Oral Nutritional Supplement Adherence and Nutritional Outcomes in Hemodialysis Patients—A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Identify socio-demographic, psychological, and clinical factors associated with ONS adherence;
- (2)
- Evaluate the effects of adherence on nutritional status, body composition, and laboratory markers; and
- (3)
- Examine the relationships between adherence, nutritional status, and mortality.
Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Adherence and Mood
3.3. Adherence to Oral Nutritional Supplement (ONS) Use and Acceptance of Nutritional Recommendations
3.4. Longitudinal Changes
3.5. Mortality Predictors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| MIA | Malnutrition–Inflammation–Atherosclerosis syndrome |
| PEW | Protein-energy wasting |
| ONS | Oral nutritional supplements |
| HD | Hemodialysis |
| MMAS-4 | 4-item Morisky Medication Adherence Scale |
| BDI | 21-item Beck Depression Inventory |
| TIBC | Total iron-binding capacity |
| CRP | C-reactive protein |
| NC | Neck circumference |
| MUAC | Mid-upper arm circumference |
| WC | Waist circumference |
| HC | Hip circumference |
| TST | Triceps skinfold thickness |
| SST | Scapular skinfold thickness |
| BW | Body weight |
| BMI | Body mass index |
| LTI | Lean tissue index |
| FTI | Fat tissue index |
| OH | Overhydration |
| BCM | Body Composition Monitor |
| MIS | Malnutrition–Inflammation Score |
| ESRD | End-stage renal disease |
| EPO | Erythropoietin |
References
- Stenvinkel, P.; Heimbürger, O.; Lindholm, B.; Kaysen, G.A.; Bergström, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transplant. 2000, 15, 953–960. [Google Scholar] [CrossRef]
- Zyga, S.; Christopoulou, G.; Malliarou, M. Malnutrition-inflammation-atherosclerosis syndrome in patients with end-stage renal disease. J. Ren. Care 2011, 37, 12–15. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin. Nephrol. 2009, 29, 3–14. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M.; Stratakis, D.; Fischer, R. Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Nephrol. Dial. Transplant. 2001, 16, 1863–1869. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: What is next? Semin. Dial. 2005, 18, 365–369. [Google Scholar] [CrossRef]
- Molfino, A.; Chiappini, M.G.; Laviano, A.; Ammann, T.; Bollea, M.R.; Alegiani, F.; Rossi Fanelli, F.; Muscaritoli, M. Effect of intensive nutritional counseling and support on clinical outcomes of hemodialysis patients. Nutrition 2012, 28, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Sadu Singh, B.K.; Khor, B.H.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2017, 36, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and Predictive Validity of a Self-Reported Measure of Medication Adherence. Med. Care 1986, 24, 67–74. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef]
- De Nicola, L.; Zoccali, C. Chronic kidney disease prevalence in the general population: Heterogeneity and concerns. Nephrol. Dial. Transplant. 2016, 31, 331–335. [Google Scholar] [CrossRef]
