Patient-Centred and Daily Life-Oriented Botulinum Toxin Treatment for Stroke Survivors with Upper Extremity Spasticity—Effects and Practical Aspects
Abstract
1. Introduction
2. Materials and Methods
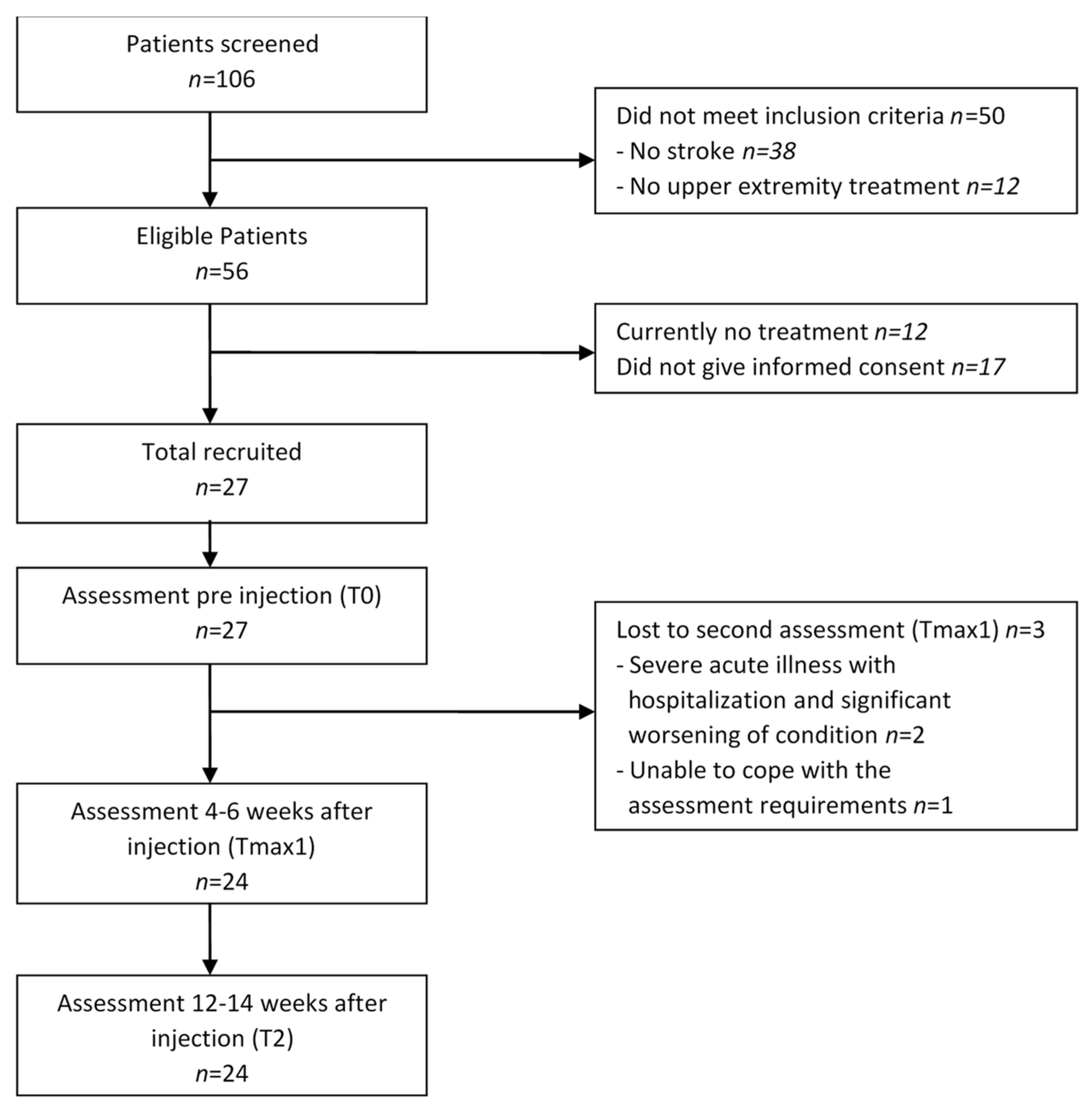
2.1. Eligibility Criteria and Study Procedures
2.2. Baseline Characteristics and Treatment Information
2.3. Outcome Measures
2.3.1. Primary Outcome Measures
2.3.2. Secondary Outcome Measures
2.4. Data Analyses
3. Results
3.1. Participants
3.2. Treatment (Botulinum Toxin A Injections, Physiotherapy, and Occupational Therapy)
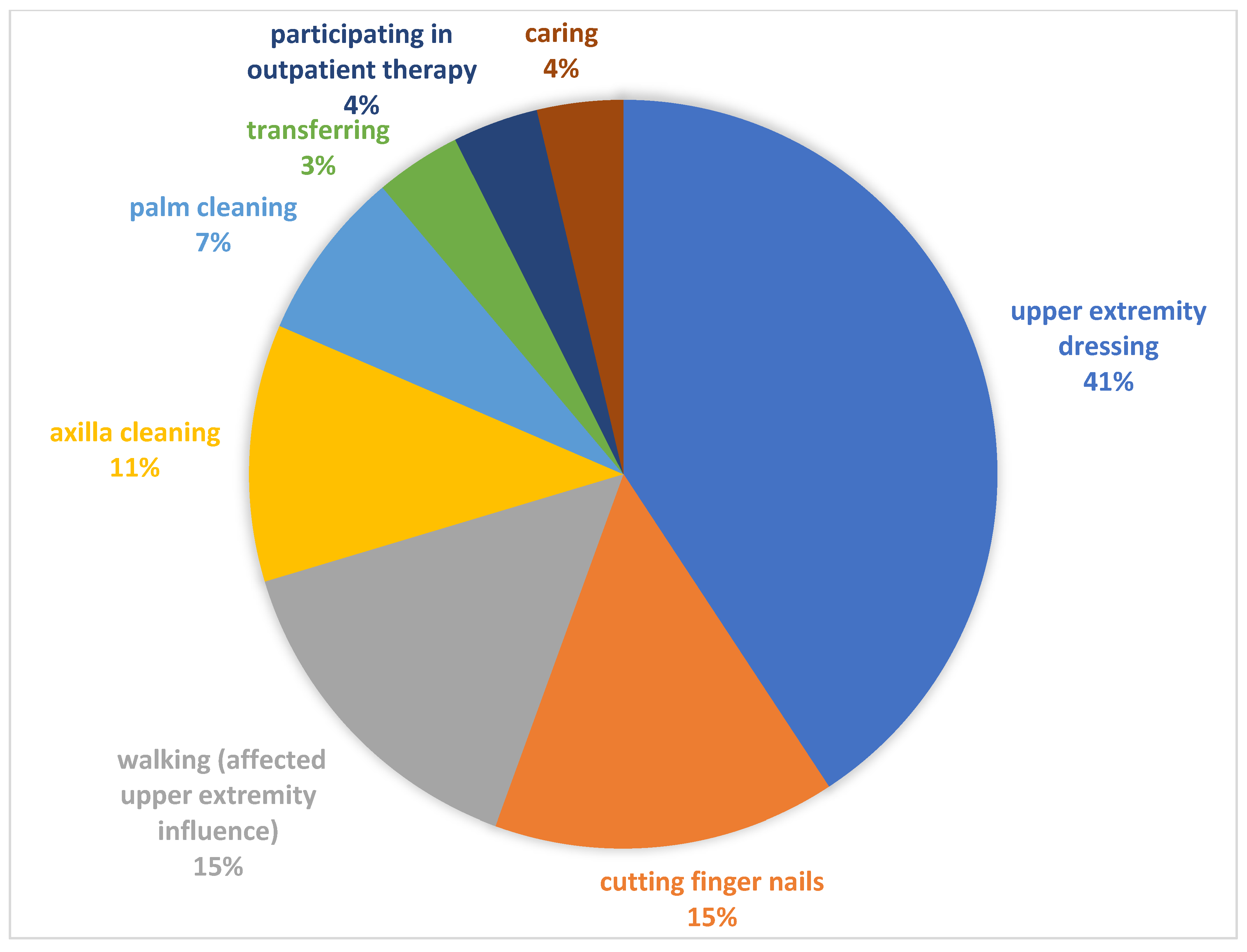
3.3. Goal Attainment Scaling
3.4. Canadian Occupational Performance Measure
3.5. Arm Activity Measure
3.6. Secondary Outcomes
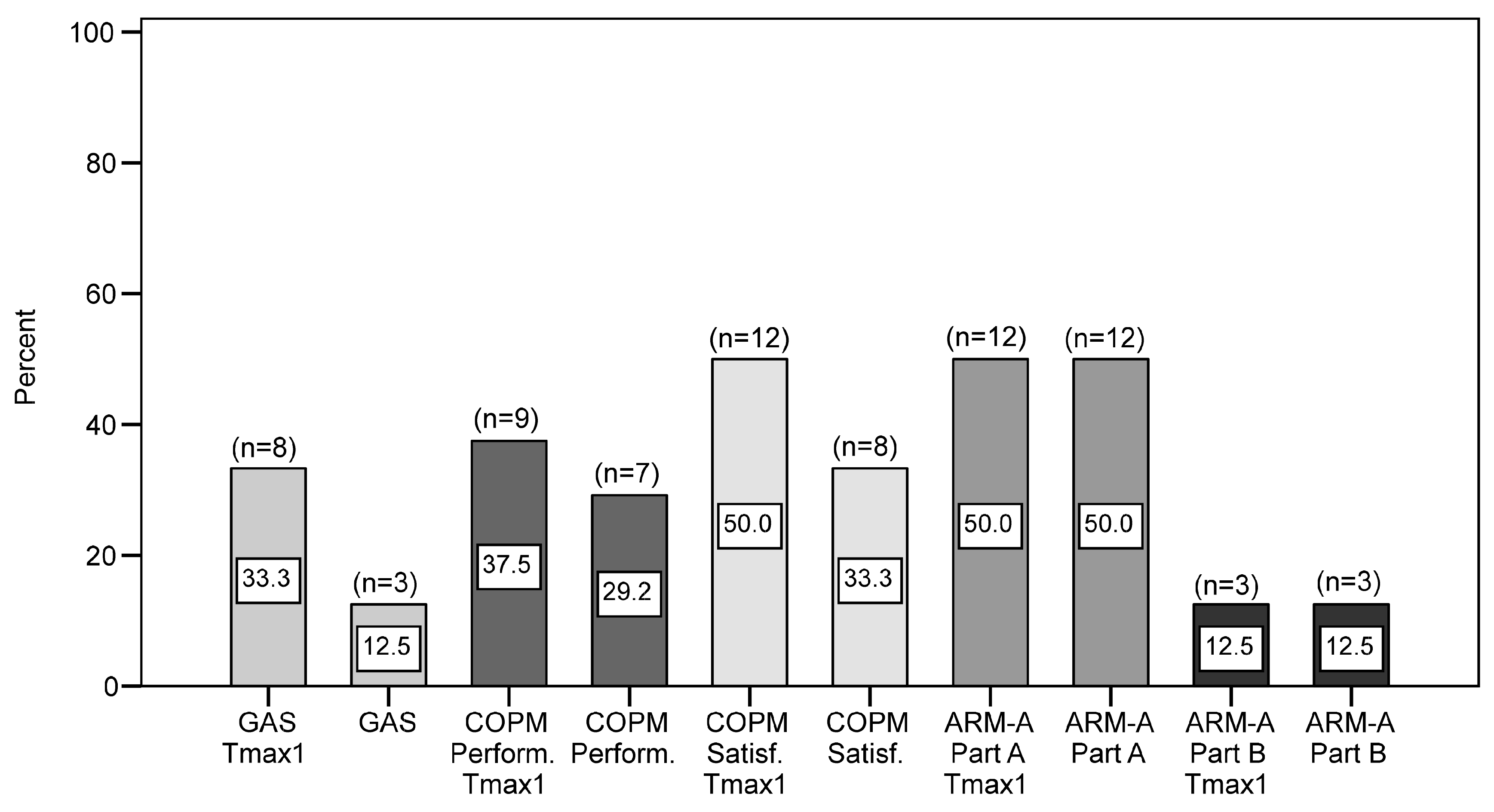
3.7. Responder Analyses (i.e., Participants Experiencing a Clinically Significant Response)
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ArmA | Arm Activity Measure |
| BoNT-A | Botulinum toxin type A |
| COPM | Canadian Occupational Performance Measure |
