Sex and Ethnic Disparities in Stroke Revascularisation Treatments and Post-Stroke Outcomes in Patients with Heart Failure: A National Inpatient Sample Study
Abstract
1. Introduction
2. Materials and Methods
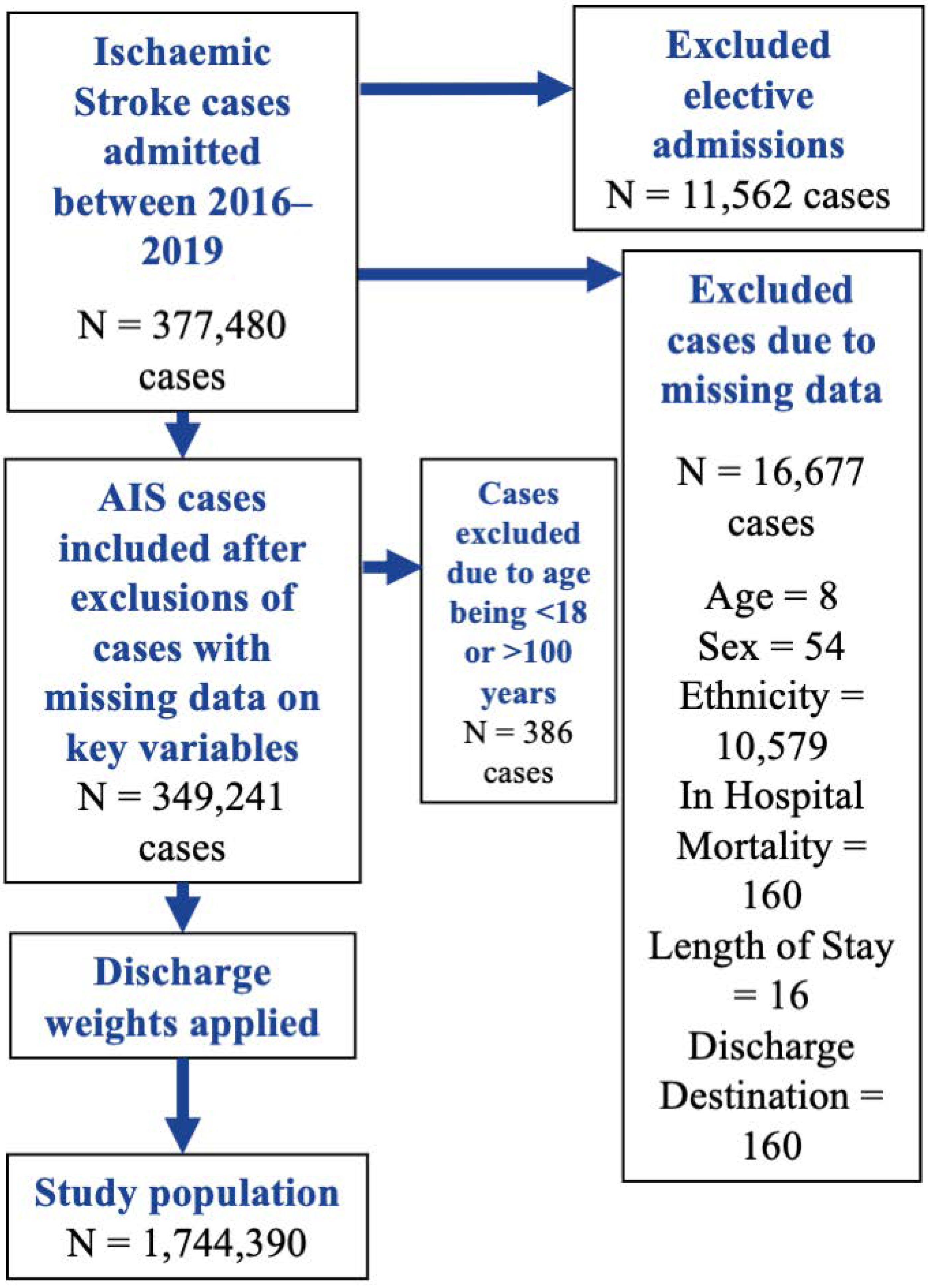
2.1. Data Source and Inclusion Criteria
2.2. Definition of Groups, Exposures, Confounders, and Outcomes
2.3. Statistical Analysis
2.4. Descriptive Statistics
2.5. Primary Analysis
2.6. Sensitivity Analyses
2.7. Adjusting Covariates
3. Results
3.1. Descriptive Statistics
3.2. Primary Analysis
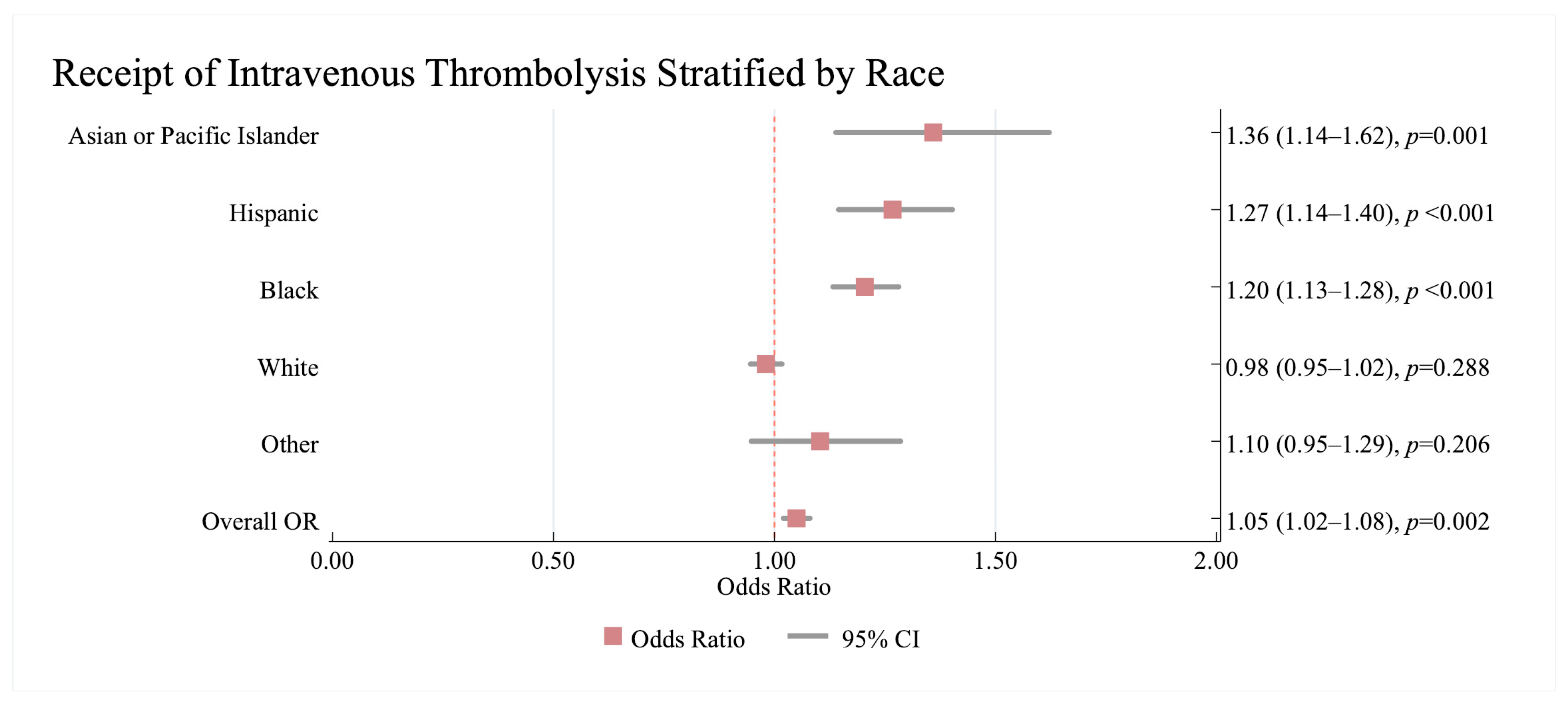
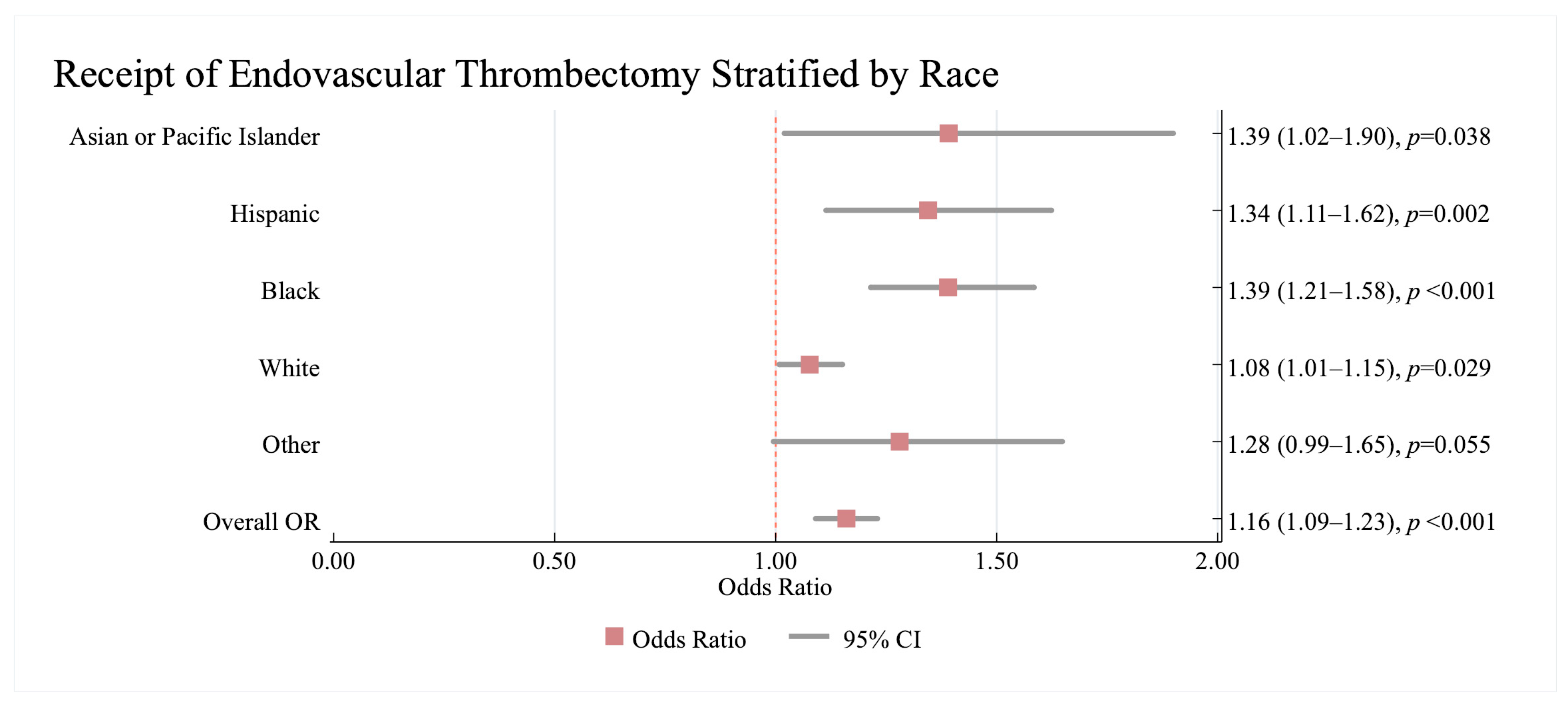
3.2.1. Association Between Revascularisation Therapy and Race/Ethnicity and Sex
3.2.2. Association Between In-Hospital Mortality and Race/Ethnicity and Sex
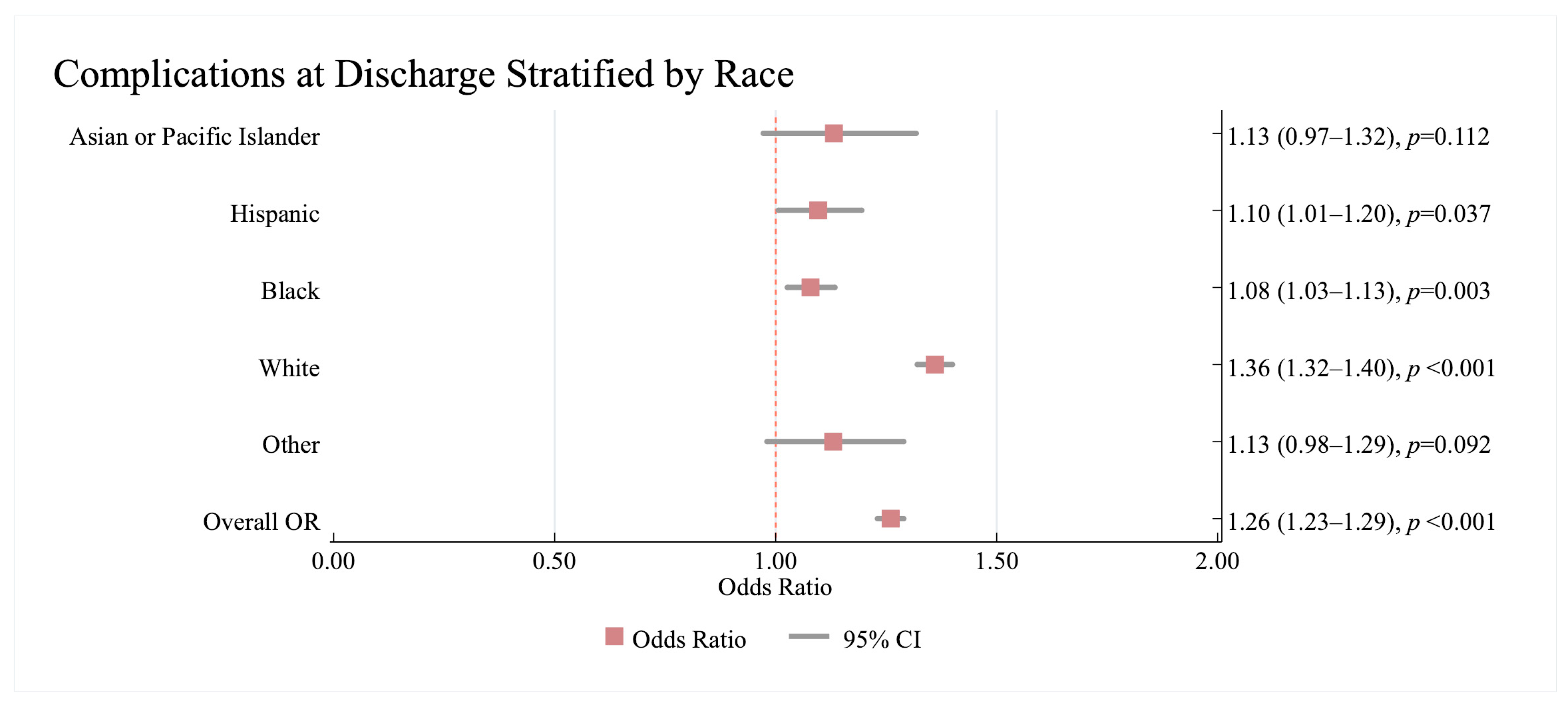