- Saran, R.; Li, Y.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Ayanian, J.; Bragg-Gresham, J.; Balkrishnan, R.; Chen, J.L.T.; Cope, E.; et al. US Renal Data System 2015 Annual Data Report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2016, 67 (Suppl. 1), S134–S434. [Google Scholar] [CrossRef]
- Fung, F.; Sherrard, D.J.; Gillen, D.L.; Wong, C.; Kestenbaum, B.; Seliger, S.; Ball, A.; Stehman-Breen, C. Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am. J. Kidney Dis. 2002, 40, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.M.; Nguyen, H.A. Nutrition education in the care of patients with chronic kidney disease and end-stage renal disease. Semin. Dial. 2018, 31, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.C.; Pope, J.F.; Hunt, A.E.; Gerald, B. The effect of diet education on the laboratory values and knowledge of hemodialysis patients with hyperphosphatemia. J. Ren. Nutr. 2004, 14, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Gast, A.; Mathes, T. Medication adherence influencing factors-an (updated) overview of systematic reviews. Syst. Rev. 2019, 8, 112. [Google Scholar] [CrossRef]
- Liljeberg, E.; Payne, L.; Skinnars Josefsson, M.; Söderström, L.; Einarsson, S. Understanding the complexity of barriers and facilitators to adherence to oral nutritional supplements among patients with malnutrition: A systematic mixed-studies review. Nutr. Res. Rev. 2024, 1–21. [Google Scholar] [CrossRef]
- Win, K.C.M.; Zhou, H.; Patton, V.; Steen, M.; Della, P. Factors Contributing to Non-Adherence to Treatment Among Adult Patients with Long-Term Haemodialysis: An Integrative Review. Nurs. Rep. 2025, 15, 314. [Google Scholar] [CrossRef]
- Vr, V.; Kaur Kang, H. The Worldwide Prevalence of Nonadherence to Diet and Fluid Restrictions Among Hemodialysis Patients: A Systematic Review and Meta-analysis. J. Ren. Nutr. 2022, 32, 658–669. [Google Scholar] [CrossRef]
- Hassan, K. Does Whey Protein Supplementation Improve the Nutritional Status in Hypoalbuminemic Peritoneal Dialysis Patients? Ther. Apher. Dial. 2017, 21, 485–492. [Google Scholar] [CrossRef]
- Sezer, S.; Bal, Z.; Tutal, E.; Uyar, M.E.; Acar, N.O. Long-term oral nutrition supplementation improves outcomes in malnourished patients with chronic kidney disease on hemodialysis. JPEN J. Parenter. Enteral. Nutr. 2014, 38, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, G.; Sun, X.; Chen, A.; Chai, Y. A Low-Cost, Intradialytic, Protein-Rich Meal Improves the Nutritional Status in Chinese Hemodialysis Patients. J. Ren. Nutr. 2020, 30, e27–e34. [Google Scholar] [CrossRef] [PubMed]
