Metabolic Predictors of CAD: Focus on Cystine, Methionine, Proline, and Threonine Circulating Levels—Exploratory Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Amino Acids Analysis
2.2. Statistical Analysis
3. Results
3.1. Laboratory Results
3.2. Echocardiography
3.3. Coronary Angiography
3.4. Amino Acid Concentration
3.4.1. Correlations Between Circulating Amino Acids and the Number of Diseased Arteries
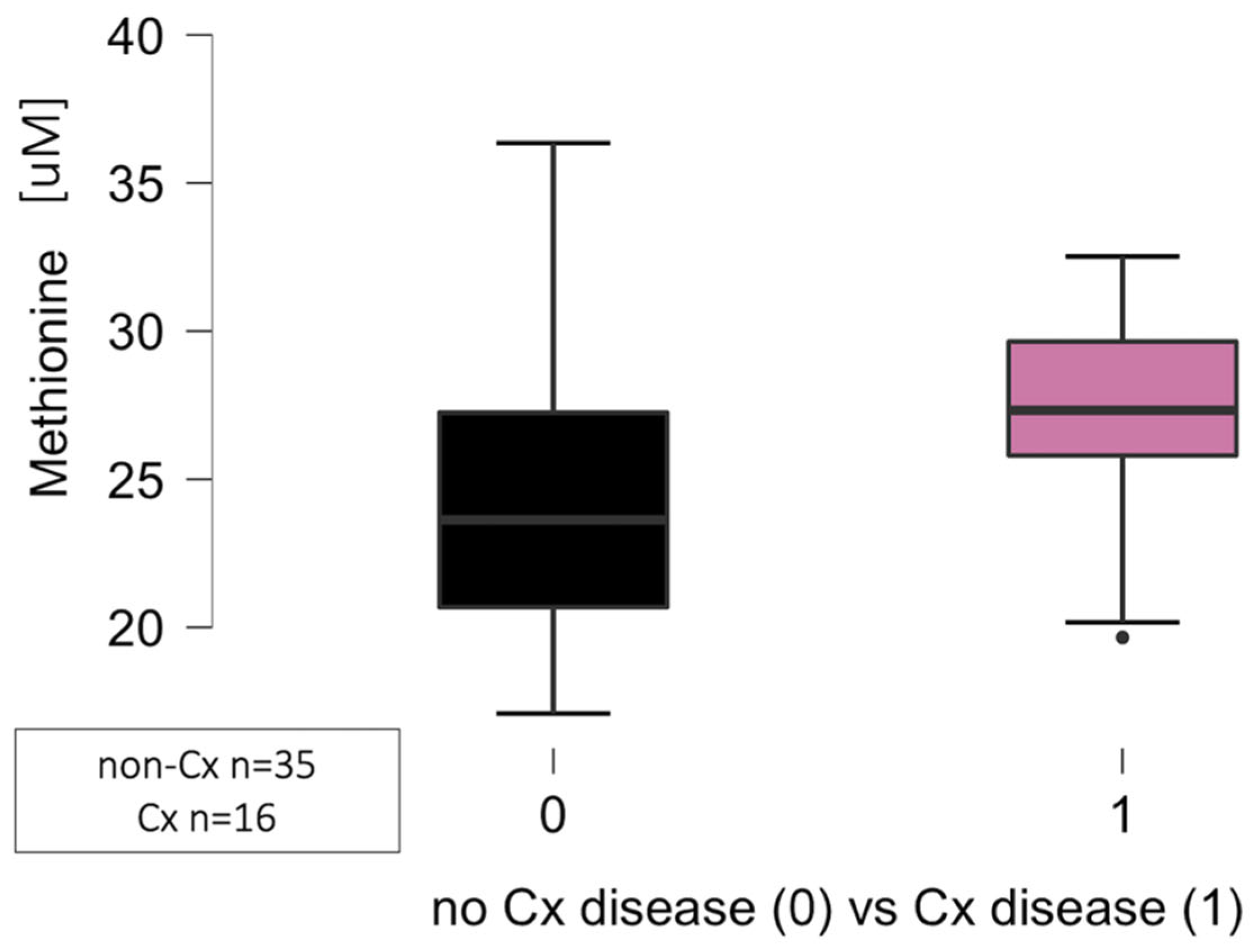
3.4.2. Certain Amino Acid Concentration and the Particular Coronary Arteries’ Atherosclerosis
Left Descending Artery
Circumflex Artery
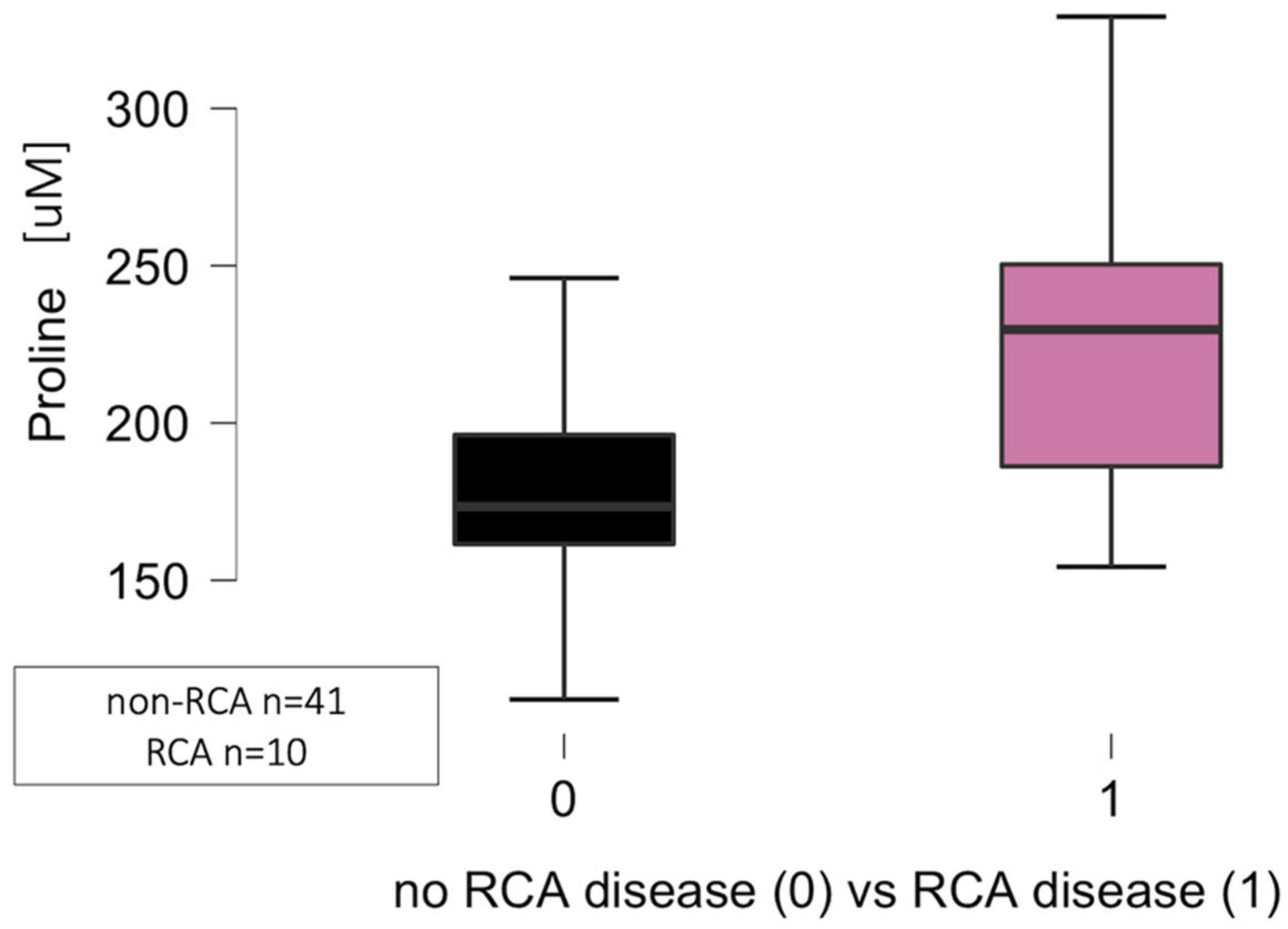
Right Coronary Artery
3.4.3. Logistic Regression Analysis for Coronary Disease Prediction
Any Significant Coronary Disease
Left Descending Artery Disease
Circumflex Artery Disease
Right Coronary Artery Disease
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguilar-Villegas, Ó.R.; Barragán-Los Santos, J.; Del Moral-Wong, L.E.; Amezcua-Guerra, L.M.; Aguirre-García, M.M. Mas allá de la placa: Implicaciones inmunológicas de los factores de riesgo en la atherosclerosis [Beyond the plaque: Immunological implications of risk factors in atherosclerosis]. Arch. Cardiol. Mex. 2024, 95, 81–95. [Google Scholar]
- Stone, P.H.; Libby, P.; Boden, W.E. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management-The Plaque Hypothesis: A Narrative Review. JAMA Cardiol. 2023, 8, 192–201. [Google Scholar] [CrossRef]
- Devesa, A.; Ibanez, B.; Malick, W.A.; Tinuoye, E.O.; Bustamante, J.; Peyra, C.; Rosenson, R.S.; Bhatt, D.L.; Stone, G.W.; Fuster, V. Primary Prevention of Subclinical Atherosclerosis in Young Adults: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 82, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131–155. [Google Scholar] [CrossRef]
- Akinyelure, O.P.; Colantonio, L.D.; Chaudhary, N.S.; Jaeger, B.C.; Judd, S.E.; Cushman, M.; Zakai, N.A.; Kabagambe, E.K.; Howard, V.J.; Safford, M.M.; et al. Inflammation biomarkers and incident coronary heart disease: The Reasons for Geographic and Racial Differences in Stroke Study. Am. Heart J. 2022, 253, 39–47. [Google