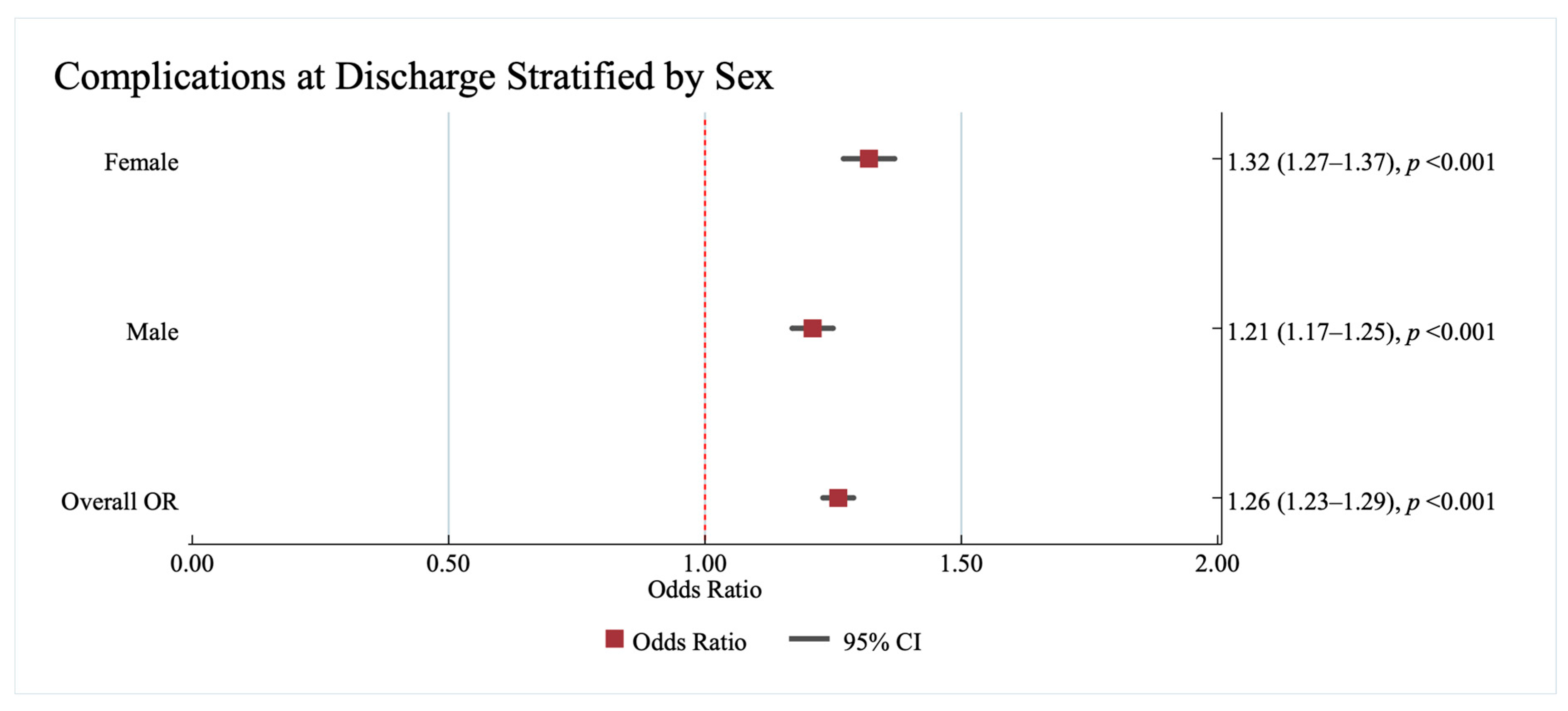
3.2.3. Association Between Complications at Discharge and Race/Ethnicity and Sex
3.2.4. Association Between Median Length of Stay and Race/Ethnicity and Sex
3.3. Sensitivity Analyses
3.3.1. Analyses Generating Absolute Risk Differences for Outcomes and Treatments
The Association Between Revascularization Therapy and Race/Ethnicity and Sex
The Association Between Complications at Discharge and Race/Ethnicity and Sex
3.3.2. Analysis Adjusted for Socioeconomic Indicators
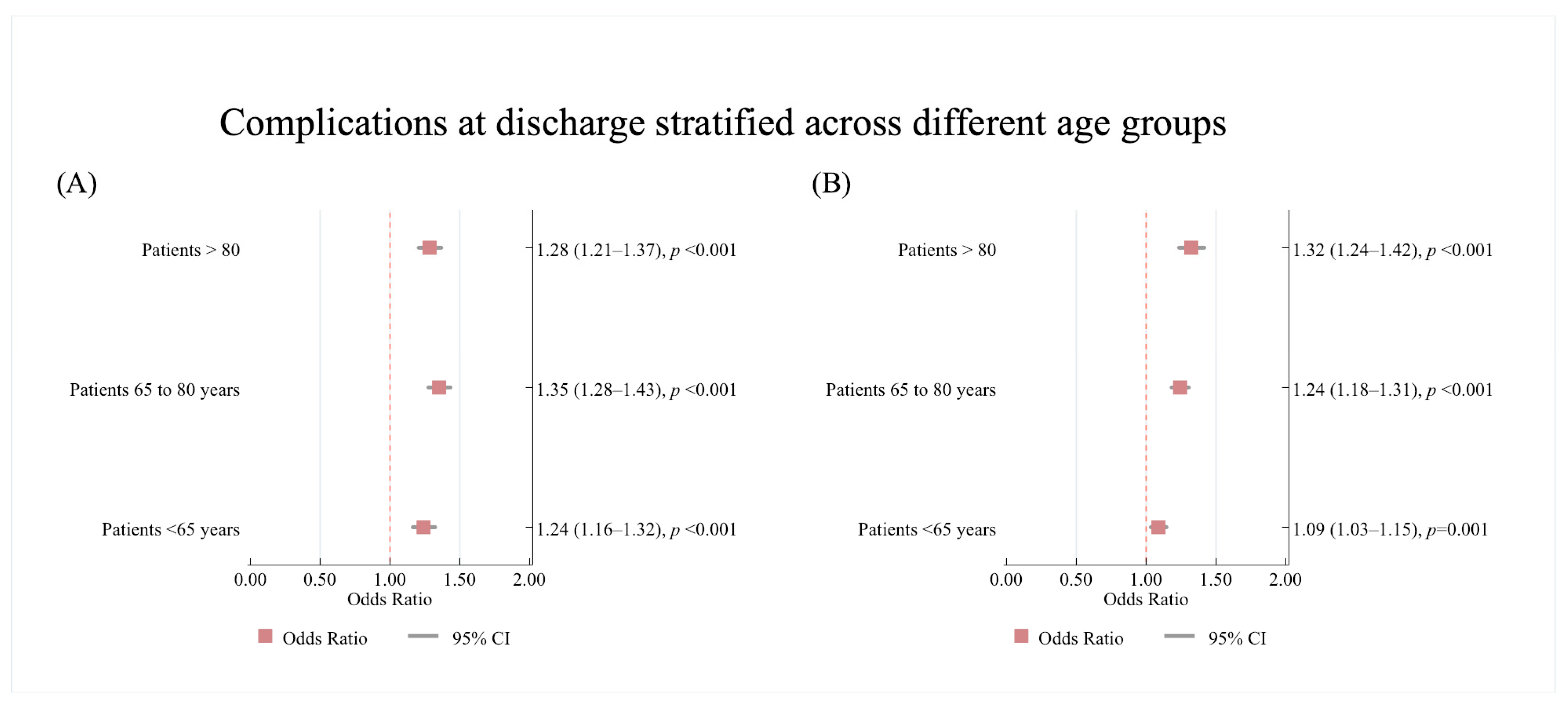
3.3.3. Age Subgroup Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HF | Heart Failure |
| AIS | Acute Ischaemic Stroke |
| AF | Atrial Fibrillation |
| HFrEF | Heart Failure with reduced ejection fraction |
| NIS | National Inpatient Sample |