- Małgorzewicz, S.; Gałęzowska, G.; Cieszyńska-Semenowicz, M.; Ratajczyk, J.; Wolska, L.; Rutkowski, P.; Jankowska, M.; Rutkowski, B.; Dębska-Ślizień, A. Amino acid profile after oral nutritional supplementation in hemodialysis patients with protein-energy wasting. Nutrition 2019, 57, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.J.; Guo, J.; Zhang, Y.; Wang, F.; Yu, K. Effects of oral nutritional supplements on the nutritional status and inflammatory markers in patients on maintenance dialysis: A systematic review and meta-analysis of randomized clinical trials. Clin. Kidney J. 2023, 16, 2271–2288. [Google Scholar] [CrossRef]
- Caglar, K.; Fedje, L.; Dimmitt, R.; Hakim, R.M.; Shyr, Y.; Ikizler, T.A. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int. 2002, 62, 1054–1059. [Google Scholar] [CrossRef]
- Fouque, D.; Vennegoor, M.; ter Wee, P.; Wanner, C.; Basci, A.; Canaud, B.; Haage, P.; Konner, K.; Kooman, J.; Martin-Malo, A.; et al. EBPG guideline on nutrition. Nephrol. Dial. Transplant. 2007, 22 (Suppl. S2), ii45–ii87. [Google Scholar] [CrossRef]
- Ghimire, S.; Castelino, R.L.; Lioufas, N.M.; Peterson, G.M.; Zaidi, S.T. Nonadherence to Medication Therapy in Haemodialysis Patients: A Systematic Review. PLoS ONE 2015, 10, e0144119. [Google Scholar] [CrossRef]
- Rambod, M.; Peyravi, H.; Shokrpour, N.; Sareban, M.T. Dietary and fluid adherence in Iranian hemodialysis patients. Health Care Manag. 2010, 29, 359–364. [Google Scholar] [CrossRef]
- Safdar, N.; Baakza, H.; Kumar, H.; Naqvi, S.A. Non-compliance to diet and fluid restrictions in haemodialysis patients. J. Pak. Med. Assoc. 1995, 45, 293–295. [Google Scholar]
- Chan, Y.M.; Zalilah, M.S.; Hii, S.Z. Determinants of compliance behaviours among patients undergoing hemodialysis in Malaysia. PLoS ONE 2012, 7, e41362. [Google Scholar] [CrossRef]
- Morgan, L. A decade review: Methods to improve adherence to the treatment regimen among hemodialysis patients. Nephrol. Nurs. J. 2000, 27, 299–304. [Google Scholar] [CrossRef]
- Krueger, K.P.; Berger, B.A.; Felkey, B. Medication adherence and persistence: A comprehensive review. Adv. Ther. 2005, 22, 313–356. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.; Fwu, C.W.; Abbott, K.C.; Moxey-Mims, M.M.; Mendley, S.; Norton, J.M.; Eggers, P.W. Psychiatric Illness and Mortality in Hospitalized ESKD Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2019, 14, 1363–1371. [Google Scholar] [CrossRef]
- Shirazian, S.; Grant, C.D.; Aina, O.; Mattana, J.; Khorassani, F.; Ricardo, A.C. Depression in Chronic Kidney Disease and End-Stage Renal Disease: Similarities and Differences in Diagnosis, Epidemiology, and Management. Kidney Int. Rep. 2016, 2, 94–107. [Google Scholar] [CrossRef]
- Hedayati, S.S.; Bosworth, H.B.; Kuchibhatla, M. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006, 69, 1662–1668. [Google Scholar] [CrossRef]
- Gerogianni, G.; Lianos, E.; Kouzoupis, A.; Polikandrioti, M.; Grapsa, E. The role of socio-demographic factors in depression and anxiety of patients on hemodialysis: An observational cross-sectional study. Int. Urol. Nephrol. 2018, 50, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.A.; Albert, J.M.; Young, E.W. Screening for depression in hemodialysis patients: Associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004, 66, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Cukor, D.; Rosenthal, D.S.; Jindal, R.M.; Brown, C.D.; Kimmel, P.L. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int. 2009, 75, 1223–1229. [Google Scholar] [CrossRef]