Scholar] [CrossRef]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Atlas Writing Group, European Society of Cardiology; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Zeitouni, M.; Clare, R.M.; Chiswell, K.; Abdulrahim, J.; Shah, N.; Pagidipati, N.P.; Shah, S.H.; Roe, M.T.; Patel, M.R.; Jones, W.S. Risk Factor Burden and Long-Term Prognosis of Patients with Premature Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e017712. [Google Scholar] [CrossRef] [PubMed]
- Helman, T.J.; Headrick, J.P.; Stapelberg, N.J.C.; Braidy, N. The sex-dependent response to psychosocial stress and ischaemic heart disease. Front. Cardiovasc. Med. 2023, 10, 1072042–11072080. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.; Cahalane, R.; Hengst, R.; Akyildiz, A.; Farrell, E.; Gijsen, F.; Aikawa, E.; van der Heiden, K.; Wissing, T. The interplay of collagen, macrophages, and microcalcification in atherosclerotic plaque cap rupture mechanics. Basic Res. Cardiol. 2024, 119, 193–213. [Google Scholar] [CrossRef]
- Gianopoulos, I.; Daskalopoulou, S.S. Macrophage profiling in atherosclerosis: Understanding the unstable plaque. Basic Res. Cardiol. 2024, 119, 35–56. [Google Scholar] [CrossRef]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline metabolism and redox; maintaining a balance in health and disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef]
- Zhang, Z.; Marrachelli, V.; Yang, W.; Trenson, S.; Huang, Q.; Wei, F.; Thijs, L.; Van Keer, J.; Monleon, D.; Verhamme, P.; et al. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a population study. Eur. J. Prev. Cardiol. 2019, 1, 22–32. [Google Scholar] [CrossRef]
- Sonkar, S.K.; Verma, J.; Sonkar, G.K.; Gupta, A.; Singh, A.; Vishwakarma, P.; Bhosale, V. Assessing the Role of Asymmetric Dimethylarginine in Endothelial Dysfunction: Insights into Cardiovascular Risk Factors. Cureus 2023, 17, e77565. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguadé-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; EVINCI Study Investigators; et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179–e002189. [Google Scholar] [CrossRef]