| ICD10 | International Classification of Disease, 10th edition |
| IVT | Intravenous Thrombolysis |
| ET | Endovascular Thrombectomy |
| LOS | Length of Stay |
| HCUP | Healthcare Cost and Utilisation Project |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| HFpEF | Heart failure with preserved ejection fraction |
References
- Lip, G.Y.H.; Rasmussen, L.H.; Skjøth, F.; Overvad, K.; Larsen, T.B. Stroke and Mortality in Patients with Incident Heart Failure: The Diet, Cancer and Health (DCH) Cohort Study. BMJ Open 2012, 2, e000975. [Google Scholar] [CrossRef]
- Witt, B.J.; Brown, R.D.; Jacobsen, S.J.; Weston, S.A.; Ballman, K.V.; Meverden, R.A.; Roger, V.L. Ischemic Stroke after Heart Failure: A Community-Based Study. Am. Heart J. 2006, 152, 102–109. [Google Scholar] [CrossRef]
- Barkhudaryan, A.; Doehner, W.; Scherbakov, N. Ischemic Stroke and Heart Failure: Facts and Numbers. An Update. J. Clin. Med. 2021, 10, 1146. [Google Scholar] [CrossRef]
- Vemmos, K.; Ntaios, G.; Savvari, P.; Vemmou, A.M.; Koroboki, E.; Manios, E.; Kounali, A.; Lip, G.Y.H. Stroke Aetiology and Predictors of Outcome in Patients with Heart Failure and Acute Stroke: A 10-year Follow-up Study. Eur. J. Heart Fail. 2012, 14, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pullicino, P.M.; Halperin, J.L.; Thompson, J.L.P. Stroke in Patients with Heart Failure and Reduced Left Ventricular Ejection Fraction. Neurology 2000, 54, 288. [Google Scholar] [CrossRef]
- Takahashi, S.; Ishizuka, K.; Hoshino, T.; Mizuno, T.; Nishimura, A.; Toi, S.; Kitagawa, K. Long-Term Outcome in Patients With Acute Ischemic Stroke and Heart Failure. Circ. J. 2023, 87, 401–408. [Google Scholar] [CrossRef]
- Pana, T.A.; Mohamed, M.O.; Clark, A.B.; Fahy, E.; Mamas, M.A.; Myint, P.K. Revascularisation Therapies Improve the Outcomes of Ischemic Stroke Patients with Atrial Fibrillation and Heart Failure. Int. J. Cardiol. 2021, 324, 205–213. [Google Scholar] [CrossRef]
- Pana, T.A.; Jesenakova, S.; Carter, B.; Hollick, R.; Mohamed, M.O.; Mamas, M.A.; Myint, P.K. Sex-Specific Outcomes of Acute Stroke in Patients with Systemic Lupus Erythematosus: A National Inpatient Sample Study. J. Clin. Med. 2023, 12, 462. [Google Scholar] [CrossRef]
- Healthcare Cost and Utilization Project NIS Database Documentation. Available online: https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp (accessed on 2 August 2024).
