A Prediction Model for Instability in Adult Distal Radius Fractures: Integrating Post-Reduction and Follow-Up Indicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Participants
2.2. Outcomes and Predictors Measurement
2.3. Sample Size
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Baseline Model
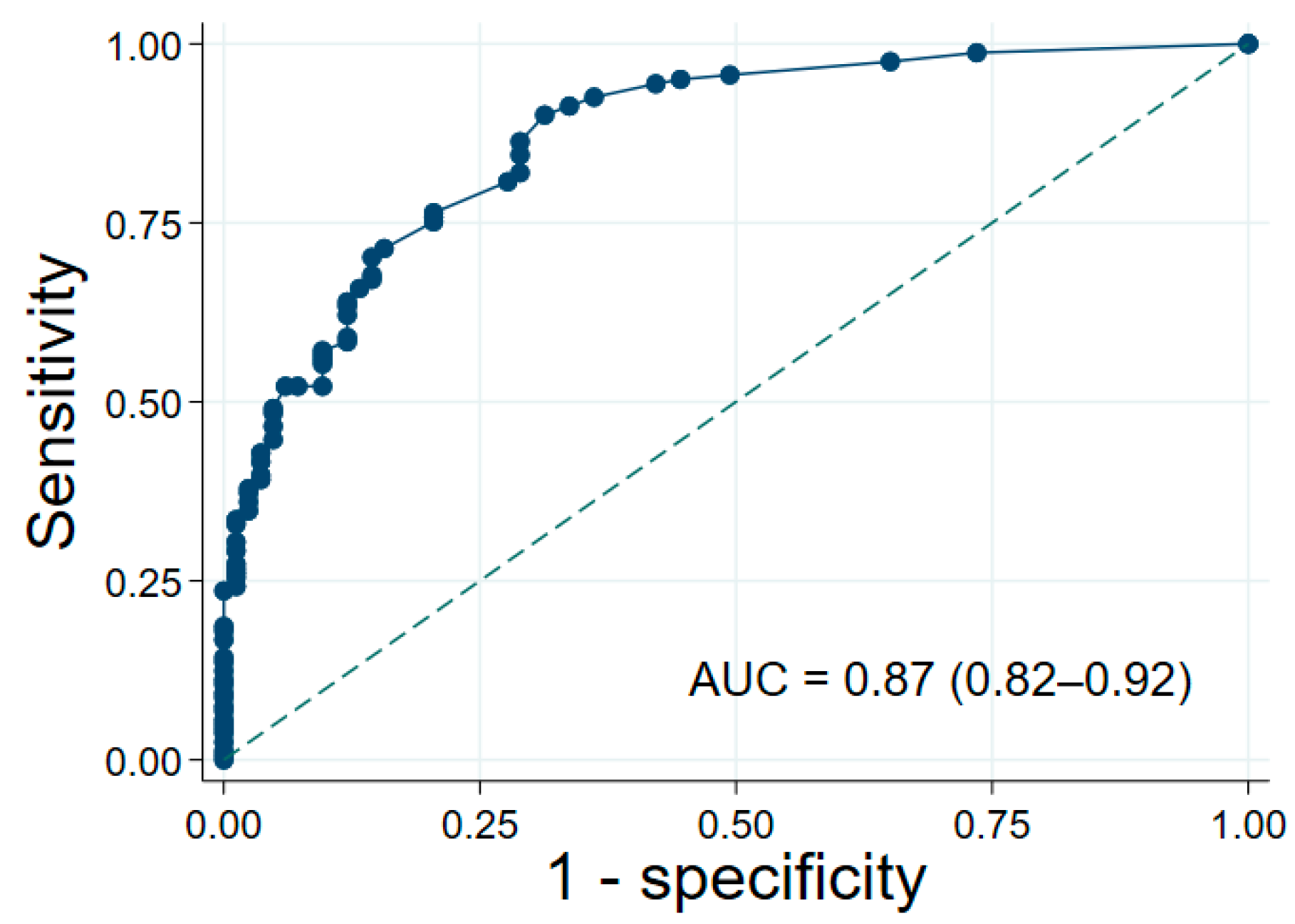
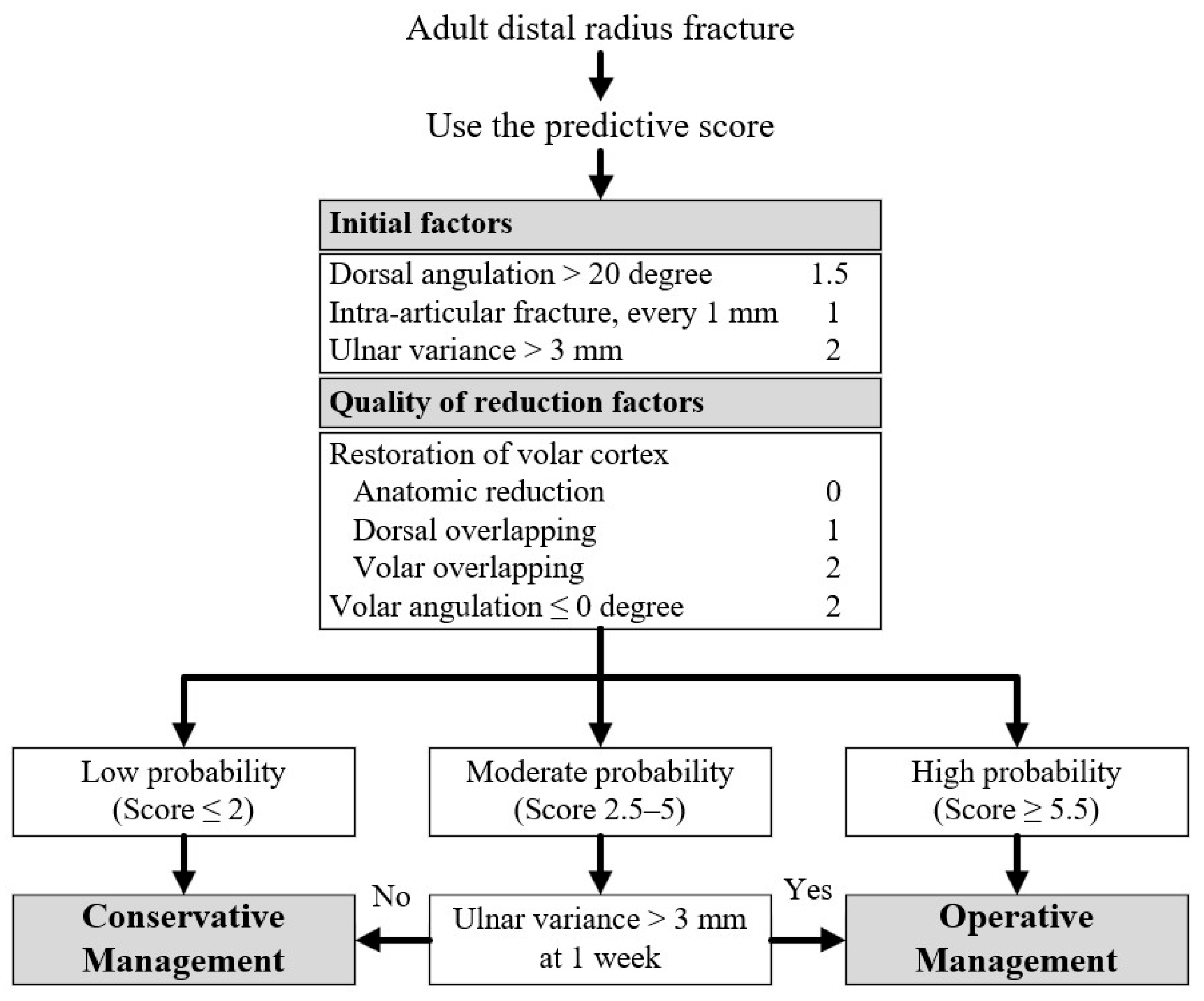
3.3. Clinical Prediction Score and Risk Stratification
3.4. One-Week Follow-Up Model
3.5. Comparison with Lafontaine Criteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2MCP | Second Metacarpal Cortical Percentage |
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| DA | Dorsal Angulation |
| DRFs | Distal Radius Fractures |
| DRUJ | Distal Radioulnar Joint |
| IAFS | Intra-articular Fracture Stepping |
| ICC | Intraclass Correlation Coefficient |
| LR+ | Positive Likelihood Ratio |
| NPV | Negative Predictive Value |
| PPV | Positive Predictive Value |
| OR | Odds Ratio |
| RI | Radial Inclination |
| RH | Radial Height |
| RS | Radial Shortening |
| RT | Radial Translation |
| UF | Ulnar Fracture |
| UV | Ulnar Variance |
References
- Pogue, D.J.; Viegas, S.F.; Patterson, R.M.; Peterson, P.D.; Jenkins, D.K.; Sweo, T.D.; Hokanson, J.A. Effects of distal radius fracture malunion on wrist joint mechanics. J. Hand Surg. 1990, 15, 721–727. [Google Scholar] [CrossRef]
- Meena, S.; Sharma, P.; Sambharia, A.; Dawar, A. Fractures of distal radius: An overview. J. Fam. Med. Prim. Care 2014, 3, 325. [Google Scholar] [CrossRef]
- Nesbitt, K.S.; Failla, J.M.; Les, C. Assessment of instability factors in adult distal radius fractures. J. Hand Surg. 2004, 29, 1128–1138. [Google Scholar] [CrossRef]
- Tahririan, M.A.; Javdan, M.; Nouraei, M.H.; Dehghani, M. Evaluation of instability factors in distal radius fractures. J. Res. Med. Sci. 2013, 18, 892–896. [Google Scholar]