| GAS | Goal Attainment Scaling |
| GCI | Global Clinical Impression |
| ICF | International Classification of Functioning, Disability and Health |
| MI | Motricity Index |
| OHS | Oxford Handicap Scale |
| REPAS | Resistance to Passive Movement Scale |
| SF-12v2 | SF-12 Health Survey, version 2 |
| VAS | Visual Analogue Scale |
References
- Mayer, N.H.; Esquenazi, A.; Childers, M.K. Common patterns of clinical motor dysfunction. Muscle Nerve 1997, 20 (Suppl. S6), S21–S35. [Google Scholar] [CrossRef]
- Bhimani, R.; Anderson, L. Clinical understanding of spasticity: Implications for practice. Rehabil. Res. Pract. 2014, 2014, 279175. [Google Scholar] [CrossRef]
- Francisco, G.E.; Wissel, J.; Platz, T.; Li, S. Post-Stroke Spasticity. In Clinical Pathways in Stroke Rehabilitation, 1st ed.; Platz, T., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 149–173. [Google Scholar]
- Royal College of Physicians; British Society of Rehabilitation Medicine; The Chartered Society of Physiotherapy; Association of Chartered Physiotherapists in Neurology; The Royal College of Occupational Therapists. Spasticity in adults: Management using botulinum toxin. In National Guidelines, 2nd ed.; RCP: London, UK, 2018. [Google Scholar]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity Poststroke Over Different ICF Domains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum neurotoxins for post-stroke spasticity in adults: A systematic review. Mov. Disord. 2009, 24, 801–812. [Google Scholar] [CrossRef]
- Amini, D.A.; Kannenberg, K.; Bodison, S.; Chang, P.-F.; Colaianni, D.; Goodrich, B.; Mahaffey, L.; Painter, M.; Urban, M.; Handley-More, D.; et al. Occupational Therapy Practice Framework: Domain and Process (3rd Edition). Am. J. Occup. Ther. 2014, 68 (Suppl. S1), S1–S48. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health (ICF); World Health Organization: Geneva, Switzerland, 2001; Available online: https://iris.who.int/handle/10665/42407 (accessed on 1 November 2025).