- Agency for Healthcare Research and Quality; Rockville, M.U. Healthcare Cost and Utilization Project (HCUP) HCUP NIS Description of Data Elements-DIED-Died During Hospitalization. Available online: https://www.hcup-us.ahrq.gov/db/vars/died/nisnote.jsp (accessed on 20 September 2023).
- Myint, P.K.; Sheng, S.; Xian, Y.; Matsouaka, R.A.; Reeves, M.J.; Saver, J.L.; Bhatt, D.L.; Fonarow, G.C.; Schwamm, L.H.; Smith, E.E. Shock Index Predicts Patient-Related Clinical Outcomes in Stroke. J. Am. Heart Assoc. 2018, 7, e007581. [Google Scholar] [CrossRef] [PubMed]
- Pana, T.A.; Dawson, D.K.; Mohamed, M.O.; Murray, F.; Fischman, D.L.; Savage, M.P.; Mamas, M.A.; Myint, P.K. Sex Differences in Ischemic Stroke Outcomes in Patients With Pulmonary Hypertension. J. Am. Heart Assoc. 2021, 10, e019341. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality; Rockville, M.U. Healthcare Cost and Utilization Project (HCUP) HCUP NIS Description of Data Elements-DISPUNIFORM-Disposition of Patient, Uniform Coding. Available online: https://www.hcup-us.ahrq.gov/db/vars/dispuniform/nisnote.jsp (accessed on 15 September 2023).
- Qureshi, A.I.; Chaudhry, S.A.; Sapkota, B.L.; Rodriguez, G.J.; Suri, M.F.K. Discharge Destination as a Surrogate for Modified Rankin Scale Defined Outcomes at 3- and 12-Months Poststroke Among Stroke Survivors. Arch. Phys. Med. Rehabil. 2012, 93, 1408–1413.e1. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality; Rockville, M.U. Healthcare Cost and Utilization Project, (HCUP), Checklist ForWorking with the NIS. Available online: https://hcup-us.ahrq.gov/db/nation/nis/nischecklist.jsp (accessed on 15 September 2023).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Attenello, F.J.; Adamczyk, P.; Wen, G.; He, S.; Zhang, K.; Russin, J.J.; Sanossian, N.; Amar, A.P.; Mack, W.J. Racial and Socioeconomic Disparities in Access to Mechanical Revascularization Procedures for Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 327–334. [Google Scholar] [CrossRef]
- Kuhrij, L.S.; Marang-van de Mheen, P.J.; van den Berg-Vos, R.M.; de Leeuw, F.-E.; Nederkoorn, P.J. Determinants of Extended Door-to-Needle Time in Acute Ischemic Stroke and Its Influence on in-Hospital Mortality: Results of a Nationwide Dutch Clinical Audit. BMC Neurol. 2019, 19, 265. [Google Scholar] [CrossRef]
- Backhouse, E.V.; Boardman, J.P.; Wardlaw, J.M. Cerebral Small Vessel Disease: Early-Life Antecedents and Long-Term Implications for the Brain, Aging, Stroke, and Dementia. Hypertension 2024, 81, 54–74. [Google Scholar] [CrossRef]
- Nagaraja, N.; Olasoji, E.B.; Patel, U.K. Sex and Racial Disparity in Utilization and Outcomes of T-PA and Thrombectomy in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104954. [Google Scholar] [CrossRef] [PubMed]
- Fargen, K.M.; Neal, D.; Blackburn, S.L.; Hoh, B.L.; Rahman, M. Health Disparities and Stroke: The Influence of Insurance Status on the Prevalence of Patient Safety Indicators and Hospital-Acquired Conditions. J. Neurosurg. 2015, 122, 870–875. [Google Scholar] [CrossRef]
- Pandey, A.; Omar, W.; Ayers, C.; LaMonte, M.; Klein, L.; Allen, N.B.; Kuller, L.H.; Greenland, P.; Eaton, C.B.; Gottdiener, J.S.; et al. Sex and Race Differences in Lifetime Risk of Heart Failure with Preserved Ejection Fraction and Heart Failure with Reduced Ejection Fraction. Circulation 2018, 137, 1814–1823. [Google Scholar] [CrossRef]
