (22.0%) | <0.001 |
| HDL < 40 mg/dL, n (%) | 40 (15.2%) | 36 (22.6%) | 28 (28.9%) | 207 (11.6%) | <0.001 |
| TG > 150 mg/dL, n (%) | 39 (14.8%) | 21 (13.2%) | 11 (11.3%) | 270 (15.2%) | 0.702 |
| Mixed LDL + TG, n (%) | 9 (3.4%) | 6 (3.8%) | 4 (4.1%) | 93 (5.2%) | 0.524 |
| Mixed LDL + HDL, n (%) | 5 (1.9%) | 2 (1.3%) | 2 (2.1%) | 22 (1.2%) | 0.769 |
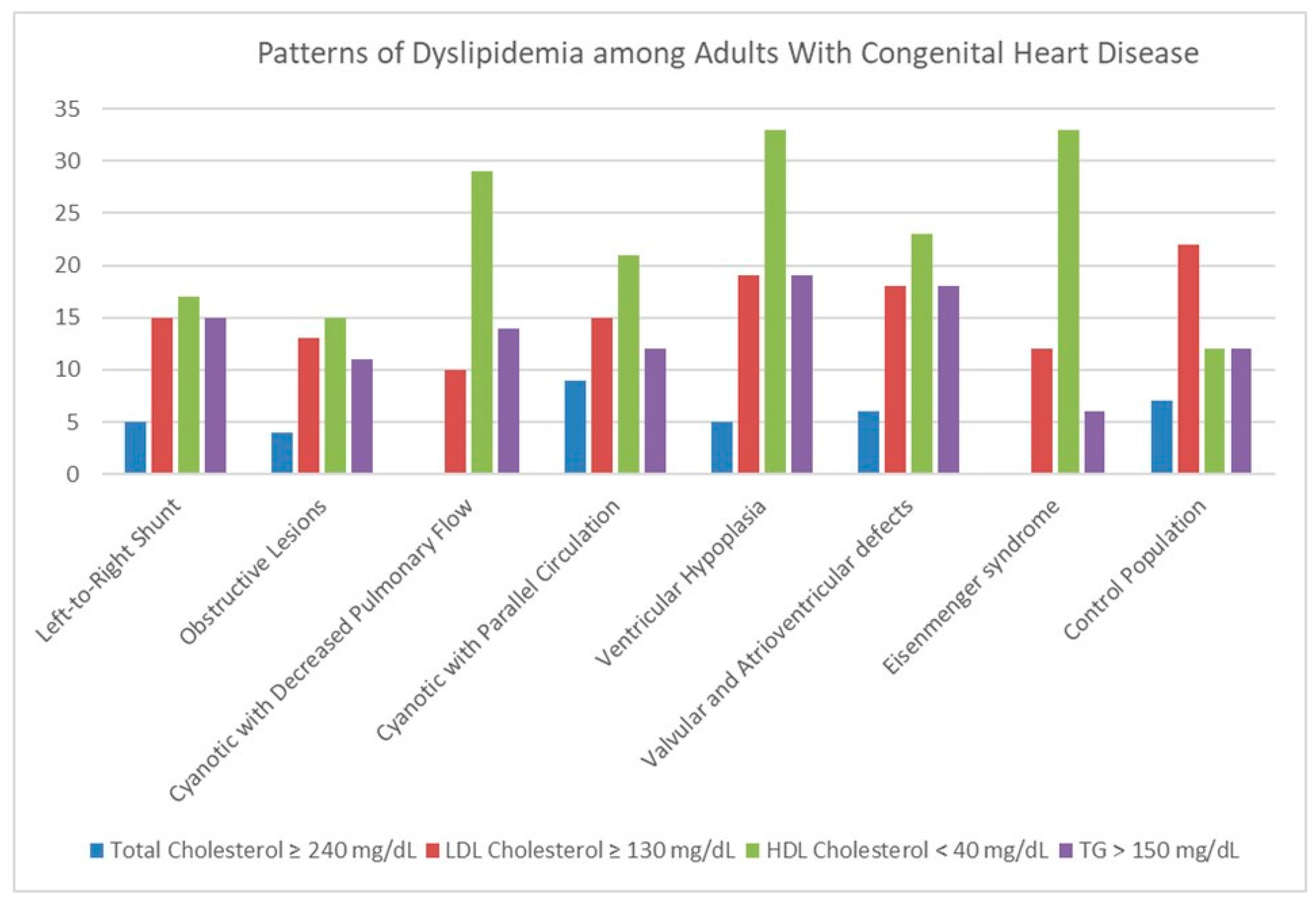
| Variables | L-R Shunt | Obstructive | Cyanotic ↓ Pulm | Cyanotic Parallel | Ventricular Hypoplasia | Valv/Atriov Defects | Eisenmenger | p |
|---|---|---|---|---|---|---|---|---|
| Patients, n (%) | 152 (29%) | 162 (31%) | 51 (10%) | 34 (7%) | 21 (4.0%) | 68 (13%) | 33 (6%) | |
| Age, years | 33 ± 15 | 34 ± 14 | 35 ± 12 | 32 ± 12 | 34 ± 11 | 34 ± 13 | 46 ± 14 | <0.001 |
| BMI, kg/m2 | 24 ± 5 | 25 ± 5 | 24 ± 5 | 25 ± 4 | 24 ± 6 | 24 ± 5 | 24 ± 6 | 0.963 |
| Male sex, n (%) | 85 (56%) | 100 (62%) | 31 (61%) | 23 (68%) | 13 (62%) | 36 (53%) | 13 (39%) | 0.201 |
| HTN, n (%) | 20 (13%) | 31 (19%) | 4 (8%) | 4 (12%) | 1 (5%) | 10 (15%) | 2 (6%) | 0.192 |
| Diabetes, n (%) | 8 (5%) | 8 (5%) | 2 (4%) | 0 (0%) | 3 (14%) | 1 (1%) | 3 (9%) | 0.174 |
| Smoking, n (%) | 8 (5%) | 9 (6%) | 6 (12%) | 1 (3%) | 0 (0%) | 3 (4%) | 1 (3%) | 0.376 |
| Atrial fibrillation, n (%) | 10 (7) | 4 (2) | 4 (8) | 1 (3) | 3 (14) | 2 (3) | 9 (27) | <0.001 |
| Arterial thrombosis, n (%) | 9 (6%) | 2 (1%) | 4 (8%) | 2 (6%) | 1 (5%) | 2 (3%) | 1 (3%) | 0.350 |
| Medical Treatment | ||||||||
| Statin, n (%) | 13 (9%) | 11 (7%) | 5 (10%) | 2 (6%) | 3 (14%) | 7 (10%) | 2 (6%) | 0.886 |
| Beta blocker, n (%) | 13 (9%) | 23 (15%) | 8 (16%) | 5 (15%) | 9 (43%) | 12 (18%) | 9 (27%) | 0.002 |
| Diuretics, n (%) | 13 (9%) | 11 (7%) | 9 (18%) | 7 (21%) | 10 (48%) | 12 (18%) | 15 (45%) | <0.001 |
| Oral anticoagulant, n (%) | 13 (9%) | 19 (12%) | 7 (14%) | 6 (18%) | 11 (52%) | 6 (9%) | 17 (52%) | <0.001 |
| Antiplatelet, n (%) | 11 (8%) | 9 (6%) | 10 (20%) | 4 (12%) | 4 (19%) | 7 (10%) | 8 (24%) | 0.006 |
| Blood Test | ||||||||
