Digital Workflow for Interim Prosthetic Rehabilitation Through the All-on-4 Concept Using 3D Printing Additive Process
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
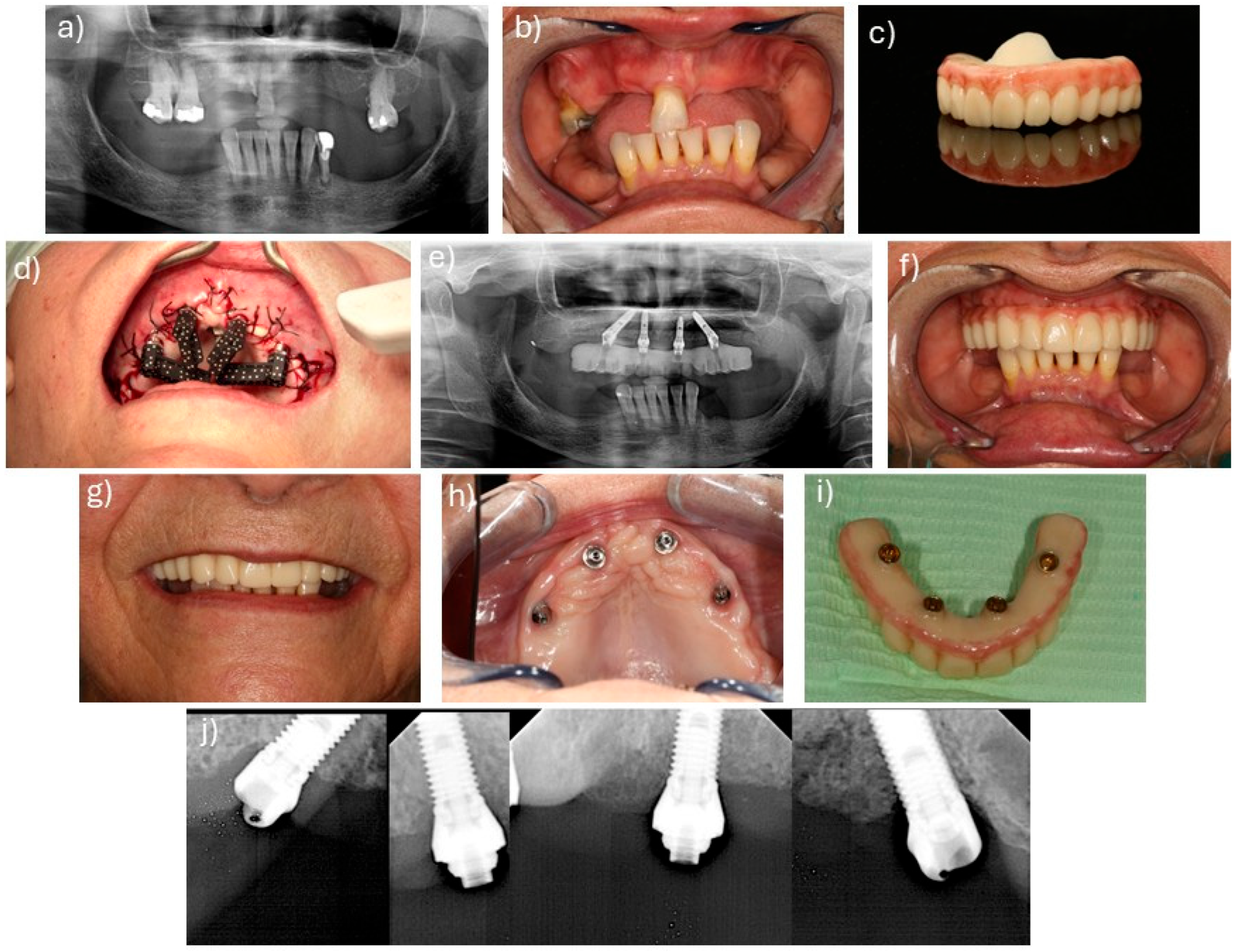
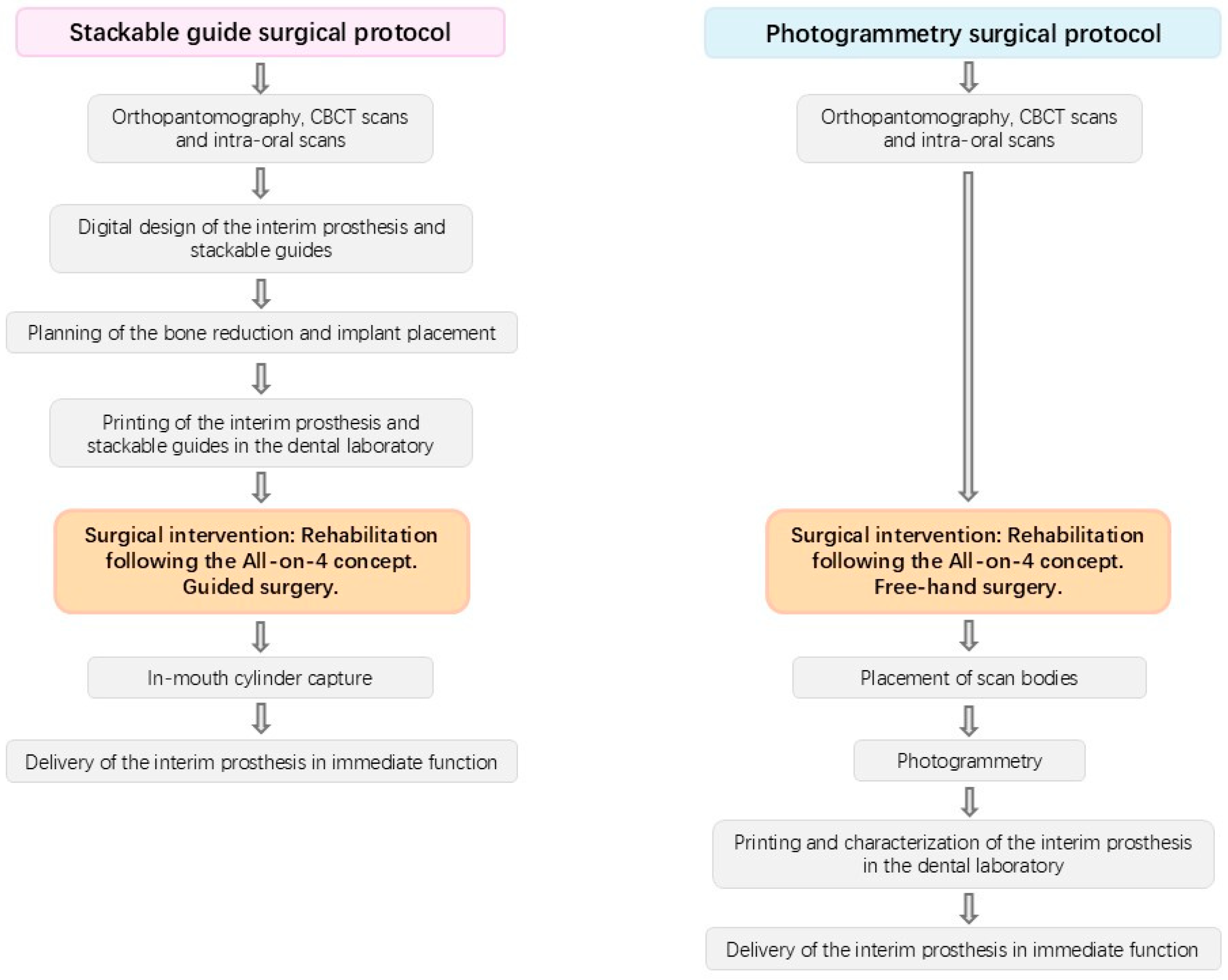
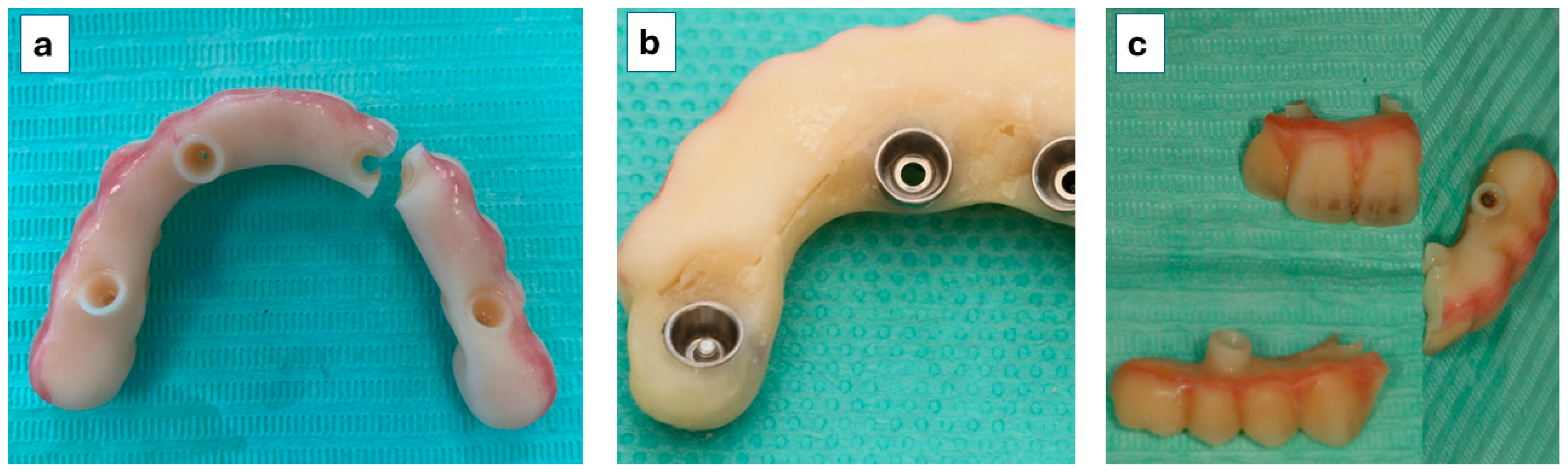
2.2. Surgical and Prosthetic Protocols
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Status Report on Oral Health. 2022. Available online: https://www.who.int/team/noncommunicable-diseases/global-status-report-on-oral-health-2022 (accessed on 2 September 2025).