- Wissel, J.; Ward, A.B.; Erztgaard, P.; Bensmail, D.; Hecht, M.J.; Lejeune, T.M.; Schnider, P.; Altavista, M.C.; Cavazza, S.; Deltombe, T.; et al. European consensus table on the use of botulinum toxin type a in adult spasticity. J. Rehabil. Med. 2009, 41, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Manack, A.; Brainin, M. Toward an epidemiology of poststroke spasticity. Neurology 2013, 80 (Suppl. S2), 13–19. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Ward, A.B. The diagnosis and management of adults with spasticity. In Handbook of Clinical Neurology. Neurological Rehabilitation, 1st ed.; Barnes, M.P., Good, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 145–160. [Google Scholar]
- Bamford, J.M.; Sandercock, P.A.; Warlow, C.P.; Slattery, J. Interobserver agreement for the assessment of handicap in stroke patients (letter). Stroke 1989, 20, 828. [Google Scholar] [CrossRef]
- Law, M.; Baptiste, S.; Carswell, A.; McColl, M.A.; Polatajko, H.; Pollock, N. COPM Canadian Occupational Performance Measure, 2nd ed.; Schulz-Kirchner: Idstein, Germany, 2011. [Google Scholar]
- Kiresuk, T.J.; Sherman, R.E. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Ment. Health J. 1968, 4, 443–453. [Google Scholar] [CrossRef]
- Bovend’Eerdt, T.J.; Botell, R.E.; Wade, D.T. Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clin. Rehabil. 2009, 23, 352–361. [Google Scholar] [CrossRef]
- Ashford, S.; Slade, M.; Turner-Stokes, L. Conceptualisation and development of the arm activity measure (ArmA) for assessment of activity in the hemiparetic arm. Disabil. Rehabil. 2013, 35, 1513–1518. [Google Scholar] [CrossRef]
- Ashford, S.; Turner-Stokes, L.; Siegert, R.; Slade, M. Initial psychometric evaluation of the Arm Activity Measure (ArmA): A measure of activity in the hemiparetic arm. Clin. Rehabil. 2013, 27, 728–740. [Google Scholar] [CrossRef]
- Law, M.; Baptiste, S.; Carswell, A.; McColl, M.A.; Polatajko, H.; Pollock, N. Canadian Occupational Performance Measure, 5th ed.; Canadian Association of Occupational Therapists (CAOT): Ottawa, ON, Canada, 2014. [Google Scholar]
- Platz, T.; Vuadens, P.; Eickhof, C.; Arnold, P.; Van Kaick, S.; Heise, K. REPAS, a summary rating scale for resistance to passive movement: Item selection, reliability and validity. Disabil. Rehabil. 2008, 30, 44–53. [Google Scholar] [CrossRef]
- Ashworth, B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964, 192, 540–542. [Google Scholar]
- Demeurisse, G.; Demol, O.; Robaye, E. Motor evaluation in vascular hemiplegia. Eur. Neurol. 1980, 19, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Wade, D. Assessing motor impairment after stroke: A pilot reliability study. J. Neurol. Neurosurg. Psychiatry 1990, 53, 576–579. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Turner-Bowker, D.M.; Gandek, B. User’s Manual for the SF-12v2® Health Survey with a Supplement Documenting SF-12® Health Survey; QualityMetric Incorporated: Lincoln, RI, USA, 2002. [Google Scholar]
- Kaňovský, P.; Slawek, J.; Denes, Z.; Platz, T.; Comes, G.; Grafe, S.; Pulte, I. Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post-stroke upper limb spasticity. J. Rehabil. Med. 2011, 43, 486–492. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Ashford, S.; Esquenazi, A.; Wissel, J.; Ward, A.B.; Francisco, G.E.; Lains, J.; Suputtitada, A.; Serrano, S.; Baguley, I.J.; et al. A comprehensive person-centred approach to adult spastic paresis: A consensus-based framework. Eur. J. Phys. Rehabil. Med. 2018, 54, 605–617. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Jacinto, J.; Fheodoroff, K.; Brashear, A.; Maisonobe, P.; Lysandropoulos, A.; Ashford, S.; Baguley, I.; Aggarwal, A.; Olver, J.; et al. Assessing the effectiveness of upper-limb spasticity management using a structured approach to goal-setting and outcome measurement: First cycle results from the ULIS-III study. J. Rehabil. Med. 2021, 53, 2731. [Google Scholar] [CrossRef] [PubMed]