| Glucose, mg/dL | 95 (89–99) | 89 (89–101) | 92 (87–99) | 92 (87–101) | 90 (84–96) | 95 (81–101) | 90 (84–106) | 0.701 |
| AST, U/L | 22 (18–27) | 21 (18–25) | 22 (16–32) | 23 (19–30) | 23 (19–30) | 22 (17–26) | 25 (19–31) | 0.096 |
| ALT, U/L | 16 (13–22) | 17 (13–22) | 18 (14–34) | 20 (16–29) | 21 (12–30) | 17 (13–32) | 18 (13–27) | 0.788 |
| Hs-CRP, mg/L | 0.2 (0.06–0.45) | 0.1 (0.05–0.4) | 0.1 (0.05–0.3) | 0.1 (0.07–0.4) | 0.2 (0.1–0.4) | 0.1 (0.06–0.5) | 0.5 (0.1–1.4) | 0.059 |
| NT-pro-BNP, pg/mL | 38 (13–80) | 41 (9–101) | 129 (48–250) | 105 (65–312) | 294 (97–1114) | 80 (24–176) | 426 (164–999) | <0.001 |
| Dyslipidemia | ||||||||
| Total Chol ≥ 240 mg/dL, n (%) | 7 (5%) | 7 (4%) | 0 (0%) | 3 (9%) | 1 (5%) | 4 (6%) | 0 (0%) | 0.421 |
| LDL ≥ 130 mg/dL, n (%) | 22 (15%) | 21 (13%) | 5 (10%) | 5 (15%) | 4 (19%) | 12 (18%) | 4 (12%) | 0.897 |
| HDL < 40 mg/dL, n (%) | 26 (17%) | 22 (14%) | 15 (29%) | 7 (21%) | 7 (33%) | 16 (24%) | 11 (33%) | 0.027 |
| TG > 150 mg/dL, n (%) | 23 (15%) | 18 (11%) | 7 (14%) | 4 (12%) | 4 (19%) | 12 (18%) | 2 (6%) | 0.623 |
| Mixed LDL + TG, n (%) | 6 (4%) | 3 (2%) | 2 (4%) | 3 (9%) | 1 (5%) | 4 (6%) | 0 (0%) | 0.378 |
| Mixed LDL + HDL, n (%) | 3 (2%) | 2 (1%) | 1 (2%) | 1 (3%) | 1 (5%) | 1 (1%) | 0 (0%) | 0.885 |
| Variables | Arterial Thrombosis | p-Value | |
|---|---|---|---|
| No | Yes | ||
| Patients, n | 500 | 21 | |
| Age, years | 33.9 ± 13.7 | 51.2 ± 14.1 | <0.001 |
| BMI, kg/m2 | 24.3 ± 5.2 | 26.3 ± 4.9 | 0.128 |
| Sex (male), n (%) | 288 (57.6%) | 12 (57.1%) | 0.967 |
| Hypertension, n (%) | 66 (13.2%) | 7 (33.3%) | 0.009 |
| Diabetes, n (%) | 22 (4.4%) | 4 (19.0%) | 0.003 |
| Smoker and ex-smoker, n (%) | 36 (7.2%) | 4 (19.0%) | 0.001 |
| Atrial fibrillation, n (%) | 29 (5.8%) | 4 (19.0%) | 0.015 |
| Mechanical valve prosthesis, n (%) | 22 (4.4%) | 0 (0.0%) | 0.255 |
| Cyanosis, n (%) | 38 (7.6%) | 6 (28.6%) | 0.001 |
| Antiaggregation, n (%) | 43 (8.6%) | 10 (47.6%) | <0.001 |
| Oral anticoagulation, n (%) | 71 (14.2%) | 9 (42.9%) | 0.001 |
| Statins, n (%) | 35 (7.0%) | 9 (42.9%) | <0.001 |