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Ferro, A.; Botto, J. The All-on-4 Treatment Concept for the Rehabilitation of the Completely Edentulous Mandible: A Longitudinal Study with 10 to 18 Years of Follow-Up. Clin. Implant. Dent. Relat. Res. 2019, 21, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Ferro, A.; Nunes, M. The All-on-4 Concept for Full-Arch Rehabilitation of the Edentulous Maxillae: A Longitudinal Study with 5–13 Years of Follow-Up. Clin. Implant. Dent. Relat. Res. 2019, 21, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridakos, P.; Chochlidakis, K.; Kang, K.; Chen, Y.; Alghfeli, A.; Kudara, Y.; Weber, H. Digital Workflow for Implant Rehabilitation with Double Full-Arch Monolithic Zirconia Prostheses. J. Prosthodont. 2020, 29, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Chochlidakis, K.; Papaspyridakos, P.; Tsigarida, A.; Romeo, D.; Chen, Y.; Natto, Z.; Ercoli, C. Digital Versus Conventional Full-Arch Implant Impressions: A Prospective Study on 16 Edentulous Maxillae. J. Prosthodont. 2020, 29, 281–286. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Vazouras, K.; Chen, Y.; Kotina, E.; Natto, Z.; Kang, K.; Chochlidakis, K. Digital vs. Conventional Implant Impressions: A Systematic Review and Meta-Analysis. J. Prosthodont. 2020, 29, 660–678. [Google Scholar] [CrossRef]
- Pommer, B.; Mailath-Pokorny, G.; Haas, R.; Busenlechner, D.; Fürhauser, R.; Watzek, G. Patients’ Preferences towards Minimally Invasive Treatment Alternatives for Implant Rehabilitation of Edentulous Jaws. Eur. J. Oral. Implantol. 2014, 7 (Suppl. 2), S91–S109. [Google Scholar]
- Papaspyridakos, P.; De Souza, A.; Bathija, A.; Kang, K.; Chochlidakis, K. Complete Digital Workflow for Mandibular Full-Arch Implant Rehabilitation in 3 Appointments. J. Prosthodont. 2021, 30, 548–552. [Google Scholar] [CrossRef]
- Bernauer, S.A.; Zitzmann, N.U.; Joda, T. The Complete Digital Workflow in Fixed Prosthodontics Updated: A Systematic Review. Healthcare 2023, 11, 679. [Google Scholar] [CrossRef]
- Schweiger, J.; Edelhoff, D.; Güth, J.-F. 3D Printing in Digital Prosthetic Dentistry: An Overview of Recent Developments in Additive Manufacturing. J. Clin. Med. 2021, 10, 2010. [Google Scholar] [CrossRef]
- Abdelmohsen, N.; Bourauel, C.; Elshazly, T.M. Interim Fixed Dental Prostheses Fabrication Techniques and Factors Affecting Their Success: A Narrative Review. Oral 2025, 5, 44. [Google Scholar] [CrossRef]
- Mühlemann, S.; Hjerppe, J.; Hämmerle, C.H.F.; Thoma, D.S. Production time, effectiveness and costs of additive and subtractive computer-aided manufacturing (CAM) of implant prostheses: A systematic review. Clin. Oral Implant. Res. 2021, 32, S289–S302. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, A.; Pala, K.; Strauss, F.J.; Hjerppe, J.; Jung, R.E.; Joda, T. Additively and subtractively manufactured implant-supported fixed dental prostheses: A systematic review. Clin. Oral Implant. Res. 2023, 34, S50–S63. [Google Scholar] [CrossRef] [PubMed]
- Erar, K.; Iliescu, A.A.; Popa, G.; Iliescu, A.; Rudnic, I.; Feier, R.; Voinea-Georgescu, R.N. Additive vs. subtractive CAD/CAM procedures in manufacturing of the PMMA interim dental crowns. A comparative in vitro study of internal fit. Rev. Chim. 2020, 71, 405–410. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Pradíes, G.; Bai, S. Factors affecting accuracy in the additive manufacturing of interim dental prostheses: A systematic review. J. Prosthet. Dent. 2024, 17, 1556–1566. [Google Scholar] [CrossRef]
- Martins, J.; Rangel, J.; de Araújo Nobre, M.; Ferro, A.; Nunes, M.; Almeida, R.; Moura Guedes, C. A New Full Digital Workflow for Fixed Prosthetic Rehabilitation of Full-Arch Edentulism Using the All-on-4 Concept. Medicina 2024, 60, 720. [Google Scholar] [CrossRef]
- Mombelli, A.; van Oosten, M.A.; Schurch, E.; Land, N.P. The Microbiota Associated with Successful or Failing Osseointegrated Titanium Implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Riva, F.; Seoane, M.; Reichenheim, M.E.; Tsakos, G.; Celeste, R.K. Adult Oral Health-Related Quality of Life Instruments: A Systematic Review. Community Dent. Oral Epidemiol. 2022, 50, 333–338. [Google Scholar] [CrossRef]