- Ashford, S.; Turner-Stokes, L. Management of shoulder and proximal upper limb spasticity using botulinum toxin and concurrent therapy interventions: A preliminary analysis of goals and outcomes. Disabil. Rehabil. 2009, 31, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Rychlik, R.; Kreimendahl, F.; Schnur, N.; Lambert-Baumann, J. Long-term efficacy and safety of incobotulinumtoxinA and conventional treatment of poststroke arm spasticity: A prospective, non-interventional, open-label, parallel-group study. BMJ Open 2015, 5, e009358. [Google Scholar] [CrossRef]
- Eftekhar, P.; Mochizuki, G.; Dutta, T.; Richardson, D.; Brooks, D. Goal Attainment Scaling in Individuals with Upper Limb Spasticity Post Stroke. Occup. Ther. Int. 2016, 23, 379–389. [Google Scholar] [CrossRef]
- Jost, W.; Hefter, H.; Reissig, A.; Kollewe, K.; Wissel, J. Efficacy and safety of botulinum toxin typ A (Dysport) for the treatment of post-stroke arm spasticity: Results of the German-Asutrian open-label post-marketing surveillance prospective study. J. Neurol. Sci. 2014, 337, 86–90. [Google Scholar] [CrossRef]
- Khan, P.; Riberto, M.; Frances, J.A.; Chueire, R.; Amorim, A.C.F.G.; Xerez, D.; Chung, T.M.; Mercuri, L.H.C.; Longo, A.L.; Lianza, S.; et al. The Effectiveness of Botulinum Toxin Type A (BoNT-A) Treatment in Brazilian Patients with Chronic Post-Stroke Spasticity: Results from the Observational, Multicenter, Prospective BCause Study. Toxins 2020, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Nott, M.T.; Barden, H.L.H.; Baguley, I.J. Goal attainment following upper-limb botulinum toxin-A injections: Are we facilitating achievement of client-centred goals? J. Rehabil. Med. 2014, 46, 864–868. [Google Scholar] [CrossRef]
- Schramm, A.; Ndayisaba, J.P.; auf dem Brinke, M.; Hecht, M.; Herrmann, C.; Huber, M.; Lobsien, E.; Mehnert, S.; Reuter, I.; Stenner, A.; et al. Spasticity treatment with onabotulinumtoxin A: Data from a prospective German real-life patient registry. J. Neural Transm. 2014, 121, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Wissel, J.; Borg, J.; Ertzgaard, P.; Herrmann, C.; Kulkarni, J.; Lindgren, K.; Reuter, I.; Sakel, M.; Säterö, P.; et al. Functional goal achievement in post-stroke spasticity patients: The Botox® Economic Spasticity Trial (BEST). J. Rehabil. Med. 2014, 46, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Fheodoroff, K.; Jacinto, J.; Maisonobe, P. Results from the Upper Limb International Spasticity Study-II (ULIS-II): A large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin a in real-life clinical management. BMJ Open 2013, 3, e002771. [Google Scholar] [CrossRef]
- Holliday, R.C.; Cano, S.; Freeman, J.A.; Playford, E.D. Should patients participate in clinical decision making? An optimised balance block design controlled study of goal setting in a rehabilitation unit. J. Neurol. Neurosurg. Psychiatry 2007, 78, 576–580. [Google Scholar] [CrossRef]
- Young, C.A.; Manmathan, G.P.; Ward, J.C.R. Perceptions of goal setting in a neurological rehabilitation unit: A qualitative study of patients, carers and staff. J. Rehabil. Med. 2008, 40, 190–194. [Google Scholar] [CrossRef]
- Reid, A.; Chesson, R. Goal Attainment Scaling: Is it appropriate for stroke patients and their physiotherapists? Physiotherapy 1998, 84, 136–144. [Google Scholar] [CrossRef]
- Stevens, A.; Beurskens, A.; Köke, A.; Van Der Weijden, T. The use of patient-specific measurement instruments in the process of goal-setting: A systematic review of available instruments and their feasibility. Clin. Rehabil. 2013, 27, 1005–1019. [Google Scholar] [CrossRef]
- Vu, M.; Law, A.V. Goal-attainment scaling: A review and applications to pharmacy practice. Res. Soc. Adm. Pharm. 2012, 8, 102–121. [Google Scholar] [CrossRef]