- Wu, J.; Chen, M.; Wang, H.; Zhu, Y.; Chen, Y.; Zhang, S.; Wang, D. Comparison of Characteristics and Outcomes Between Acute Ischemic Stroke Patients with Different Types of Heart Failure. Int. Heart J. 2024, 65, 22–717. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.P.; Jia, H.; Williams, L.S.; Vogel, W.B.; Duncan, P.W. Ethnic Disparities in Stroke. Stroke 2005, 36, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Horner, R.D.; Edwards, L.J.; Hoff, J.; Armstrong, S.B.; Smith-Hammond, C.A.; Matchar, D.B.; Oddone, E.Z. Racial Variation in Initial Stroke Severity. Stroke 2000, 31, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Duncan, P.W.; Nguyen-Huynh, M.N.; Ogedegbe, O.G. Interventions Targeting Racial/Ethnic Disparities in Stroke Prevention and Treatment. Stroke 2020, 51, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Dehlendorff, C.; Andersen, K.K.; Olsen, T.S. Sex Disparities in Stroke: Women Have More Severe Strokes but Better Survival Than Men. J. Am. Heart Assoc. 2015, 4, e001967. [Google Scholar] [CrossRef] [PubMed]
- Owais, S.B.; Bulwa, Z.B.; Ammar, F. El Differences in Stroke Clinical Presentation among Sexes. J. Stroke Cerebrovasc. Dis. 2024, 33, 107807. [Google Scholar] [CrossRef]
| Total | No HF | HF | p Value | |
|---|---|---|---|---|
| N | 1,744,390 | 1,459,035 | 285,355 | |
| Age, y, median (IQR), | 71.0 (61.0–82.0) | 71.0 (60.0–81.0) | 76.0 (65.0–85.0) | <0.001 |
| Length of stay, d, median (IQR) | 3.0 (2.0–6.0) | 3.0 (2.0–5.0) | 4.0 (3.0–7.0) | <0.001 |
| Mortality, n, (%) | 61,295 (3.5) | 42,980 (3.0) | 18,315 (6.4) | <0.001 |
| Sex, n, (%) | ||||
| Female | 876,100 (50.2) | 731,590 (50.1) | 144,510 (50.6) | 0.029 |
| Male | 868,290 (49.8) | 727,445 (49.9) | 140,845 (49.4) | 0.029 |
| Race/Ethnicity, n, (%) | ||||
| White | 1,203,595 (69.0) | 1,012,210 (69.4) | 191,385 (67.1) | <0.001 |
| Black | 311,600 (17.9) | 249,985 (17.1) | 61,615 (21.6) | <0.001 |
| Hispanic | 129,460 (7.4) | 111,000 (7.6) | 18,460 (6.5) | <0.001 |
| Asian or Pacific Islander | 48,650 (2.8) | 42,665 (2.9) | 5985 (2.1) | <0.001 |
| Other | 51,085 (2.9) | 43,175 (3.0) | 7910 (2.8) | <0.001 |
| Comorbidities, n, (%) | ||||
| Atrial Fibrillation | 442,345 (25.36) | 305,625 (20.95) | 136,720 (47.91) | <0.001 |
| Coronary Heart Disease | 499,795 (28.65) | 346,050 (23.72) | 153,745 (53.88) | <0.001 |
| Deep Vein Thrombosis | 25,160 (1.44) | 19,840 (1.36) | 5320 (1.86) | <0.001 |
| Infective Endocarditis | 6110 (0.35) | 4450 (0.30) | 1660 (0.58) | <0.001 |
| Peripheral vascular disease | 146,915 (8.42) | 115,170 (7.89) | 31,745 (11.12) | <0.001 |
| Previous Valve Surgery | 32,920 (1.89) | 21,420 (1.47) | 11,500 (4.03) | <0.001 |
| Ventricular Tachycardia | 27,365 (1.57) | 15,645 (1.07) | 11,720 (4.11) | <0.001 |
| Venous Thromboembolism | 100,805 (5.78) | 78,840 (5.40) | 21,965 (7.70) | <0.001 |
| Alcoholism | 77,050 (4.42) | 66,960 (4.59) | 10,090 (3.54) | <0.001 |
| Anaemia | 240,785 (13.80) | 177,930 (12.20) | 62,855 (22.03) | <0.001 |
| Use of Anticoagulant(s) | 176,290 (10.11) | 124,365 (8.52) | 51,925 (18.20) | <0.001 |
| Use of antiplatelet(s) | 504,935 (28.95) | 417,395 (28.61) | 87,540 (30.68) | <0.001 |
| Arthritis | 195,385 (11.20) | 157,950 (10.83) | 37,435 (13.12) | <0.001 |
| Bleeding disorder | 62,290 (3.57) | 47,135 (3.23) | 15,155 (5.31) | <0.001 |
| Chronic lung disease | 285,390 (16.36) | 210,160 (14.40) | 75,230 (26.36) | <0.001 |
| Dementia | 205,225 (11.76) | 162,610 (11.15) | 42,615 (14.93) | <0.001 |
| Diabetes mellitus | 681,665 (39.08) | 548,365 (37.58) | 133,300 (46.71) | <0.001 |