- Crespi, R.; Fabris, G.B.M.; Crespi, G.; Toti, P.; Marconcini, S.; Covani, U. Effects of different loading protocols on the bone remodeling volume of immediate maxillary single implants: A 2- to 3-year follow-up. Int. J. Oral Maxillofac. Implant. 2019, 34, 953–962. [Google Scholar] [CrossRef]
- La Monaca, G.; Pranno, N.; Annibali, S.; Di Carlo, S.; Pompa, G.; Cristalli, M.P. Immediate Flapless Full-Arch Rehabilitation of Edentulous Jaws on 4 or 6 Implants According to the Prosthetic-Driven Planning and Guided Implant Surgery: A Retrospective Study on Clinical and Radiographic Outcomes up to 10 Years of Follow-Up. Clin. Implant. Dent. Relat. Res. 2022, 24, 831–844. [Google Scholar] [CrossRef]
- Ferro, A.; de Araújo Nobre, M.A. All-on-4 Concept Using TiUltra Surface Implants and Multi-Unit Xeal Abutments: Pilot Study Report. In Proceedings of the European Association for Osseointegration Congress, Milan, Italy, 29 September–1 October 2021. [Google Scholar]
- Maló, P.; Rangert, B.; de Araújo Nobre, M. “All-on-Four” Immediate-Function Concept with Brånemark System® Implants for Completely Edentulous Mandibles: A Retrospective Clinical Study. Clin. Implant. Dent. Relat. Res. 2003, 5, 2–9. [Google Scholar] [CrossRef]
- Maló, P.; Rangert, B.; de Araújo Nobre, M. All-on-4 Immediate-Function Concept with Brånemark System Implants for Completely Edentulous Maxillae: A 1-Year Retrospective Clinical Study. Clin. Implant. Dent. Relat. Res. 2005, 7 (Suppl. 1), S88–S94. [Google Scholar] [CrossRef]
- Abdelfattah, M.Y.; Al Humayyani, N.; Alwthinani, F.K.; Alzahrani, A.H.; Alotaibi, A.O.; Yousef, M.; Sayed Ahmed, A.; Ali, A. In Vitro Evaluation of the Mechanical and Optical Properties of 3D Printed vs CAD/CAM Milled Denture Teeth Materials. Saudi Dent. J. 2024, 36, 1227–1232. [Google Scholar] [CrossRef]
- Myagmar, G.; Lee, J.-H.; Ahn, J.-S.; Yeo, I.-S.L.; Yoon, H.-I.; Han, J.-S. Wear of 3D Printed and CAD/CAM Milled Interim Resin Materials after Chewing Simulation. J. Adv. Prosthodont. 2021, 13, 144. [Google Scholar] [CrossRef]
- Stober, T.; Lutz, T.; Gilde, H.; Rammelsberg, P. Wear of Resin Denture Teeth by Two-Body Contact. Dent. Mater. 2006, 22, 243–249. [Google Scholar] [CrossRef]
- Takaki, P.; Vieira, M.; Bommarito, S. Maximum Bite Force Analysis in Different Age Groups. Int. Arch. Otorhinolaryngol. 2014, 18, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Nagni, M.; Pirani, F.; D’Orto, B.; Ferrini, F.; Cappare, P. Clinical and Radiographic Follow-Up of Full-Arch Implant Prosthetic Rehabilitations: Retrospective Clinical Study at 6-Year Follow-Up. Appl. Sci. 2023, 13, 11143. [Google Scholar] [CrossRef]
- Capparè, P.; Tetè, G.; D’Orto, B.; Nagni, M.; Gherlone, E.F. Immediate Loaded Full-Arch Mandibular Rehabilitations in Younger vs. Elderly Patients: A Comparative Retrospective Study with 7-Year Follow-Up. J. Clin. Med. 2023, 12, 4524. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.M.; Packer, M.E.; Watson, R.M. Maintenance Requirements of Implant-Supported Fixed Prostheses Opposed by Implant-Supported Fixed Prostheses, Natural Teeth, or Complete Dentures: A 5-Year Retrospective Study. Int. J. Prosthodont. 2003, 16, 521–523. [Google Scholar] [CrossRef]
- Shen, H.; Di, P.; Luo, J.; Lin, Y. Clinical Assessment of Implant-Supported Full-Arch Immediate Prostheses over 6 Months of Function. Clin. Implant. Dent. Relat. Res. 2019, 21, 473–481. [Google Scholar] [CrossRef]
- Chen, C.; Lai, H.; Zhu, H.; Gu, X. Digitally Prefabricated versus Conventionally Fabricated Implant-Supported Full-Arch Provisional Prosthesis: A Retrospective Cohort Study. BMC Oral Health 2022, 22, 335. [Google Scholar] [CrossRef]
- Kwak, Y.T.; Han, I.-W.; Lee, P.H.; Yoon, J.-K.; Suk, S.-H. Associated Conditions and Clinical Significance of Awake Bruxism. Geriatr. Gerontol. Int. 2009, 9, 382–390. [Google Scholar] [CrossRef]