- Chilcot, J.; Davenport, A.; Wellsted, D. An association between depressive symptoms and survival in incident dialysis patients. Nephrol. Dial. Transplant. 2011, 26, 1628–1634. [Google Scholar] [CrossRef]
- Akman, B.; Uyar, M.; Afsar, B.; Sezer, S.; Ozdemir, F.N.; Haberal, M. Adherence, depression and quality of life in patients on a renal transplantation waiting list. Transpl. Int. 2007, 20, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Murali, K.M.; Lonergan, M. Breaking the adherence barriers: Strategies to improve treatment adherence in dialysis patients. Semin. Dial. 2020, 33, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, B.; Prabhu, R.; Unnikrishnan, B.; Ballal, S.; Mundkur, S.C.; Chandra Sekaran, V.; Shetty, A.; Moreira, P. Effect of Educational Intervention on Knowledge and Level of Adherence among Hemodialysis Patients: A Randomized Controlled Trial. Glob. Health Epidemiol Genom. 2023, 2023, 4295613. [Google Scholar] [CrossRef]
- Okoyo Opiyo, R.; Nyawade, S.A.; McCaul, M.; Nyasulu, P.S.; Lango, D.B.; Were, A.J.O.; Nabakwe, E.C.; Bukania, Z.N.; Olenja, J.M. Perceptions on Adherence to Dietary Prescriptions for Adults with Chronic Kidney Disease on Hemodialysis: A Qualitative Study. Diseases 2020, 8, 29. [Google Scholar] [CrossRef]
- Opiyo, R.O.; Nyasulu, P.S.; Olenja, J.; Zunza, M.; Nguyen, K.A.; Bukania, Z.; Nabakwe, E.; Mbogo, A.; Were, A.O. Factors associated with adherence to dietary prescription among adult patients with chronic kidney disease on hemodialysis in national referral hospitals in Kenya: A mixed-methods survey. Ren. Replace Ther. 2019, 5, s41100-019-0237-4. [Google Scholar] [CrossRef]
- Campbell, K.L.; Ash, S.; Davies, P.S.; Bauer, J.D. Randomized controlled trial of nutritional counseling on body composition and dietary intake in severe CKD. Am. J. Kidney Dis. 2008, 51, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, K.L.; Aruna, M.; Narayana Rao, S.V.L.; Mohan, P.R. Dietary Counseling by Renal Dietician Improves the Nutritional Status of Hemodialysis Patients. Indian J. Nephrol. 2019, 29, 179–185. [Google Scholar] [CrossRef]
- Murali, K.M.; Mullan, J.; Roodenrys, S.; Hassan, H.C.; Lambert, K.; Lonergan, M. Strategies to improve dietary, fluid, dialysis or medication adherence in patients with end stage kidney disease on dialysis: A systematic review and meta-analysis of randomized intervention trials. PLoS ONE 2019, 14, e0211479. [Google Scholar] [CrossRef]
- Safi, F.; Areshtanab, H.N.; Ghafourifard, M.; Ebrahimi, H. The association between self-efficacy, perceived social support, and family resilience in patients undergoing hemodialysis: A cross-sectional study. BMC Nephrol. 2024, 25, 207. [Google Scholar] [CrossRef]