- Beutner, F.; Ritter, C.; Scholz, M.; Teren, A.; Holdt, L.; Teupser, D.; Becker, S.; Thiele, H.; Gielen, S.; Thiery, J.; et al. A metabolomic approach to identify the link between sports activity and atheroprotection. Eur. J. Prev. Cardiol. 2022, 29, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Gajecki, D.; Gawryś, J.; Szahidewicz-Krupska, E.; Doroszko, A. Role of Erythrocytes in Nitric Oxide Metabolism and Paracrine Regulation of Endothelial Function. Antioxidants 2022, 11, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.J.; Tommasi, S.; Faull, R.; Gleadle, J.M.; Mangoni, A.A.; Selvanayagam, J.B. Arginine Metabolites as Biomarkers of Myocardial Ischaemia, Assessed with Cardiac Magnetic Resonance Imaging in Chronic Kidney Disease. Biomolecules 2021, 11, 416–428. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, R.; Liu, Y.; Lou, X.; Zhang, W.; Cui, Z.; Huang, Y.; Wang, T. Perylene-Mediated Electron Leakage in Respiratory Chain to Trigger Endogenous ROS Burst for Hypoxic Cancer Chemo-Immunotherapy. Adv. Sci. 2023, 10, e2204498. [Google Scholar] [CrossRef]
- Liu, K.; Borreggine, R.; Gallart-Ayala, H.; Ivanisevic, J.; Marques-Vidal, P. Branched chain amino acids and body composition in healthy, non-obese women. Eur. J. Prev. Cardiol. 2024, 31, zwae175.123. [Google Scholar] [CrossRef]
- Hu, X.; Ma, Z.; Xu, B.; Li, S.; Yao, Z.; Liang, B.; Wang, J.; Liao, W.; Lin, L.; Wang, C.; et al. Glutamine metabolic microenvironment drives M2 macrophage polarization to mediate trastuzumab resistance in HER2-positive gastric cancer. Cancer Commun. 2023, 43, 909–937. [Google Scholar] [CrossRef]
- McCarty, M.F. Supplementation with Phycocyanobilin, Citrulline, Taurine, and Supranutritional Doses of Folic Acid and Biotin-Potential for Preventing or Slowing the Progression of Diabetic Complications. Healthcare 2017, 5, 15–33. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Milton, J.E.; Packard, D.P.; Shuler, L.A.; Short, R.A. Dietary amino acids and blood pressure: A cohort study of patients with cardiovascular disease. Am. J. Kidney Dis. 2012, 59, 803–809. [Google Scholar] [CrossRef]