- Krasny-Pacini, A.; Hiebel, J.; Pauly, F.; Godon, S.; Chevignard, M. Goal Attainment Scaling in rehabilitation: A literature-based update. Ann. Phys. Rehabil. Med. 2013, 56, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L. Goal attainment scaling (GAS) in rehabilitation: A practical guide. Clin. Rehabil. 2009, 23, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Polatajko, H.; Pollock, N.; McColl, M.A.; Carswell, A.; Baptiste, S. Pilot testing of the Canadian Occupational Performance Measure: Clinical and measurement issues. Can. J. Occup. Ther. 1994, 61, 191–197. [Google Scholar] [CrossRef]
- Toomey, M.; Nicholson, D.; Carswell, A. The clinical utillity of the Canadian occupational Performance Measure. Carnadian J. Occup. Ther. 1995, 62, 242–249. [Google Scholar] [CrossRef]
- Townsend, E.A.; Polatajko, H.J. Enabling Occupation II: Advancing an Occupational Therapy Vision for Health, Well-Being & Justice Through Occupation; Canadian Association of Occupational Therapists (CAOT): Ottawa, ON, Canada, 2007. [Google Scholar]
- Ashford, S.; Slade, M.; Nair, A.; Turner-Stokes, L. Arm Activity measure (ArmA) application for recording functional gain following focal spasticity treatment. Int. J. Ther. Rehabil. 2014, 21, 10–17. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, T.; Hu, X.; Wang, T. Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: A systematic review with meta-analysis and trial sequential analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Zhang, C.; Liu, Y.; Magat, E.; Verduzco-Gutierrez, M.; Francisco, G.E.; Zhou, P.; Zhang, Y.; Li, S. The effects of botulinum toxin injections on spasticity and motor performance in chronic stroke with spastic hemiplegia. Toxins 2020, 12, 492. [Google Scholar] [CrossRef]
- Baker, J.A.; Pereira, G. The efficacy of Botulinum Toxin A for spasticity and pain in adults: A systematic review and meta-analysis using the Grades of Recommendation, Assessment, Development and Evaluation approach. Clin. Rehabil. 2013, 27, 1084–1096. [Google Scholar] [CrossRef]
- Elovic, E.P.; Brashear, A.; Kaelin, D.; Liu, J.; Millis, S.R.; Barron, R.; Turkel, C. Repeated treatments with botulinum toxin type A produce sustained decreases in the limitations associated with focal upper-limb poststroke spasticity for caregivers and patients. Arch. Phys. Med. Rehabil. 2008, 89, 799–806. [Google Scholar] [CrossRef]
- McCrory, P.; Turner-Stokes, L.; Baguley, I.J.; De Graaff, S.; Katrak, P.; Sandanam, J.; Davies, L.; Munns, M.; Hughes, A. Botulinum toxin a for treatment of upper limb spasticity following stroke: A multi-centre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J. Rehabil. Med. 2009, 41, 536–544. [Google Scholar] [CrossRef]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L. BoTULS: A multicentre randomized controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol. Assess. 2010, 14, 1–13. [Google Scholar] [CrossRef]
- Elovic, E.P.; Munin, M.C.; Kaňovský, P.; Hanschmann, A.; Hiersemenzel, R.; Marciniak, C. Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity. Muscle Nerve 2016, 53, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Marque, P.; Denis, A.; Gasq, D.; Chaleat-Valayer, E.; Yelnik, A.; Colin, C.; Pérennou, D. Botuloscope: 1-year follow-up of upper limb post-stroke spasticity treated with botulinum toxin. Ann. Phys. Rehabil. Med. 2019, 62, 207–213. [Google Scholar] [CrossRef]
- Gracies, J.-M.; Jech, R.; Valkovic, P.; Marque, P.; Vecchio, M.; Denes, Z.; Vilain, C.; Delafont, B.; Picaut, P. When can maximal efficacy occur with repeat botulinum toxin injection in upper limb spastic paresis? Brain Commun. 2021, 3, fcaa201. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Molteni, F.; Cannaviello, G.; Lansaman, T.; Roche, N.; Bensmail, D. Does botulinum toxin treatment improve upper limb active function? Ann. Phys. Rehabil. Med. 2019, 62, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, R.; Kreimendahl, F.; Schnur, N.; Lambert-Baumann, J.; Dressler, D. Quality of life and costs of spasticity treatment in German stroke patients. Health Econ. Rev. 2016, 6, 27. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Baguley, I.J.; De Graaff, S.; Katrak, P.; Davies, L.; McCrory, P.; Hughes, A. Goal attainment scaling in the evaluation of treatment of upper limb spasticity with botulinum toxin: A secondary analysis from a double-blind placebo-controlled randomized clinical trial. J. Rehabil. Med. 2010, 42, 81–89. [Google Scholar] [CrossRef]