- Dantas, L.G.; Cruz, C.; Rocha, M.; Moura JAJr Paschoalin, E.; Paschoalin, S.; Marcilio de Souza, C. Prevalence and predictors of nonadherence to hemodialysis. Nephron Clin. Pract. 2013, 124, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sousa, H.; Ribeiro, O.; Paúl, C.; Costa, E.; Miranda, V.; Ribeiro, F.; Figueiredo, D. Social support and treatment adherence in patients with end-stage renal disease: A systematic review. Semin. Dial. 2019, 32, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Alatawi, A.A.; Alaamri, M.; Almutary, H. Social Support and Adherence to Treatment Regimens among Patients Undergoing Hemodialysis. Healthcare 2024, 12, 1958. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef]
- Stosovic, M.; Stanojevic, M.; Simic-Ogrizovic, S.; Jovanovic, D.; Djukanovic, L. The predictive value of anthropometric parameters on mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 1367–1374. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Streja, E.; Kovesdy, C.P.; Oreopoulos, A.; Noori, N.; Jing, J.; Nissenson, A.R.; Krishnan, M.; Kopple, J.D.; Mehrotra, R.; et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin. Proc. 2010, 85, 991–1001. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 101) |
|---|---|
| Demographic | |
| Gender M/F (%) | 56.4/43.6% |
| Age (mean, std; years) | 60.8 ± 16.15 |
| HD vintage (median; months) | 51 (range 9–456) |
| Primary kidney disease (%) | |
| Glomerulonephritis | 27.7% |
| Diabetic nephropathy | 21.8% |
| Hypertensive kidney disease | 14.9% |
| Other | 35.6% |
| Sociodemographic and psychosocial determinants | |
| Educational level (%) | |
| Primary education | 26.7% |
| Secondary education | 52.5% |
| Higher education | 18.8% |
| Marital status (%) | |
| Married | 56.4% |
| Single/divorced/widowed | 40.6% |
| Did not specify | 3.0% |
| MMAS-4 (mean, std) | 1.5 ± 1.2 |
| Categories (%): | |
| Low adherence | 18.8% |
| Moderate adherence | 44.6% |
| High adherence | 36.6% |
| BDI (mean, std) | 11.2 ± 6.8 |
| Categories (%): | |
| Minimal/normal mood oscillation | 46.5% |
| Mild depression | 31.7% |
| Borderline depression | 14.9% |
| Moderate depression | 6.9% |
| Severe depression | 0% |
| Nutritional parameters (mean, std) | |
| MIS | 7.8 ± 3.4 |
| BMI (kg/m2) | 25.2 ± 5.4 |
| BW (kg) | 69.8 ± 17.2 |
| NC (cm) | 38.3 ± 4.3 |
| MUAC (cm) | 27.6 ± 4.5 |
| WC (cm) | 94.3 ± 14.8 |
| HC (cm) | 100.7 ± 10.9 |
| TST (mm) | 15.5 ± 7.7 |
| SST (mm) | 15.4 ± 7.4 |
| LTI (kg/m2) | 12.5 ± 3.1 |
| FTI (kg/m2) | 12.3 ± 5.3 |
| OH (L) | 2.6 ± 1.6 |
| Laboratory parameters (mean, std) | |
| Hemoglobin (g/L) | 107.6 ± 12.2 |
| Creatinine (µmol/L) | 771.1 ± 198.3 |
| Urea (mmol/L) | 20.9 ± 5.5 |
| Albumin (g/L) | 37.8 ± 3.3 |
| Prealbumin (g/L) | 0.5 ± 0.2 |
| Calcium (mmol/L) | 2.2 ± 0.2 |
| Phosphorus (mmol/L) | 1.4 ± 0.4 |
| Potassium (mmol/L) | 4.9 ± 0.6 |
| Cholesterol (mmol/L) | 4.2 ± 1.1 |