- Anand, S.K.; Governale, T.A.; Zhang, X.; Razani, B.; Yurdagul, A., Jr.; Pattillo, C.B.; Rom, O. Amino Acid Metabolism and Atherosclerotic Cardiovascular Disease. Am. J. Pathol. 2024, 194, 510–524. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Wang, Y.; Xiang, H.; Yang, S.Y.; Guo, Z.; Wang, R.; Chen, W.; Xing, D.; Chen, B.; et al. The Role of IL-6 and TMEM100 in Lumbar Discogenic Pain and the Mechanism of the Glycine-Serine-Threonine Metabolic Axis: A Metabolomic and Molecular Biology Study. J. Pain. Res. 2023, 16, 437–461. [Google Scholar] [CrossRef]
- Chadee, A.; Mohammad, M.; Vanlerberghe, G.C. Evidence that mitochondrial alternative oxidase respiration supports carbon balance in source leaves of Nicotiana tabacum. J. Plant Physiol. 2022, 279, 153840–153851. [Google Scholar] [CrossRef]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J. Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926–102943. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A. The antioxidant glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [PubMed]
- Kaludercic, N.; Arusei, R.J.; Di Lisa, F. Recent advances on the role of monoamine oxidases in cardiac pathophysiology. Basic Res. Cardiol. 2023, 118, 41–51. [Google Scholar] [CrossRef]
- Boneberg, R.; Pardun, A.; Hannemann, L.; Hildebrandt, O.; Koehler, U.; Kinscherf, R.; Hildebrandt, W. High Plasma Cystine Levels Are Associated with Blood Pressure and Reversed by CPAP in Patients with Obstructive Sleep Apnea. J. Clin. Med. 2021, 10, 1387–1403. [Google Scholar] [CrossRef]
- Patel, R.S.; Ghasemzadeh, N.; Eapen, D.J.; Sher, S.; Arshad, S.; Ko, Y.A.; Veledar, E.; Samady, H.; Zafari, A.M.; Sperling, L.; et al. Novel Biomarker of Oxidative Stress Is Associated with Risk of Death in Patients with Coronary Artery Disease. Circulation 2016, 133, 361–369. [Google Scholar] [CrossRef]
- Suzuki, S.; Shino, M.; Fujikawa, T.; Itoh, Y.; Ueda, E.; Hashimoto, T.; Kuji, T.; Kobayashi, N.; Ohnishi, T.; Hirawa, N.; et al. Plasma Cystine Levels and Cardiovascular and All-Cause Mortality in Hemodialysis Patients. Ther. Apher. Dial. 2018, 22, 476–484. [Google Scholar] [CrossRef]