- Neritan, M. Factors Predicting Late Collapse of Distal Radius Fractures. Malays. Orthop. J. 2011, 5, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, M.M.J.; Mulders, M.A.M.; Van Hilst, J.; Goslings, J.C.; Schep, N.W.L. Prediction of Distal Radius Fracture Redisplacement: A Validation Study. J. Orthop. Trauma 2018, 32, e92–e96. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, M.M.J.; Aydin, S.; Mulders, M.A.M.; Goslings, J.C.; Schep, N.W.L. Predictors of unstable distal radius fractures: A systematic review and meta-analysis. J. Hand Surg. (Eur. Vol.) 2016, 41, 501–515. [Google Scholar] [CrossRef]
- Mackenney, P.J. Prediction of Instability in Distal Radial Fractures. J. Bone Jt. Surg. 2006, 88, 1944–1951. [Google Scholar]
- Lafontaine, M.; Hardy, D.; Delince, P. Stability assessment of distal radius fractures. Injury 1989, 20, 208–210. [Google Scholar] [CrossRef]
- Alemdaroǧlu, K.B.; Iltar, S.; Aydoǧan, N.H.; Say, F.; Kilinç, C.Y.; Tiftikçi, U. Three-point index in predicting redisplacement of extra-articular distal radial fractures in adults. Injury 2010, 41, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.K.; Thomas, F.; Kaplan, D.; Liporace, F.; Paksima, N.; Koval, K.J. An evaluation of two scoring systems to predict instability in fractures of the distal radius. J. Trauma Acute Care Surg. 2004, 57, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- LaMartina, J.; Jawa, A.; Stucken, C.; Merlin, G.; Tornetta, P. Predicting Alignment After Closed Reduction and Casting of Distal Radius Fractures. J. Hand Surg. 2015, 40, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Ghodasra, J.H.; Yousaf, I.S.; Sanghavi, K.K.; Rozental, T.D.; Means, K.R.; Giladi, A.M. Assessing the Relationship Between Bone Density and Loss of Reduction in Nonsurgical Distal Radius Fracture Treatment. J. Hand Surg. 2021, 46, 377–385.e2. [Google Scholar] [CrossRef]
- Hove, L.M.; Solheim, E.; Skjeie, R.; Sorensen, F.K. Prediction of Secondary Displacement in Colles’ Fracture. J. Hand Surg. 1994, 19, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Yamazaki, H.; Hayashi, M.; Isobe, F.; Kitamura, Y. The Predictive Factors of Displacement of Adult Distal End Radius Fracture Treated with Casting. J. Hand Surg. (Asian-Pac. Vol.) 2021, 26, 525–534. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Jenkins, N.H. The unstable Colles’ fracture. J. Hand. Surg. 1989, 14, 149–154. [Google Scholar]