| Triglycerides (mmol/L) | 1.7 ± 0.9 |
| Glucose (mmol/L) | 6.5 ± 2.7 |
| Iron (µmol/L) | 11.8 ± 4.8 |
| TIBC | 40.3 ± 8.2 |
| Ferritin (µg/L) | 388.3 ± 189.2 |
| CRP (mg/L) | 8.2 ± 12.7 |
| Parameter | High Adherence (MMAS-4 = 0) | Moderate Adherence (MMAS-4 = 1–2) | Low Adherence (MMAS-4 = 3–4) | p-Value | Test |
|---|---|---|---|---|---|
| Age group, n (%) | 0.71 | χ2 | |||
| ≤55 years | 10 | 11 | 5 | ||
| 56–65 years | 9 | 14 | 8 | ||
| 66–75 years | 10 | 13 | 2 | ||
| ≥75 years | 8 | 7 | 4 | ||
| Sex (F/M), n | 13/24 | 27/18 | 4/15 | 0.007 | χ2 |
| Marital status, n (%) | 0.16 | χ2 | |||
| Married/partnered | 18 | 29 | 10 | ||
| Single/widowed/divorced | 19 | 13 | 9 | ||
| Living situation, n (%) | 0.21 | χ2 | |||
| Living alone | 14 | 12 | 3 | ||
| Living with others | 23 | 33 | 16 | ||
| Education level, n (%) | 0.19 | χ2 | |||
| Primary | 15 | 10 | 2 | ||
| Secondary | 17 | 22 | 14 | ||
| Higher | 2 | 3 | 2 | ||
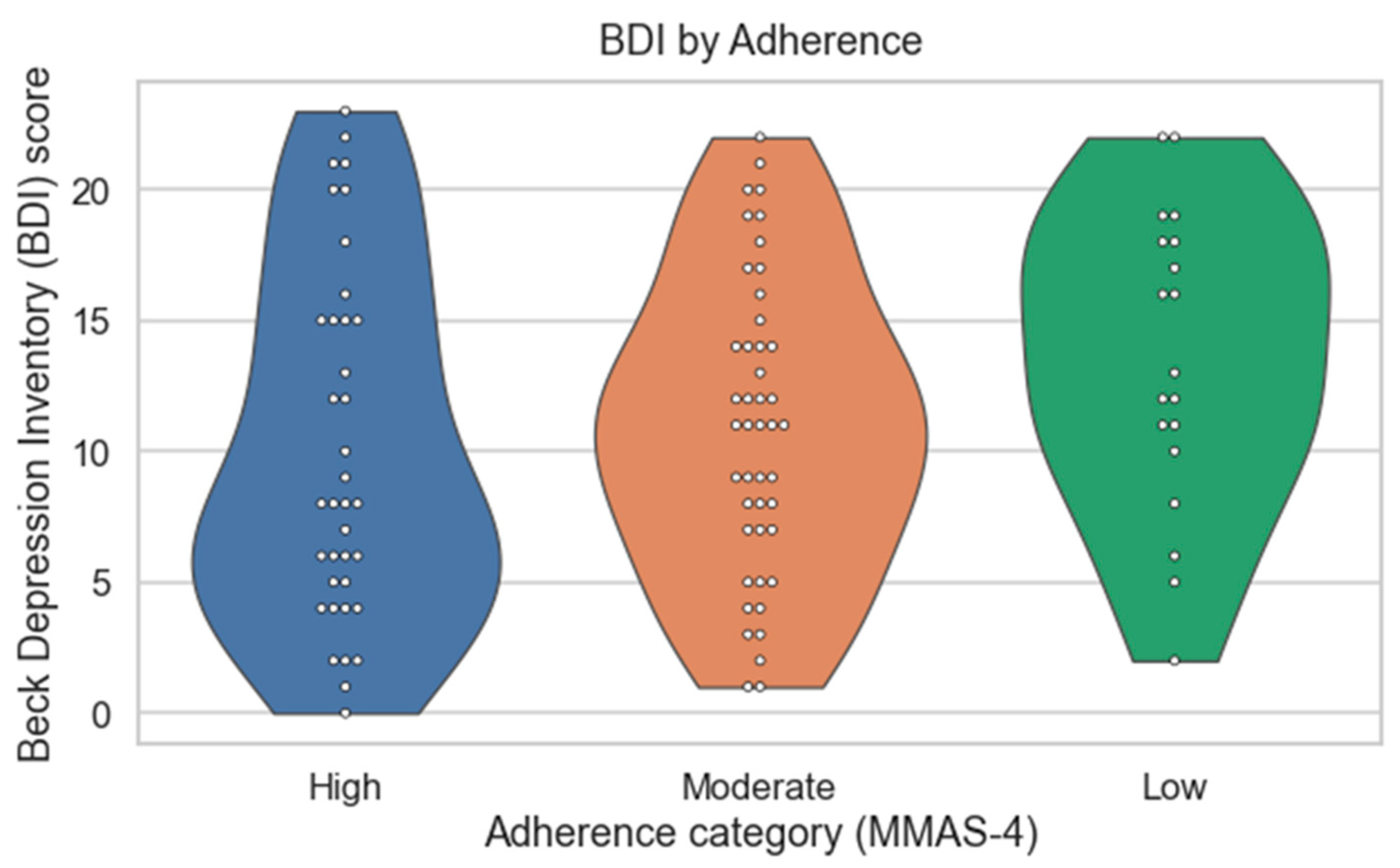
| BDI category, n (%) | 0.19 | χ2 | |||
| Minimal (0–10) | 22 | 20 | 5 | ||
| Mild (11–16) | 8 | 16 | 7 | ||
| Borderline (17–20) | 3 | 7 | 5 | ||
| Moderate (21–29) | 4 | 2 | 2 | ||
| Severe (≥30) | 0 | 0 | 0 | ||
| MIS, median (IQR) | 8 (5–10) | 8 (5–10.8) | 8.5 (6.3–10.5) | 0.46 | Kruskal–Wallis |
| Albumin (g/L) | 39 (37–40) | 38 (36–40) | 37 (33–40) | 0.41 | ANOVA |
| LDL (mmol/L) | 2.1 (1.7–2.4) | 2.5 (2.0–3.0) | 1.8 (1.2–2.6) | 0.038 | ANOVA |
| Proportion of Participants by ONS Intake (N) | p * | |||
|---|---|---|---|---|
| ONS Non-Users | ONS Users | Total | ||
| MIS first visit | ||||
| Low and moderate inflammation | 26 (59) | 18 (35) | 44 (46) | 0.02 |
| High inflammation | 18 (41) | 33 (65) | 51 (54) | |
| Total | 44 (100) | 51 (100) | 95 (100) | |
| MIS second visit | ||||
| Low and moderate inflammation | 21 (62) | 18 (34) | 39 (45) | 0.01 |
| High inflammation | 13 (38) | 35 (66) | 48 (55) | |
| Total | 34 (100) | 53 (100) | 87 (100) | |
| MIS third visit | ||||
| Low and moderate inflammation | 19 (73) | 26 (60) | 45 (65) | 0.29 |
| High inflammation | 7 (27) | 17 (40) | 24 (35) | |
| Total | 26 (100) | 43 (100) | 69 (100) | |
| Median (IQR) Total | p * | |||
|---|---|---|---|---|
| 1st Visit | 2nd Visit | 3rd Visit | ||
| Creatinine (µmol/L) | 749 (617–905.5) | 738 (602.8–927.3) | 736.5 (635.3–951.5) | 0.57 |
| Urea (mmol/L) | 21 (18.4–23.6) | 20.9 (18.3–24.9) | 22.65 (18.6–25) | 0.26 |
| Total protein (g/L) | 66 (64–70) | 66 (64–70.5) | 67 (64–71) | 0.29 |
| Albumin (g/L) | 38.25 (35.7–40) | 38.6 (36.2–40.9) | 38.4 (35.9–40.9) | 0.37 |
| Prealbumin (g/L) | 0.5 (0.4–0.6) | 0.5 (0.5–0.7) | 0.5 (0.4–0.7) | 0.02 |
| Hemoglobin (g/L) | 106 (99–116) | 110 (104–117.25) | 114 (103–120) | 0.04 |
| Leucocytes (×109/L) | 5.9 (4.8–7.4) | 5.8 (4.88–6.93) | 5.7 (4.7–6.4) | 0.32 |
| Thrombocytes (×109/L) | 171 (137.5–212) | 164.5 (130.75–200.25) | 166 (131–209.3) | 0.19 |
| Iron (µmol/L) | 11 (8–14) | 11 (8–14) | 11.5 (9–14.3) | 0.71 |