- Schwörer, S.; Berisa, M.; Violante, S.; Qin, W.; Zhu, J.; Hendrickson, R.C.; Cross, J.R.; Thompson, C.B. Proline biosynthesis is a vent for TGFβ-induced mitochondrial redox stress. EMBO J. 2020, 39, e103334. [Google Scholar] [CrossRef]
- Lv, Q.; Li, D.; Zhao, L.; Yu, P.; Tao, Y.; Zhu, Q.; Wang, Y.; Wang, M.; Fu, G.; Shang, M.; et al. Proline metabolic reprogramming modulates cardiac remodeling induced by pressure overload in the heart. Sci. Adv. 2024, 10, eadl3549. [Google Scholar] [CrossRef]
- Xie, W.; Parker, J.L.; Heaps, C.L. Effect of exercise training on nitric oxide and superoxide/H2O2 signaling pathways in collateral-dependent porcine coronary arterioles. J. Appl. Physiol. 2021, 112, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Self, T.S.; Bray, J.F.; Heaps, C.L. H2O2-mediated relaxation in a swine model of ischemic heart disease and exercise training: Mechanistic insights and the role of Kv7 channels. Basic Res. Cardiol. 2025; ahead of print. [Google Scholar]
- Bertero, E.; Popoiu, T.A.; Maack, C. Mitochondrial calcium in cardiac ischemia/reperfusion injury and cardioprotection. Basic Res. Cardiol. 2024, 119, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Preckel, B.; Zuurbier, C.J.; Weber, N.C. Effects of SGLT2 inhibitors on ion channels in heart failure: Focus on the en-dothelium. Basic Res. Cardiol. 2025; ahead of print. [Google Scholar]
- Otero-Cacho, A.; Aymerich, M.; Flores-Arias, M.T.; Abal, M.; Álvarez, E.; Pérez-Muñuzuri, V.; Muñuzuri, A.P. Determination of hemodynamic risk for vascular disease in planar artery bifurcations. Sci. Rep. 2018, 8, 2795–3002. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Hanć, A.; Frąckowiak, J.; Piecek, J.; Spasenenko, I.; Olasińska-Wiśniewska, A.; Krasińska, B.; Tykarski, A. The Hypothesis of Trace Elements Involvement in the Coronary Arteries Atherosclerotic Plaques’ Location. J. Clin. Med. 2024, 13, 6933–6944. [Google Scholar] [CrossRef]

| Parameters | CAD Group n = 27 | Non-CAD Group n = 27 | p |
|---|---|---|---|
| Demographics: | |||
| Age [years] (median (Q1–Q3)) | 66 (62–72) | 68 (61–72) | 0.633 |