- Burke, D.J.; Seitz, A.; Aladesuru, O.; Robbins, M.S.; Ch’ang, J.H. Bruxism in Acute Neurologic Illness. Curr. Pain. Headache Rep. 2021, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, M.C.; Lobbezoo, F.; Wetselaar, P.; Aarab, G.; Koutris, M. Parkinson’s Disease, Temporomandibular Disorders and Bruxism: A Pilot Study. J. Oral Rehabil. 2018, 45, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Uchima Koecklin, K.H.; Aliaga-Del Castillo, A.; Li, P. The Neural Substrates of Bruxism: Current Knowledge and Clinical Implications. Front. Neurol. 2024, 15, 1451183. [Google Scholar] [CrossRef] [PubMed]
- Garrett, A.R.; Hawley, J.S. SSRI-Associated Bruxism. Neurol. Clin. Pract. 2018, 8, 135–141. [Google Scholar] [CrossRef]
- Ionfrida, J.A.; Stiller, H.L.; Kämmerer, P.W.; Walter, C. Dental Implant Failure Risk in Patients with Bruxism-A Systematic Review and Meta-Analysis of the Literature. Dent. J. 2024, 13, 11. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, J.; Luo, L.; Wang, Y. Does Bruxism Contribute to Dental Implant Failure? A Systematic Review and Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2016, 18, 410–420. [Google Scholar] [CrossRef]
- Soto-Penaloza, D.; Zaragozí-Alonso, R.; Penarrocha-Diago, M.; Penarrocha-Diago, M. The All-on-Four Treatment Concept: Systematic Review. J. Clin. Exp. Dent. 2017, 9, e474–e488. [Google Scholar] [CrossRef]
- Sbricoli, L.; Bazzi, E.; Stellini, E.; Bacci, C. Systemic Diseases and Biological Dental Implant Complications: A Narrative Review. Dent. J. 2022, 11, 10. [Google Scholar] [CrossRef]
- Moura-Grec, P.G.D.; Marsicano, J.A.; Carvalho, C.A.P.D.; Sales-Peres, S.H.D.C. Obesity and Periodontitis: Systematic Review and Meta-Analysis. Cien Saude Colet 2014, 19, 1763–1772. [Google Scholar] [CrossRef]
- Suvan, J.; D’Aiuto, F.; Moles, D.R.; Petrie, A.; Donos, N. Association between Overweight/Obesity and Periodontitis in Adults. A Systematic Review. Obes. Rev. 2011, 12, e381–e404. [Google Scholar] [CrossRef]
- Turri, A.; Rossetti, P.H.O.; Canullo, L.; Grusovin, M.G.; Dahlin, C. Prevalence of Peri-Implantitis in Medically Compromised Patients and Smokers: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2016, 31, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between Diabetes Mellitus/Hyperglycaemia and Peri-Implant Diseases: Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and Risk Factors of Peri-Implantitis: A Systematic Review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhu, Y.; Liu, Z.; Tian, Z.; Zhu, S. Association between Diabetes and Dental Implant Complications: A Systematic Review and Meta-Analysis. Acta Odontol. Scand. 2021, 79, 9–18. [Google Scholar] [CrossRef]
- Parihar, A.S.; Madhuri, S.; Devanna, R.; Sharma, G.; Singh, R.; Shetty, K. Assessment of Failure Rate of Dental Implants in Medically Compromised Patients. J. Fam. Med. Prim. Care 2020, 9, 883–885. [Google Scholar] [CrossRef]
- Naujokat, H.; Kunzendorf, B.; Wiltfang, J. Dental Implants and Diabetes Mellitus-a Systematic Review. Int. J. Implant. Dent. 2016, 2, 5. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. J. Dent. 2015, 43, 487–498. [Google Scholar] [CrossRef]
- Mustapha, A.; Salame, Z.; Chrcanovic, B. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. Medicina 2021, 58, 39. [Google Scholar] [CrossRef]
- Stiller, H.L.; Ionfrida, J.; Kämmerer, P.W.; Walter, C. The Effects of Smoking on Dental Implant Failure: A Current Literature Update. Dent. J. 2024, 12, 311. [Google Scholar] [CrossRef]
- Corbella, S.; Del Fabbro, M.; Taschieri, S.; De Siena, F.; Francetti, L. Clinical Evaluation of an Implant Maintenance Protocol for the Prevention of Peri-Implant Diseases in Patients Treated with Immediately Loaded Full-Arch Rehabilitations. Int. J. Dent. Hyg. 2011, 9, 216–222. [Google Scholar] [CrossRef]
- Healthiest Teeth Index. Available online: https://www.qunomedical.com/en/research/healthiest-teeth-index (accessed on 27 October 2025).