- Walenkamp, M.; Vos, L.; Strackee, S.; Goslings, J.; Schep, N. The Unstable Distal Radius Fracture—How Do We Define It? A Systematic Review. J. Wrist Surg. 2015, 4, 307–316. [Google Scholar] [CrossRef]
- Winayak, A.; Gossat, A.; Cooper, J.; Ritchie, P.; Lim, W.; Klim, S.; Kelly, A.-M. Do instability markers predict satisfactory reduction and requirement for later surgery in emergency department patients with wrist fracture? Emerg. Med. Australas. 2018, 30, 42–46. [Google Scholar] [CrossRef]
- Abbaszadegan, H.; Jonsson, U.; Sivers, K. Prediction of instability of Codes’ fractures. Acta Orthop. Scand. 1989, 60, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Souza, K.E.; Kellam, P.J.; Stephens, A.R.; Kazmers, N.H. Evaluation of Risk Factors for Loss of Acceptable Alignment for Distal Radius Fractures That Are Nondisplaced or Minimally Displaced on Initial Presentation. J. Hand Surg. 2022, 47, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.S.; Martyn, T.L.B.; Rao, K.S.; MacLean, S.B.M. The Volar Cortical Hinge: An Independent Risk Factor for Distal Radius Fracture Displacement. J. Wrist Surg. 2024, 13, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Chun, K.J.; Kim, J.Y.; Lee, J.S. Necessity of acceptable radiologic alignment by preoperative closed reduction for unstable distal radius fractures treated with volar locking plates. Eur. J. Trauma Emerg. Surg. 2021, 47, 1881–1887. [Google Scholar] [CrossRef]
- Dissaneewate, P.; Thanavirun, P.; Tangjaroenpaisan, Y.; Dissaneewate, K. External validation and revision of the Lafontaine criteria for unstable distal radius fractures: A retrospective study. J. Orthop. Surg. Res. 2025, 20, 146. [Google Scholar] [CrossRef]

| Factors | Total (n = 244) | Stability (n = 83) | Instability (n = 161) | p Valve |