| Sex (male (%)/female (%)) | 18 (67)/9 (33) | 15 (56)/12 (44) | 0.413 |
| BMI [kg/m2] (median (Q1–Q3)) | 27.2 (25.2–30.4) | 27.8 (25.9–33.5) | 0.242 |
| BMI > 30 (n (%)) | 7 (26)/20 (74) | 9 (33)/18 (67) | 0.622 |
| Comorbidities: | |||
| Arterial hypertension (n (%)) | 18 (67) | 17 (63) | 0.893 |
| Diabetes. mellitus (n (%)) | 9 (33) | 4 (15) | 0.155 |
| Dyslipidemia (n (%)) | 25 (93) | 20 (74) | 0.142 |
| COPD (n (%)) | 2 (7) | 3 (11) | 0.582 |
| Kidney disease * (n (%)) | 10 (37) | 5 (19) | 0.224 |
| Family history (n (%)) | 3 (11) | 6 (22) | 0.233 |
| Nicotine: | |||
| Active smoking (n (%)) | 5 (19) | 2 (7) | 0.254 |
| Former smoker (n (%)) | 9 (33) | 7 (26) | 0.621 |
| Parameters | CAD Group n = 27 | Non-CAD Group n = 27 | p |
|---|---|---|---|
| Whole blood count | |||
| WBC [10 × 9/dL] (median (Q1–Q3)) | 7.91 (6.14–10.31) | 6.89 (6.44–7.93) | 0.342 |
| Hb [10 × 9/dL] (median (Q1–Q3)) | 9.00 (8.30–9.60) | 8.95 (8.50–9.30) | 0.696 |
| Hct [%] (median (Q1–Q3)) | 44 (40–45) | 43 (42–45) | 0.643 |
| Plt [10 × 9/dL] (median (Q1–Q3)) | 238 (201–266) | 268 (168–321) | 0.682 |
| RDW [%] (median (Q1–Q3)) | 13.2 (12.9–13.6) | 12.7 (12.4–13.2) | 0.032 |
| Kidney function tests: | |||
| Serum creatinine [umol/L] (median (Q1–Q3)) | 91 (84–106) | 74 (69–85) | 0.017 |
| GFR [mL/min/m2] (median (Q1–Q3)) | 68 (51–81) | 73 (69–80) | 0.144 |
| Liver function tests: | |||
| ALT [IU/mL] (median (Q1–Q3)) | 24 (21–51) | 26 (21–47) | 0.823 |
| NT–pro–BNP [pg/mL] (median (Q1–Q3)) | 691 (402–1781) | 511 (242–906) | 0.352 |
| Lipoprotein (a) [mg/dL] (median (Q1–Q3)) | 2.20 (1.76–4.33) | 3.30 (2.43–5.10) | 0.424 |
| CK-MB [ng/mL] (median (Q1–Q3)) | 2.70 (1.55–2.96) | 1.30 (1.00–1.80) | <0.001 |
| Lipidogram: | |||
| Total cholesterol [mmol/L] (median (Q1–Q3)) | 5.10 (3.23–6.15) | 4.47 (4.07–7.17) | 0.567 |
| HDL [mmol/L] (median (Q1–Q3)) | 1.23 (1.12–4.23) | 1.46 (1.35–1.96) | 0.728 |
| LDL [mmol/L] (median (Q1–Q3)) | 3.18 (1.54–4.50) | 2.85 (1.88–5.13) | 0.435 |
| Triglycerides [mmol/L] (median (Q1–Q3)) | 1.68 (1.41–1.89) | 1.72 (1.43–1.83) | 0.747 |
| Amino Acids [uM] (Median (Q1–Q3)) | LOD Empirical | LOD Analyte | CAD Group n = 27 | Non-CAD Group n = 27 | p |