- de Araújo Nobre, M.; Moura Guedes, C.; Almeida, R.; Silva, A.; Sereno, N. The All-on-4 Concept Using Polyetheretherketone (PEEK)-Acrylic Resin Prostheses: Follow-Up Results of the Development Group at 5 Years and the Routine Group at One Year. Biomedicines 2023, 11, 3013. [Google Scholar] [CrossRef]
- Legg, A.; Hare, K.; Campbell, C. Immediately Loaded Full Arch Implant Rehabilitation and Oral Health-Related Quality of Life: A Retrospective Cohort Study from Primary Dental Care. Clin. Implant. Dent. Relat. Res. 2023, 25, 1103–1111. [Google Scholar] [CrossRef]
- Babbush, C.A. Posttreatment Quantification of Patient Experiences With Full-Arch Implant Treatment Using a Modification of the OHIP-14 Questionnaire. J. Oral Implantol. 2012, 38, 251–260. [Google Scholar] [CrossRef]


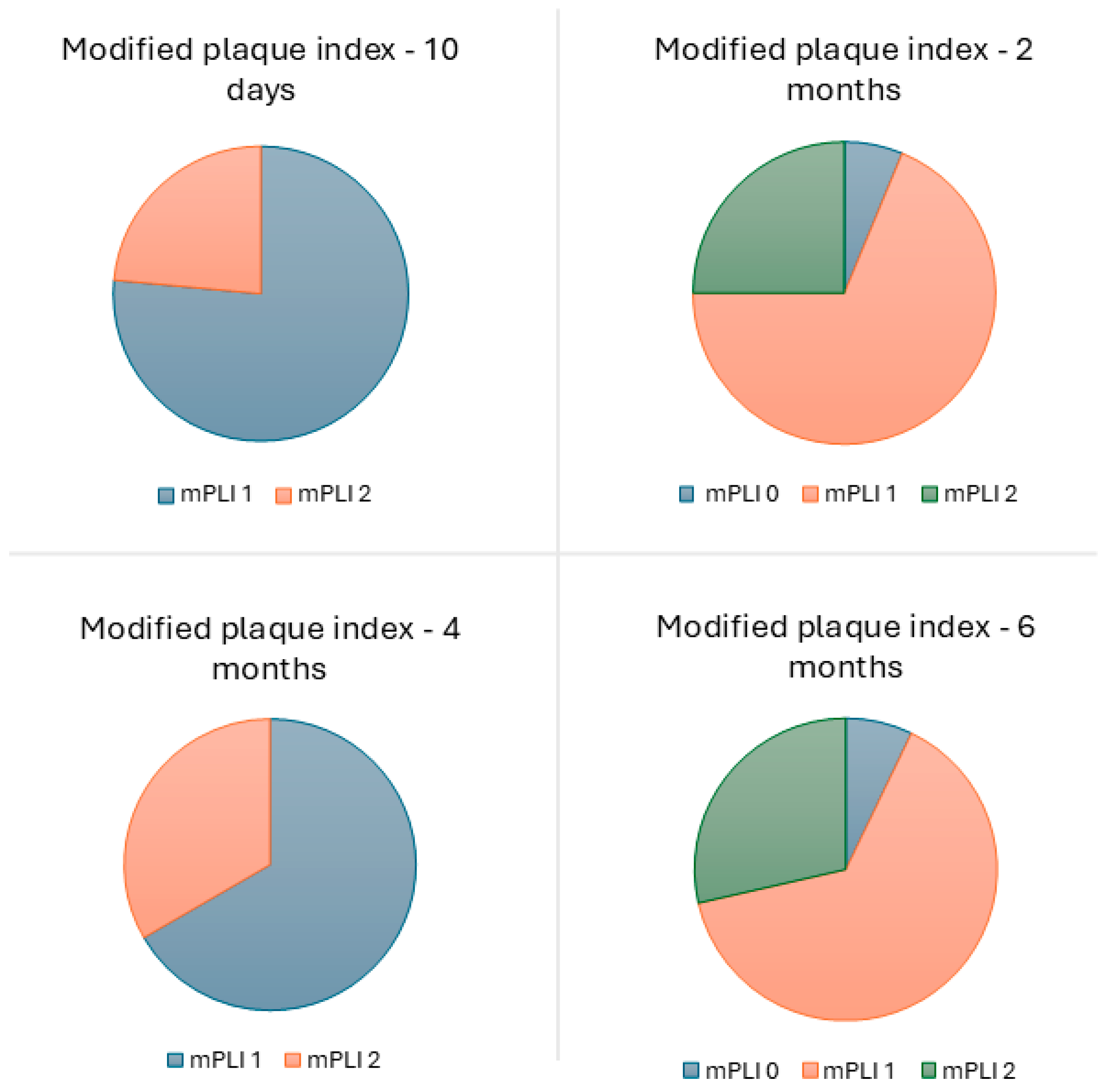
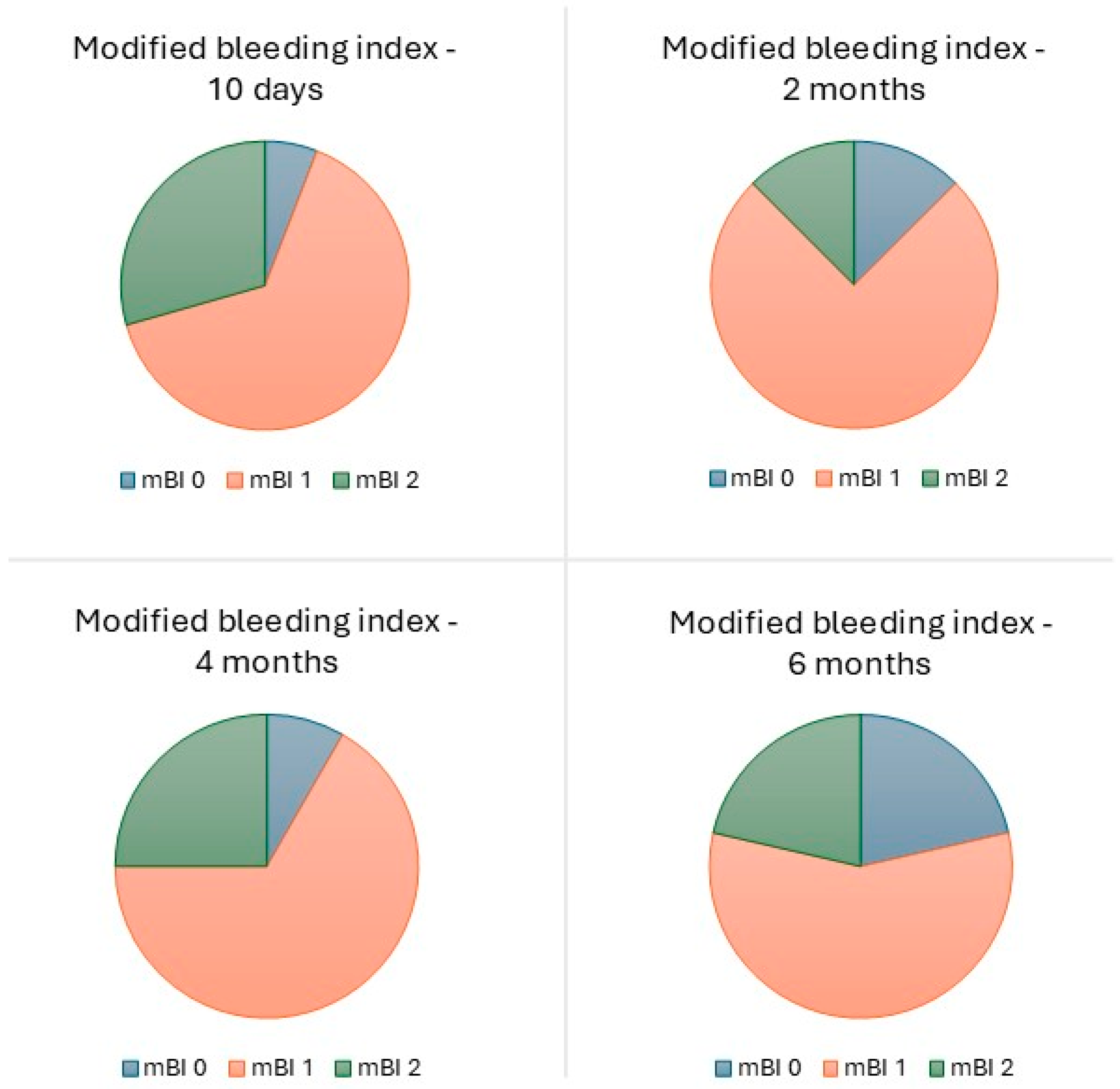
| Time (Months) | Total Number of Prostheses | Lost to Follow-Up | Lost * | Survival Rate (%) | Cumulative Survival Rate (%) |
|---|---|---|---|---|---|
| 0–2 | 20 | 0 | 1 | 95 | 95 |
| 2–4 | 19 | 0 | 1 | 95 | 90 |
| 4–6 | 18 | 0 | 0 | 100 | 90 |
| Time (Months) | Total Number of Implants | Lost to Follow-Up | Lost * | Survival Rate (%) | Cumulative Survival Rate (%) |
|---|---|---|---|---|---|
| 0–2 | 80 | 0 | 1 | 98.8 | 98.8 |
| 2–4 | 79 | 0 | 1 | 98.7 | 97.5 |
| 4–6 | 78 | 0 | 0 | 100 | 97.5 |
| 4-Month Follow-Up | Overall Bone Loss by Implant |
|---|---|
| Average (mm) | 0.31 CI 95% [0.19–0.44] * |
| SD (mm) | 0.52 |
| Number (%) of implants with readable radiographs | 74 (92.5%) |
| Frequencies | N |
| <0 mm | 11 |
| 0 mm | 22 |
| 0.1–1 mm | 31 |
| 1.1–2 mm | 10 |
| Patient Subjective Evaluation | 10-Day Mean (Standard Deviation) | 2-Month Mean (Standard Deviation) | 4-Month Mean (Standard Deviation) | 6-Month Mean (Standard Deviation) |