| Dyslipidemia | |||
| Total Chol ≥ 240, n (%) | 21 (4.2%) | 1 (4.8%) | 0.900 |
| LDL ≥ 130 mg/dL, n (%) | 70 (14.0%) | 3 (14.3%) | 0.971 |
| HDL < 40 mg/dL, n (%) | 99 (19.8%) | 2 (9.5%) | 0.625 |
| TG > 150 mg/dL, n (%) | 68 (13.6%) | 2 (9.5%) | 0.929 |
| Mixed LDL + TG, n (%) | 19 (3.8%) | 0 (0.0%) | 0.363 |
| Mixed LDL + HDL, n (%) | 7 (1.4%) | 2 (9.5%) | 0.005 |
| Covariates | OR (Crude) (95% CI) | p | OR (Adjusted) (95% CI) | p |
|---|---|---|---|---|
| Age, years | 1.06 (1.04–1.08) | <0.001 | 1.04 (1.01–1.08) | 0.032 |
| Hypertension, n (%) | 3.29 (1.28–8.45) | 0.013 | 0.98 (0.22–2.94) | 0.745 |
| Diabetes, n (%) | 5.11 (1.59–16.47) | 0.006 | 2.18 (0.54–8.83) | 0.273 |
| Smoker, n (%) | 2.61 (1.29–5.25) | 0.007 | 1.91 (0.84–4.34) | 0.124 |
| Atrial fibrillation, n (%) | 3.81 (1.20–12.07) | 0.023 | 1.14 (0.27–4.75) | 0.853 |
| Cyanosis, n (%) | 4.86 (1.71–13.26) | 0.002 | 6.81 (2.12–21.87) | <0.001 |
| Statins, n (%) | 10.52 (4.08–27.08) | <0.001 | 4.31 (1.25–14.89) | 0.021 |
| Mixed LDL + HDL, n (%) | 7.41 (1.44–38.10) | 0.016 | 4.45 (0.75–26.31) | 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Quintana, E.; Rodríguez-González, F. Dyslipidemia Patterns in Adults with Congenital Heart Disease: Focus on HDL Cholesterol. J. Clin. Med. 2025, 14, 8357. https://doi.org/10.3390/jcm14238357
Martínez-Quintana E, Rodríguez-González F. Dyslipidemia Patterns in Adults with Congenital Heart Disease: Focus on HDL Cholesterol. Journal of Clinical Medicine. 2025; 14(23):8357. https://doi.org/10.3390/jcm14238357
Chicago/Turabian StyleMartínez-Quintana, Efrén, and Fayna Rodríguez-González. 2025. "Dyslipidemia Patterns in Adults with Congenital Heart Disease: Focus on HDL Cholesterol" Journal of Clinical Medicine 14, no. 23: 8357. https://doi.org/10.3390/jcm14238357
APA StyleMartínez-Quintana, E., & Rodríguez-González, F. (2025). Dyslipidemia Patterns in Adults with Congenital Heart Disease: Focus on HDL Cholesterol. Journal of Clinical Medicine, 14(23), 8357. https://doi.org/10.3390/jcm14238357