|---|---|---|---|---|---|
| 1-methylhistidine | 0.560 | 0.560 | 7.82 (6.21–10.42) | 6.21 (4.77–9.06) | 0.141 |
| Alfa-aminobutyric acid | 1.415 | 1.415 | 20.75 (18.48–27.39) | 22.47 (19.51–27.93) | 0.562 |
| Alanine | 9.834 | 9.834 | 412 (378–514) | 403 (349–445) | 0.261 |
| Allo-isoleucine | 0.615 | 0.615 | 1.95 (1.39–2.60) | 1.67 (1.39–2.01) | 0.223 |
| Asparganine | 10.642 | 10.642 | 46.47 (45.60–56.69) | 46.67 (41.03–51.47) | 0.203 |
| Glutamine | 67.153 | 67.153 | 595 (555–658) | 593 (519–626) | 0.397 |
| Histidine | 20.440 | 115.657 | 92.02 (87.05–113.75) | 99.06 (84.45–99.12) | 0.416 |
| Taurine | 9.807 | 19.409 | 132 (102–167) | 151 (114–164) | 0.436 |
| Beta-aminoisobutyric acid | 0.170 | 0.170 | 2.67 (1.94–4.19) | 2.56 (1.37–4.42) | 0.500 |
| Arginine | 6.509 | 27.542 | 84.84 (76.54–97.17) | 82.13 (71.41–103.25) | 0.628 |
| Aspartic acid | 7.321 | 7.321 | 22.25 (18.54–27.96) | 26.74 (20.56–33.23) | 0.183 |
| Ethanolamine | 0.389 | 0.389 | 9.34 (8.76–10.45) | 9.84 (8.31–11.25) | 0.141 |
| Glycine | 124.104 | 124.104 | 250 (208–277) | 256 (215–282) | 0.706 |
| Lysine | 20.570 | 115.831 | 206 (171–221) | 196 (172–225) | 0.856 |
| Sarcosine | 1.004 | 1.004 | 1.45 (1.23–1.80) | 1.40 (1.11–1.85) | 0.684 |
| Valine | 28.335 | 28.335 | 247 (212–280) | 246 (232–264) | 0.815 |
| Beta-Alanine | 2.583 | 2.583 | 2.82 (2.43–3.36) | 3.00 (2.56–4.30) | 0.400 |
| Citrulline | 6.952 | 6.952 | 33.72 (29.21–42.16) | 35.80 (33.36–37.35) | 0.545 |
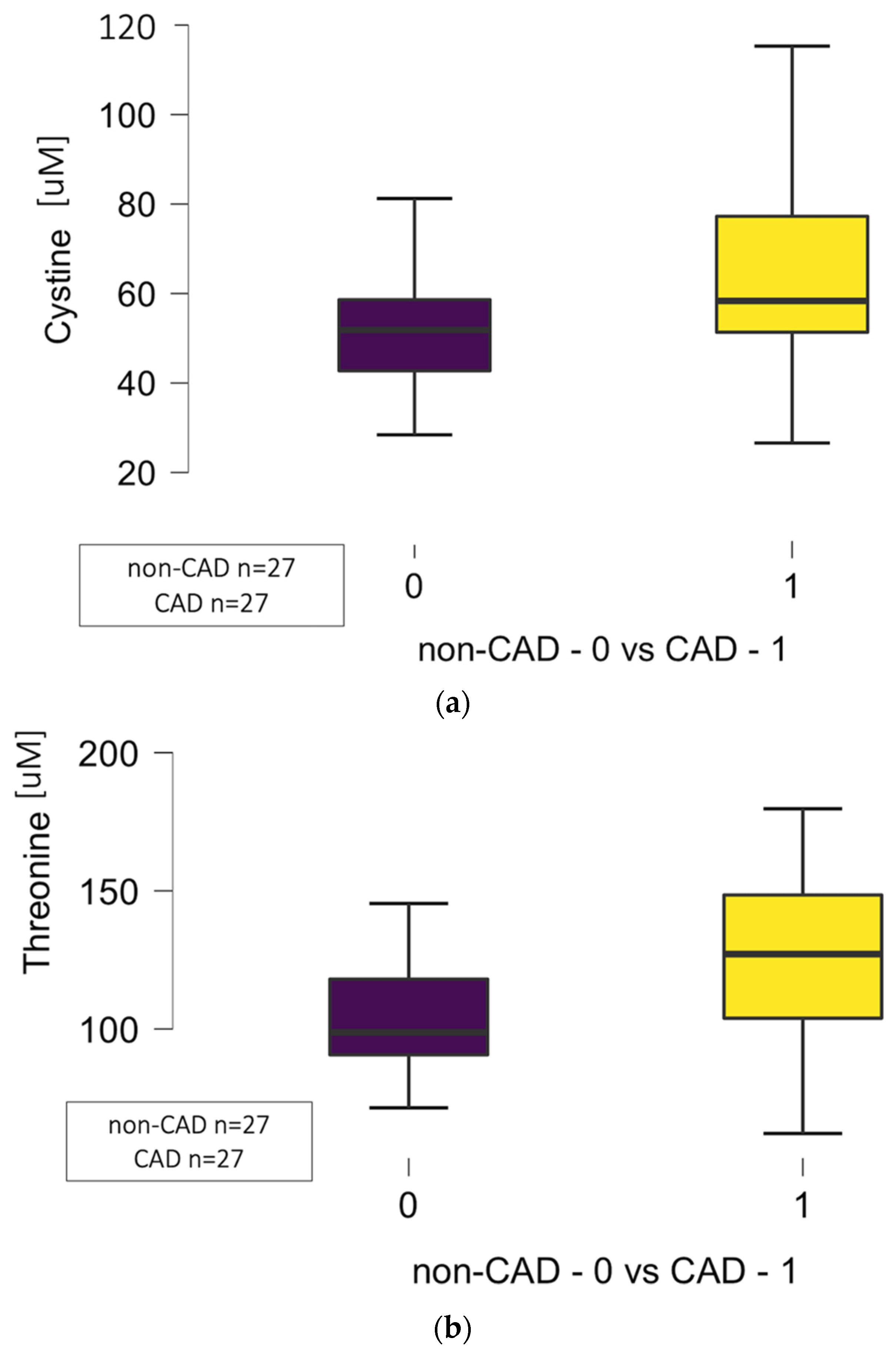
| Cystine | 3.246 | 15.003 | 58.34 (51.34–77.26) | 51.84 (42.72–58.63) | 0.036 * |
| Leucine | 37.650 | 91.335 | 141 (123–159) | 142 (119–146) | 0.571 |
| Serine | 9.283 | 9.283 | 142 (120–167) | 144 (123–155) | 0.659 |
| Threonine | 17.027 | 17.027 | 127 (104–149) | 99 (91–118) | 0.006 * |
| Tyrosine | 5.338 | 5.338 | 70.31 (59.52–81.03) | 66.82 (60.60–76.31) | 0.706 |
| 4-Hydroxyproline | 0.443 | 0.443 | 11.82 (8.34–14.25) | 13.53 (7.39–16.28) | 0.684 |
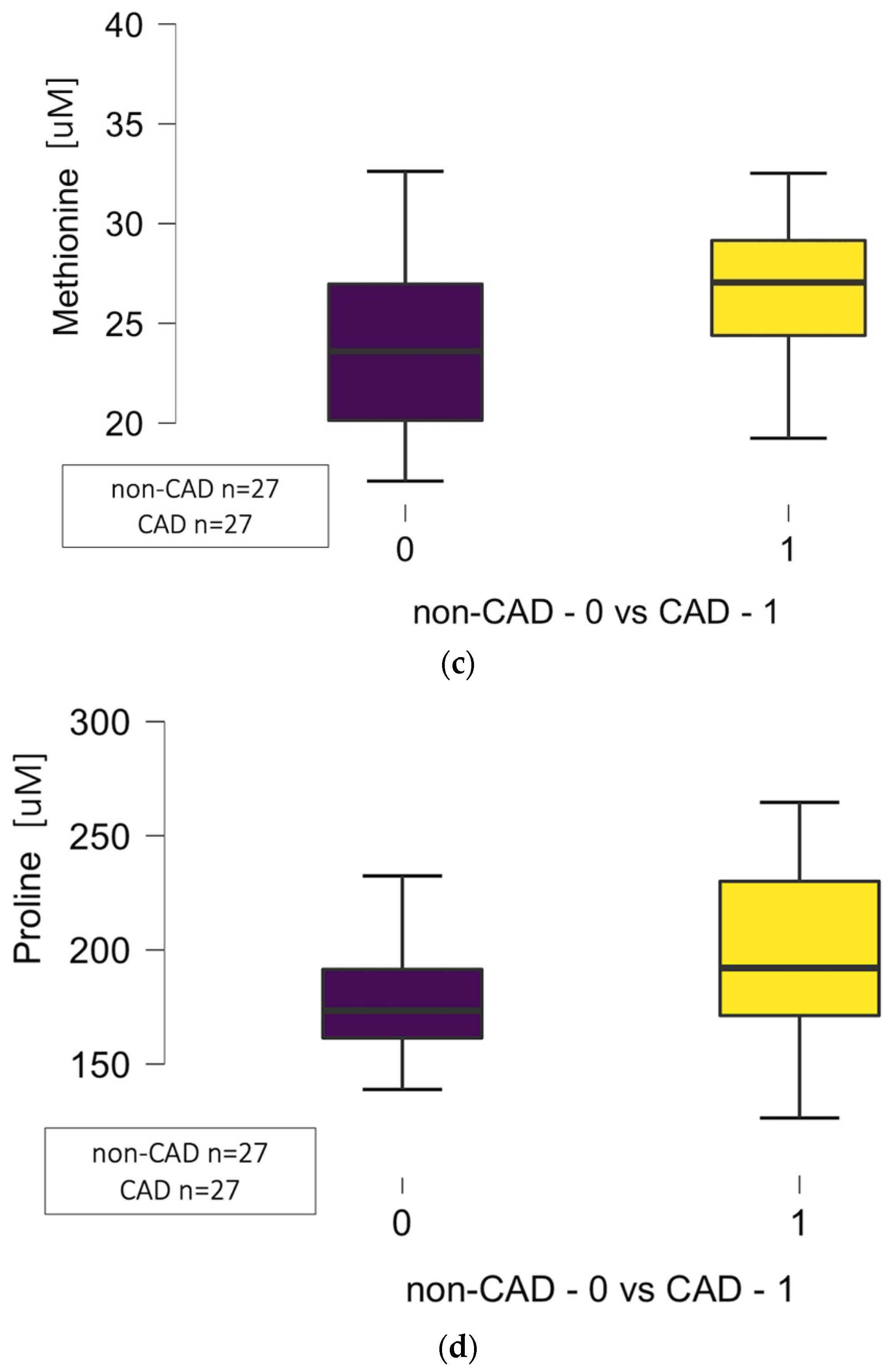
| Methionine | 4.375 | 4.375 | 27.05 (24.39–29.16) | 23.60 (20.14–26.97) | 0.013 * |