|---|---|---|---|---|
| In-mouth comfort | ||||
| Overall | 9.18 (1.1) | 8.16 (2.5) | 8.68 (2.7) | 9.35 (1.3) |
| Stackable guides | 9.37 (0.9) | 8.10 (3.0) | 8.3 (3.2) | 9.5 (1.4) |
| Photogrammetry | 9.10 (1.2) | 8.5 (1.6) | 9.4 (0.8) | 9.3 (1.0) |
| Overall chewing feeling | ||||
| Overall | 8.25 (2.4) | 7.74 (2.3) | 8.93 (1.9) | 8.79 (1.7) |
| Stackable guides | 7.56 (2.8) | 8.5 (2.5) | 9.9 (0.3) | 9.3 (1.3) |
| Photogrammetry | 9.10 (1.6) | 6.9 (1.7) | 7.6 (2.3) | 8.0 (1.7) |
| OHIP-14 Evaluation Parameters | 10-Day Mean (Standard Deviation) | 2-Month Mean (Standard Deviation) | 4-Month Mean (Standard Deviation) | 6-Month Mean (Standard Deviation) |
|---|---|---|---|---|
| Functional limitation Have you had trouble pronouncing any words? Have you felt that your sense of taste has worsened? | 0.65 (1.11) | 0.88 (1.11) | 0.54 (1.08) | 0.63 (1.08) |
| Physical pain Have you had painful aching in your mouth? Have you found it uncomfortable to eat any foods? | 0.82 (1.12) | 0.41 (0.74) | 0.42 (0.81) | 0.23 (0.56) |
| Psychological discomfort Have you been self-conscious? Have you felt tense? | 0.44 (0.88) | 0.69 (1.01) | 0.50 (1.04) | 0.24 (0.57) |
| Physical disability Has your diet been unsatisfactory? Have you had to interrupt meals? | 0.76 (1.16) | 0.56 (1.14) | 0.42 (0.42) | 0.33 (0.75) |
| Psychological disability Have you found it difficult to relax? Have you been a bit embarrassed? | 0.21 (0.53) | 0.38 (0.70) | 0.25 (0.60) | 0.07 (0.36) |
| Social disability Have you been a bit irritable with other people? Have you had difficulty doing your usual jobs? | 0.35 (0.72) | 0.09 (0.38) | 0.08 (0.40) | 0.10 (0.40) |