|---|---|---|---|---|
| Center, n (%) | ||||
| Rajavithi hospital | 215 (88.1) | 68 (81.9) | 147 (91.3) | 0.03 |
| Tharongchang hospital | 29 (11.9) | 15 (18.1) | 14 (8.7) | |
| Female, n (%) | 184 (75.4) | 67 (80.7) | 117 (72.7) | 0.17 |
| Occupation, n (%) | ||||
| Labor | 70 (28.7) | 26 (31.3) | 44 (27.3) | 0.004 |
| Maid | 58 (23.8) | 27 (32.5) | 31 (19.3) | |
| Officer | 34 (13.9) | 14 (16.9) | 20 (12.4) | |
| Other | 82 (33.6) | 16 (19.3) | 66 (41.0) | |
| Dominant hand, n (%) | ||||
| Right | 239 (98.0) | 81 (97.6) | 158 (98.1) | 1.00 |
| Left | 5 (2.0) | 2 (2.4) | 3 (1.9) | |
| Injury hand, n (%) | ||||
| Right | 118 (48.4) | 40 (48.2) | 78 (48.4) | 0.97 |
| Left | 126 (51.6) | 43 (51.8) | 83 (51.6) | |
| Initial factors | ||||
| Age, year | 58.5 ± 16.7 | 55.7 ± 14.7 | 59.9 ± 17.5 | 0.06 |
| Age > 60 years, n (%) | 111 (45.5) | 31 (37.3) | 80 (49.7) | 0.07 |
| Ulnar fracture | 106 (43.4) | 31 (37.3) | 75 (46.6) | 0.17 |
| Dorsal angulation, degree | −10.5 ± 17.2 | −6.5 ± 12.7 | −12.5 ± 18.8 | 0.008 |
| Dorsal angulation > 20 degree | 71 (29.1) | 10 (12.0) | 61 (37.9) | <0.001 |
| Intra-articular fracture, n (%) | 98 (40.2) | 18 (21.7) | 80 (49.7) | <0.001 |
| Intra-articular fracture stepping *, mm | 0 [0, 2] | 0 [0, 0] | 0 [0, 2] | <0.001 |
| Dorsal comminution | 175 (71.7) | 47 (56.6) | 128 (79.5) | <0.001 |
| Ulnar variance *, mm | 3.0 [1.5, 5.0] | 2.0 [1.0, 3.0] | 4.0 [2.0, 5.0] | <0.001 |
| Ulnar variance > 3 mm | 101 (41.4) | 14 (16.9) | 87 (54.0) | <0.001 |
| 2MCP measurement ≤ 50 percentage | 72 (29.5) | 22 (26.5) | 50 (31.1) | 0.46 |
| Radial inclination, degree | 18.6 ± 7.2 | 22.3 ± 5.8 | 16.7 ± 7.1 | <0.001 |
| Radial inclination ≤ 10 degree | 32 (13.1) | 3 (3.6) | 29 (18.0) | 0.001 |
| Radial height, mm | 8.7 ± 3.3 | 10.3 ± 2.8 | 7.9 ± 3.3 | <0.001 |
| Radial height ≤ 11 mm | 198 (81.1) | 56 (67.5) | 142 (88.2) | <0.001 |
| Metaphyseal comminution | 85 (34.8) | 9 (10.8) | 76 (47.2) | <0.001 |
| Distal radioulnar joint separation | 124 (50.8) | 25 (30.1) | 99 (61.5) | <0.001 |