| Phenyloalanine | 4.741 | 4.741 | 76.43 (65.43–88.24) | 76.16 (68.31–87.43) | 0.917 |
| Pipecolic acid | 0.891 | 0.891 | 1.43 (1.10–1.93) | 1.37 (0.92–1.66) | 0.337 |
| 3-methylhistidine | 4.045 | 5.370 | 13.39 (8.36–22.59) | 11.26 (5.86–23.00) | 0.595 |
| Alpha-aminoadipic acid | 0.219 | 0.219 | 1.10 (0.90–1.42) | 1.06 (0.82–1.28) | 0.736 |
| Glutamic acid | 23.869 | 23.869 | 73.70 (62.78–100.43) | 73.24 (55.96–107.35) | 0.972 |
| Proline | 16.765 | 16.765 | 192 (171–231) | 173 (162–192) | 0.042 * |
| Tryptophane | 0.716 | 0.716 | 58.3 (49.61–68.74) | 61.18 (52.33–68.24) | 0.643 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowicz, T.; Pietkiewicz, D.; Plewa, S.; Krasińska, B.; Spasenenko, I.; Gabriel, K.; Jezierska, K.; Krasiński, Z.; Kowalewski, M.; Matysiak, J.; et al. Metabolic Predictors of CAD: Focus on Cystine, Methionine, Proline, and Threonine Circulating Levels—Exploratory Pilot Study. J. Clin. Med. 2025, 14, 8356. https://doi.org/10.3390/jcm14238356
Urbanowicz T, Pietkiewicz D, Plewa S, Krasińska B, Spasenenko I, Gabriel K, Jezierska K, Krasiński Z, Kowalewski M, Matysiak J, et al. Metabolic Predictors of CAD: Focus on Cystine, Methionine, Proline, and Threonine Circulating Levels—Exploratory Pilot Study. Journal of Clinical Medicine. 2025; 14(23):8356. https://doi.org/10.3390/jcm14238356
Chicago/Turabian StyleUrbanowicz, Tomasz, Dagmara Pietkiewicz, Szymon Plewa, Beata Krasińska, Ievgen Spasenenko, Katarzyna Gabriel, Karolina Jezierska, Zbigniew Krasiński, Mariusz Kowalewski, Jan Matysiak, and et al. 2025. "Metabolic Predictors of CAD: Focus on Cystine, Methionine, Proline, and Threonine Circulating Levels—Exploratory Pilot Study" Journal of Clinical Medicine 14, no. 23: 8356. https://doi.org/10.3390/jcm14238356
APA StyleUrbanowicz, T., Pietkiewicz, D., Plewa, S., Krasińska, B., Spasenenko, I., Gabriel, K., Jezierska, K., Krasiński, Z., Kowalewski, M., Matysiak, J., & Tykarski, A. (2025). Metabolic Predictors of CAD: Focus on Cystine, Methionine, Proline, and Threonine Circulating Levels—Exploratory Pilot Study. Journal of Clinical Medicine, 14(23), 8356. https://doi.org/10.3390/jcm14238356