| TIBC | 39 (35.5–44) | 38 (34–42.25) | 38.5 (34.8–42) | 0.19 |
| Ferritin (µg/L) | 391.1 (255.9–513.6) | 397.5 (249.53–523.18) | 350.75 (206.1–487.5) | 0.17 |
| EPO dose (IU per month) | 24,000 (16,000–48,000) | 32,000 (20,000–48,000) | 32,000 (23,000–48,000) | 0.87 |
| Iron therapy (mg per month) | 100 (0–200) | 125 (0–125) | 125 (31.3–125) | 0.18 |
| Calcium (mmol/L) | 2.2 (2.12–2.3) | 2.23 (2.1–2.3) | 2.21 (2.1–2.3) | 0.23 |
| Phosphorus (mmol/L) | 1.4 (1.14–1.71) | 1.46 (1.2–1.8) | 1.57 (1.3–1.8) | 0.13 |
| Potassium (mmol/L) | 5 (4.45–5.4) | 5.05 (4.4–5.5) | 5.3 (4.7–5.9) | <0.001 |
| Glucose (mmol/L) | 5.7 (4.8–7.7) | 5.9 (4.6–7.7) | 5 (4.3–7.4) | 0.02 |
| CRP (mg/L) | 4.75 (1.2–10.6) | 3.5 (1.3–8.5) | 4.1 (2.1–8) | 0.26 |
| Cholesterol (mmol/L) | 4.05 (3.43–4.88) | 3.9 (3.3–4.8) | 3.9 (3.3–4.5) | 0.21 |
| Triglycerides (mmol/L) | 1.57 (1.03–2.22) | 1.45 (1–2.3) | 1.29 (1–1.7) | 0.64 |
| HDL (mmol/L) | 1.01 (0.82–1.27) | 1.01 (0.8–1.3) | 1 (0.8–1.2) | 0.69 |
| LDL (mmol/L) | 2.23 (1.7–2.8) | 2.01 (1.7–2.6) | 2.16 (1.6–2.7) | 0.32 |
| BW (kg) | 69.3 (56.3–81.7) | 68.6 (56.5–81.5) | 69.5 (57.5–79.4) | 0.28 |
| BMI (kg/m2) | 24.5 (21.1–27.9) | 24.3 (21.2–27.7) | 25.4 (21.2–28.2) | 0.50 |
| MIS | 8 (6–10) | 8 (5–12) | 6 (4.5–8.5) | 0.003 |
| OH (L) | 2.4 (1.6–3.4) | 2.4 (1.3–3.8) | 1.9 (0.8–3) | 0.01 |
| LTI (kg/m2) | 12.4 (10.2–14.3) | 11.1 (9.8–13.6) | 11.8 (9.8–14.1) | 0.009 |
| FTI (kg/m2) | 11.3 (8.5–15.1) | 12.05 (9.4–15.8) | 11.9 (8.8–15.9) | 0.08 |
| NC (cm) | 38 (35–41) | 40 (35–41) | 39 (35–42) | 0.03 |
| MUAC (cm) | 27 (24–30) | 28 (25–31.3) | 29 (26–32) | <0.001 |
| WC (cm) | 93 (83–104.5) | 95 (85–105) | 98 (85–106) | 0.02 |
| HC (cm) | 100 (93–107) | 101 (95.5–107) | 100.5 (95.9–109.3) | 0.16 |
| SST (mm) | 14 (10–21) | 13 (9–18.5) | 15 (10–18.5) | 0.01 |
| TST (mm) | 14 (10–20) | 14 (8.5–21) | 15 (9.5–20) | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katalinic, L.; Juric, I.; Atic, A.; Jelakovic, B.; Basic-Jukic, N. Oral Nutritional Supplement Adherence and Nutritional Outcomes in Hemodialysis Patients—A Prospective Study. J. Clin. Med. 2025, 14, 8337. https://doi.org/10.3390/jcm14238337
Katalinic L, Juric I, Atic A, Jelakovic B, Basic-Jukic N. Oral Nutritional Supplement Adherence and Nutritional Outcomes in Hemodialysis Patients—A Prospective Study. Journal of Clinical Medicine. 2025; 14(23):8337. https://doi.org/10.3390/jcm14238337
Chicago/Turabian StyleKatalinic, Lea, Ivana Juric, Armin Atic, Bojan Jelakovic, and Nikolina Basic-Jukic. 2025. "Oral Nutritional Supplement Adherence and Nutritional Outcomes in Hemodialysis Patients—A Prospective Study" Journal of Clinical Medicine 14, no. 23: 8337. https://doi.org/10.3390/jcm14238337
APA StyleKatalinic, L., Juric, I., Atic, A., Jelakovic, B., & Basic-Jukic, N. (2025). Oral Nutritional Supplement Adherence and Nutritional Outcomes in Hemodialysis Patients—A Prospective Study. Journal of Clinical Medicine, 14(23), 8337. https://doi.org/10.3390/jcm14238337