| Quality of reduction factors | ||||
| Restoration of volar cortex | ||||
| Anatomic type | 92 (37.7) | 48 (57.8) | 44 (27.3) | <0.001 |
| Dorsal overlapping type | 93 (38.1) | 27 (32.5) | 66 (41.0) | |
| Volar overlapping type | 59 (24.2) | 8 (9.6) | 51 (31.7) | |
| Volar angulation, degrees | 3.2 ± 10.2 | 5.1 ± 6.5 | 2.2 ± 11.6 | 0.040 |
| Volar angulation ≤ 0 degree | 88 (36.1) | 16 (19.3) | 72 (44.7) | <0.001 |
| Distal radioulnar joint separation | 63 (25.8) | 12 (14.5) | 51 (31.7) | 0.004 |
| Radial translation *, mm | 1 [0, 3] | 0 [0, 1] | 1 [0, 3] | <0.001 |
| Three-point molding distance, mm | −1.9 ± 15.0 | −2.2 ± 15.3 | −1.7 ± 14.8 | 0.79 |
| Factors at 1 week | ||||
| Ulnar variance *, mm | 2.0 [1.0, 3.0] | 1.0 [0.0, 2.0] | 3.0 [1.0, 4.0] | <0.001 |
| Ulnar variance > 3 mm | 59 (24.2) | 1 (1.2) | 58 (36.0) | <0.001 |
| Volar angulation, degree | 1.3 ± 10.8 | 4.1 ± 7.0 | −0.1 ± 12.1 | 0.004 |
| Volar angulation ≤ 0 degree | 103 (42.2) | 19 (22.9) | 84 (52.2) | <0.001 |
| Prognostic Factors | OR (95% CI) | p Valve |
|---|---|---|
| Initial factors | ||
| Age, every 1 year | 1.02 (1.00–1.03) | 0.07 |
| Ulnar fracture | 1.46 (0.85–2.52) | 0.17 |
| Dorsal angulation > 20 degree | 4.45 (2.14–9.27) | <0.001 |
| Intra-articular fracture stepping, every 1 mm | 1.95 (1.43–2.67) | <0.001 |
| Dorsal comminution | 2.97 (1.67–5.30) | <0.001 |
| Ulnar variance > 3 mm | 5.79 (3.02–11.13) | <0.001 |
| 2MCP measurement ≤ 50 percentage | 1.25 (0.69–2.25) | 0.46 |
| Quality of reduction factors | ||
| Restoration of volar cortex | ||
| Anatomic reduction | Reference | - |
| Dorsal overlapping | 2.67 (1.45–4.89) | 0.002 |
| Volar overlapping | 6.95 (2.97–16.27) | <0.001 |
| Volar angulation ≤ 0 degree | 3.39 (1.81–6.35) | <0.001 |
| DRUJ separation | 2.74 (1.37–5.50) | 0.004 |
| Radial translation | 1.59 (1.29–1.97) | <0.001 |
| Three-point molding distance | 1.00 (0.98–1.02) | 0.79 |
| Prognostic Factors | OR (95% CI) | p Valve | Coefficient | Score |
|---|---|---|---|---|
| Initial factors | ||||