| Drug abuse | 65,110 (3.73) | 55,215 (3.78) | 9895 (3.47) | <0.001 |
| Epilepsy | 74,145 (4.25) | 61,060 (4.18) | 13,085 (4.59) | <0.001 |
| Human Immunodeficiency virus | 3565 (0.20) | 3035 (0.21) | 530 (0.19) | 0.268 |
| Hypertension | 1,501,600 (86.08) | 1,236,840 (84.77) | 264,760 (92.78) | <0.001 |
| Hypotension | 62,690 (3.59) | 46,375 (3.18) | 16,315 (5.72) | <0.001 |
| Hyperlipidaemia | 1,055,995 (60.54) | 880,600 (60.35) | 175,395 (61.47) | <0.001 |
| Liver disease | 28,410 (1.63) | 21,610 (1.48) | 6800 (2.38) | <0.001 |
| Malignancy | 79,295 (4.55) | 66,615 (4.57) | 12,680 (4.44) | 0.191 |
| Malnutrition | 128,770 (7.38) | 101,860 (6.98) | 26,910 (9.43) | <0.001 |
| Smoking cigarettes | 331,265 (18.99) | 291,250 (19.96) | 40,015 (14.02) | <0.001 |
| Non-rheumatic valve disease | 95,605 (5.48) | 67,320 (4.61) | 28,285 (9.91) | <0.001 |
| Obesity | 244,100 (13.99) | 197,595 (13.54) | 46,505 (16.30) | <0.001 |
| Pneumonia | 98,410 (5.64) | 68,160 (4.67) | 30,250 (10.60) | <0.001 |
| Previous coronary artery bypass graft | 114,705 (6.58) | 78,445 (5.38) | 36,260 (12.71) | <0.001 |
| Psychiatric disease | 353,320 (20.25) | 294,905 (20.21) | 58,415 (20.47) | 0.180 |
| Renal disease | 442,575 (25.37) | 314,180 (21.53) | 128,395 (44.99) | <0.001 |
| Respiratory failure | 118,925 (6.82) | 76,205 (5.22) | 42,720 (14.97) | <0.001 |
| Rheumatic heart disease | 52,285 (3.00) | 34,285 (2.35) | 18,000 (6.31) | <0.001 |
| Sepsis | 26,950 (1.54) | 18,295 (1.25) | 8655 (3.03) | <0.001 |
| Thyroid disease | 285,940 (16.39) | 231,995 (15.90) | 53,945 (18.90) | <0.001 |
| Viral hepatitis | 16,075 (0.92) | 13,245 (0.91) | 2830 (0.99) | 0.059 |
| Thrombolysis | 220,830 (12.66) | 184,630 (12.65) | 36,200 (12.69) | 0.837 |
| Endovascular thrombectomy | 45,690 (2.62) | 34,790 (2.38) | 10,900 (3.82) | <0.001 |
| Patient Admission year, n, (%) | ||||
| 2016 | 438,825 (25.2) | 372,205 (25.5) | 66,620 (23.4) | <0.001 |
| 2017 | 461,005 (26.4) | 386,720 (26.5) | 74,285 (26.0) | <0.001 |
| 2018 | 327,720 (18.8) | 272,665 (18.7) | 55,055 (19.3) | <0.001 |
| 2019 | 516,840 (29.6) | 427,445 (29.3) | 89,395 (31.3) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Batat, A.L.; Pana, T.A.; Mamas, M.A.; Kontopantelis, E.; Myint, P.K. Sex and Ethnic Disparities in Stroke Revascularisation Treatments and Post-Stroke Outcomes in Patients with Heart Failure: A National Inpatient Sample Study. J. Clin. Med. 2025, 14, 8354. https://doi.org/10.3390/jcm14238354
Al-Batat AL, Pana TA, Mamas MA, Kontopantelis E, Myint PK. Sex and Ethnic Disparities in Stroke Revascularisation Treatments and Post-Stroke Outcomes in Patients with Heart Failure: A National Inpatient Sample Study. Journal of Clinical Medicine. 2025; 14(23):8354. https://doi.org/10.3390/jcm14238354
Chicago/Turabian StyleAl-Batat, Ali L., Tiberiu A. Pana, Mamas A. Mamas, Evangelos Kontopantelis, and Phyo K. Myint. 2025. "Sex and Ethnic Disparities in Stroke Revascularisation Treatments and Post-Stroke Outcomes in Patients with Heart Failure: A National Inpatient Sample Study" Journal of Clinical Medicine 14, no. 23: 8354. https://doi.org/10.3390/jcm14238354
APA StyleAl-Batat, A. L., Pana, T. A., Mamas, M. A., Kontopantelis, E., & Myint, P. K. (2025). Sex and Ethnic Disparities in Stroke Revascularisation Treatments and Post-Stroke Outcomes in Patients with Heart Failure: A National Inpatient Sample Study. Journal of Clinical Medicine, 14(23), 8354. https://doi.org/10.3390/jcm14238354