- Gracies, J.M.; O’Dell, M.; Vecchio, M.; Hedera, P.; Kocer, S.; Rudzinska-Bar, M.; Rubin, B.; Timerbaeva, S.L.; Lusakowska, A.; Boyer, F.C.; et al. Effects of repeated abobotulinumtoxinA injections in upper limb spasticity. Muscle Nerve 2018, 57, 245–254. [Google Scholar] [CrossRef]
- Rosales, R.L.; Balcaitiene, J.; Berard, H.; Maisonobe, P.; Goh, K.J.; Kumthornthip, W.; Mazlan, M.; Latif, L.A.; Santos, M.M.D.D.; Chotiyarnwong, C.; et al. Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study. Toxins 2018, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Mach, H.; Frohlich, S.; Behrend, S.; Werner, C.; Melzer, I. An early botulinum toxin A treatment in subacute stroke patients may prevent a disabling finger flexor stiffness six months later: A randomized controlled trial. Clin. Rehabil. 2012, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.; Ispoglou, S.; Helliwell, B.; Hicklin, D.; Sturman, S.; Pandyan, A. Can the early use of botulinum toxin in post stroke spasticity reduce contracture development? A randomised controlled trial. Clin. Rehabil. 2021, 35, 399–409. [Google Scholar] [CrossRef]
- Abo, M.; Shigematsu, T.; Hara, H.; Matsuda, Y.; Nimura, A.; Yamashita, Y.; Takahashi, K. Efficacy and Safety of OnabotulinumtoxinA 400 Units in Patients with Post-Stroke Upper Limb Spasticity: Final Report of a Randomized, Double-Blind, Placebo-Controlled Trial with an Open-Label Extension Phase. Toxins 2020, 12, 127. [Google Scholar] [CrossRef]
- Baricich, A.; Picelli, A.; Santamato, A.; Carda, S.; de Sire, A.; Smania, N.; Cisari, C.; Invernizzi, M. Safety Profile of High-Dose Botulinum Toxin Type A in Post-Stroke Spasticity Treatment. Clin. Drug Investig. 2018, 38, 991–1000. [Google Scholar] [CrossRef]
- Intiso, D.; Simone, V.; Bartolo, M.; Santamato, A.; Ranieri, M.; Gatta, M.T.; Di Rienzo, F. High dosage of botulinum toxin type a in adult subjects with spasticity following acquired central nervous system damage: Where are we at? Toxins 2020, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M.; GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke upper limb spasticity. Curr. Med. Res. Opin. 2010, 26, 1983–1992. [Google Scholar] [CrossRef]
- O’Dell, M.W.; Brashear, A.; Jech, R.; Lejeune, T.; Marque, P.; Bensmail, D.; Ayyoub, Z.; Simpson, D.M.; Volteau, M.; Vilain, C.; et al. Dose-Dependent Effects of AbobotulinumtoxinA (Dysport) on Spasticity and Active Movements in Adults With Upper Limb Spasticity: Secondary Analysis of a Phase 3 Study. PM R 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Wissel, J.; Bensmail, D.; Ferreira, J.J.; Molteni, F.; Satkunam, L.; Moraleda, S.; Rekand, T.; McGuire, J.; Scheschonka, A.; Flatau-Baqué, B.; et al. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity: The TOWER study. Neurology 2017, 88, 1321–1328. [Google Scholar] [CrossRef]
- Roschka, S. Do Stroke Survivors with Upper Extremity Spasticity Benefit from Botulinum Toxin? An Observational Study Exploring Daily Activities. Master’s Thesis, University of Birmingham, Birmingham, UK, 2019. [Google Scholar]
| Muscles | Number of Participants (n) | Type of BoNT-A | Units | |
|---|---|---|---|---|
| Mean (SD) | Range | |||
| Biceps brachii | 10 (37.0%) | Incobotulinum toxin A (n = 7) | 32.9 (9.5) | (20–50) |
| Onabotulinum toxin A (n = 3) | 36.7 (15.3) | (20–50) | ||
| Brachialis | 14 (51.9%) | Incobotulinum toxin A (n = 12) | 29.2 (5.1) | (20–40) |
| Onabotulinum toxin A (n = 2) | 40.0 (0.0) | (40–40) | ||
| Brachioradialis | 1 (3.7%) | Onabotulinum toxin A (n = 1) | 20 | |
| Deltoideus | 2 (7.4%) | Incobotulinum toxin A (n = 2) | 30.0 (0.0) | (30–30) |
| Extensor digitorum communis | 1 (3.7%) | Incobotulinum toxin A (n = 1) | 30.0 | |