| Dorsal angulation > 20 degree | 3.74 (1.56–8.96) | 0.003 | 1.32 | 1.5 |
| Intra-articular fracture *, every 1 mm | 2.27 (1.51–3.41) | <0.001 | 0.82 | 1 |
| Ulnar variance > 3 mm | 5.97 (2.77–12.88) | <0.001 | 1.79 | 2 |
| Quality of reduction factors | ||||
| Restoration of volar cortex | ||||
| Anatomic reduction | Reference | - | Reference | 0 |
| Dorsal overlapping | 2.34 (1.10–4.98) | 0.03 | 0.85 | 1 |
| Volar overlapping | 4.35 (1.59–11.89) | 0.004 | 1.47 | 2 |
| Volar angulation ≤ 0 degree | 4.78 (2.20–10.36) | <0.001 | 1.56 | 2 |
| Instability Score | Probability Category | LR+ | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| ≤2 | Low | 0.19 | 13.7% | 28.9% | 27.2% | 14.7% |
| 2.5–5 | Moderate | 1.84 | 46.6% | 74.7% | 78.1% | 41.9% |
| ≥5.5 | High | 11.00 | 39.8% | 96.4% | 95.5% | 45.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khajonvittayakul, N.; Supichyangur, K.; Apivatgaroon, A.; Tantiyavarong, P. A Prediction Model for Instability in Adult Distal Radius Fractures: Integrating Post-Reduction and Follow-Up Indicators. J. Clin. Med. 2025, 14, 8336. https://doi.org/10.3390/jcm14238336
Khajonvittayakul N, Supichyangur K, Apivatgaroon A, Tantiyavarong P. A Prediction Model for Instability in Adult Distal Radius Fractures: Integrating Post-Reduction and Follow-Up Indicators. Journal of Clinical Medicine. 2025; 14(23):8336. https://doi.org/10.3390/jcm14238336
Chicago/Turabian StyleKhajonvittayakul, Nuttapol, Kittiwan Supichyangur, Adinun Apivatgaroon, and Pichaya Tantiyavarong. 2025. "A Prediction Model for Instability in Adult Distal Radius Fractures: Integrating Post-Reduction and Follow-Up Indicators" Journal of Clinical Medicine 14, no. 23: 8336. https://doi.org/10.3390/jcm14238336
APA StyleKhajonvittayakul, N., Supichyangur, K., Apivatgaroon, A., & Tantiyavarong, P. (2025). A Prediction Model for Instability in Adult Distal Radius Fractures: Integrating Post-Reduction and Follow-Up Indicators. Journal of Clinical Medicine, 14(23), 8336. https://doi.org/10.3390/jcm14238336