| Handicap Have you been unable to function? Have you felt life in general was less satisfying? | 0.06 (0.24) | 0.03 (0.17) | 0.00 (0.00) | 0.00 (0.00) |
| Total sum | 3.29 (0.27) SG: 4.17 (0.34) P: 2.56 (0.22) | 3.03 (0.28) SG: 2.67 (0.25) P: 3.56 (0.32) | 2.21 (0.19) SG: 1.63 (0.18) 2.90 (0.35) | 1.61 (0.20) SG: 1.01 (0.20) P: 2.50 (0.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo Nobre, M.; Almeida, R.; Moura Guedes, C.; Alvarez, G.; Antunes, C.; Ferro, A.; Nunes, M.; Lopes, A.; Rangel, J.; Martins, J.P.; et al. Digital Workflow for Interim Prosthetic Rehabilitation Through the All-on-4 Concept Using 3D Printing Additive Process. J. Clin. Med. 2025, 14, 8353. https://doi.org/10.3390/jcm14238353
de Araújo Nobre M, Almeida R, Moura Guedes C, Alvarez G, Antunes C, Ferro A, Nunes M, Lopes A, Rangel J, Martins JP, et al. Digital Workflow for Interim Prosthetic Rehabilitation Through the All-on-4 Concept Using 3D Printing Additive Process. Journal of Clinical Medicine. 2025; 14(23):8353. https://doi.org/10.3390/jcm14238353
Chicago/Turabian Stylede Araújo Nobre, Miguel, Ricardo Almeida, Carlos Moura Guedes, Gonçalo Alvarez, Carolina Antunes, Ana Ferro, Mariana Nunes, Armando Lopes, João Rangel, João Pedro Martins, and et al. 2025. "Digital Workflow for Interim Prosthetic Rehabilitation Through the All-on-4 Concept Using 3D Printing Additive Process" Journal of Clinical Medicine 14, no. 23: 8353. https://doi.org/10.3390/jcm14238353
APA Stylede Araújo Nobre, M., Almeida, R., Moura Guedes, C., Alvarez, G., Antunes, C., Ferro, A., Nunes, M., Lopes, A., Rangel, J., Martins, J. P., Santos, D., & Gouveia, M. (2025). Digital Workflow for Interim Prosthetic Rehabilitation Through the All-on-4 Concept Using 3D Printing Additive Process. Journal of Clinical Medicine, 14(23), 8353. https://doi.org/10.3390/jcm14238353