| Flexor carpi radialis | 14 (51.9%) | Incobotulinum toxin A (n = 13) | 28.9 (8.7) | (20–50) |
| Onabotulinum toxin A (n = 1) | 20 | |||
| Flexor carpi ulnaris | 6 (22.2%) | Incobotulinum toxin A (n = 6) | 25.0 (8.4) | (20–40) |
| Flexor digitorum profundus | 20 (74.1%) | Incobotulinum toxin A (n = 15) | 25.0 (6.8) | (15–40) |
| Onabotulinum toxin A (n = 4) | 22.5 (5.0) | (20–30) | ||
| Abotulinum toxin A (n = 1) | 200 | |||
| Flexor digitorum superficialis | 18 (66.7%) | Incobotulinum toxin A (n = 15) | 47.3 (12.2) | (30–70) |
| Onabotulinum toxin A (n = 2) | 40.0 (0.0) | (40–40) | ||
| Abotulinum toxin A (n = 1) | 300 | |||
| Flexor pollicis brevis | 2 (7.4%) | Incobotulinum toxin A (n = 2) | 5.0 (0.0) | (5–5) |
| Flexor pollicis longus | 7 (25.9%) | Incobotulinum toxin A (n = 4) | 12.5 (5.0) | (10–20) |
| Onabotulinum toxin A (n = 2) | 10.0 (0.0) | (10–10) | ||
| Abotulinum toxin A (n = 1) | 100 | |||
| Lumbricales | 1 (3.7%) | Incobotulinum toxin A (n = 1) | 15.0 | |
| Opponens pollicis | 3 (11.1%) | Incobotulinum toxin A (n = 3) | 8.3 (2.9) | (5–10) |
| Pectorales | 15 (55.6%) | Incobotulinum toxin A (n = 14) | 31.1 (7.9) | (20–40) |
| Onabotulinum toxin A (n = 1) | 40 | |||
| Pronator teres | 10 (37.0%) | Incobotulinum toxin A (n = 10) | 28.0 (6.3) | (20–40) |
| Triceps brachii | 7 (25.9%) | Incobotulinum toxin A (n = 7) | 37.1 (19.8) | (20–70) |
| Outcome Measure | T0 (Baseline) | Tmax1 (4–6 Weeks) | T2 (12–14 Weeks) | p-Value * | p-Value † | ||
|---|---|---|---|---|---|---|---|
| T0–Tmax1 | T0–T2 | Tmax1–T2 | |||||
| COPM (n = 24) | |||||||
| Performance | 4.3 (3.0–6.0) | 6.0 (4.5–7.7) | 5.3 (4.6–7.0) | <0.0001 | <0.001 | <0.004 | 0.052 |
| Satisfaction | 3.8 (2.6–5.9) | 6.0 (4.4–7.5) | 5.7 (4.0–7.3) | <0.0001 | <0.001 | <0.001 | 0.129 |
| ArmA (n = 24) | |||||||
| Passive function | 12.5 (6.0–16.0) | 7.0 (4.3–10.8) | 9.5 (5.0–12.0) | <0.0001 | <0.0001 | 0.001 | 0.196 |
| Active function | 37.0 (30.3–47.3) | 36.5 (30.0–47.0) | 36.0 (30.3–46.5) | 0.011 | 0.020 | 0.586 | 0.197 |
| REPAS (n = 21) | 9.0 (7.0–12.0) | 7.0 (4.5–9.5) | 8.0 (4.5–10.0) | <0.001 | 0.002 | 0.001 | 0.054 |
| MI (n = 21) | 40 (26.5–56.0) | 40 (34.5–61.0) | 40 (29.0–59.0) | 0.157 | - | - | - |
| SF-12v2 (n = 24) | |||||||
| PCS | 35.5 (28.0–38.8) | 37.2 (31.3–41.0) | 33.3 (31.8–39.1) | 0.180 | - | - | - |
| MCS | 47.8 (43.6–53.4) | 51.0 (44.6–57.2) | 50.7 (41.8–57.4) | 0.819 | - | - | - |
| GCI (n = 24) | |||||||
| Participant/carer | - | 2.0 (2.0–2.4) | 2.0 (2.0–2.5) | - | - | - | 0.286 |
| Physician/therapist | - | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | - | - | - | 0.248 |
| Outpatient therapist | - | 2.5 (2.0–3.0) | - | - | - | - | - |
| VAS (mean (SD)) (n = 24) | |||||||
| Importance | - | - | 70.2 (22.6) | - | - | - | - |
| Satisfaction | - | - | 73.6 (18.2) | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roschka, S.; Punt, D.; Platz, T. Patient-Centred and Daily Life-Oriented Botulinum Toxin Treatment for Stroke Survivors with Upper Extremity Spasticity—Effects and Practical Aspects. J. Clin. Med. 2025, 14, 8339. https://doi.org/10.3390/jcm14238339
Roschka S, Punt D, Platz T. Patient-Centred and Daily Life-Oriented Botulinum Toxin Treatment for Stroke Survivors with Upper Extremity Spasticity—Effects and Practical Aspects. Journal of Clinical Medicine. 2025; 14(23):8339. https://doi.org/10.3390/jcm14238339
Chicago/Turabian StyleRoschka, Sybille, David Punt, and Thomas Platz. 2025. "Patient-Centred and Daily Life-Oriented Botulinum Toxin Treatment for Stroke Survivors with Upper Extremity Spasticity—Effects and Practical Aspects" Journal of Clinical Medicine 14, no. 23: 8339. https://doi.org/10.3390/jcm14238339
APA StyleRoschka, S., Punt, D., & Platz, T. (2025). Patient-Centred and Daily Life-Oriented Botulinum Toxin Treatment for Stroke Survivors with Upper Extremity Spasticity—Effects and Practical Aspects. Journal of Clinical Medicine, 14(23), 8339. https://doi.org/10.3390/jcm14238339






