Clinical Outcomes Associated with Statin Use in Pulmonary Embolism: A Systematic Review of Observational Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy, Study Selection, and Data Extraction
2.2. Quality Assessment and Risk of Bias
2.3. Data Synthesis and Analysis
3. Results
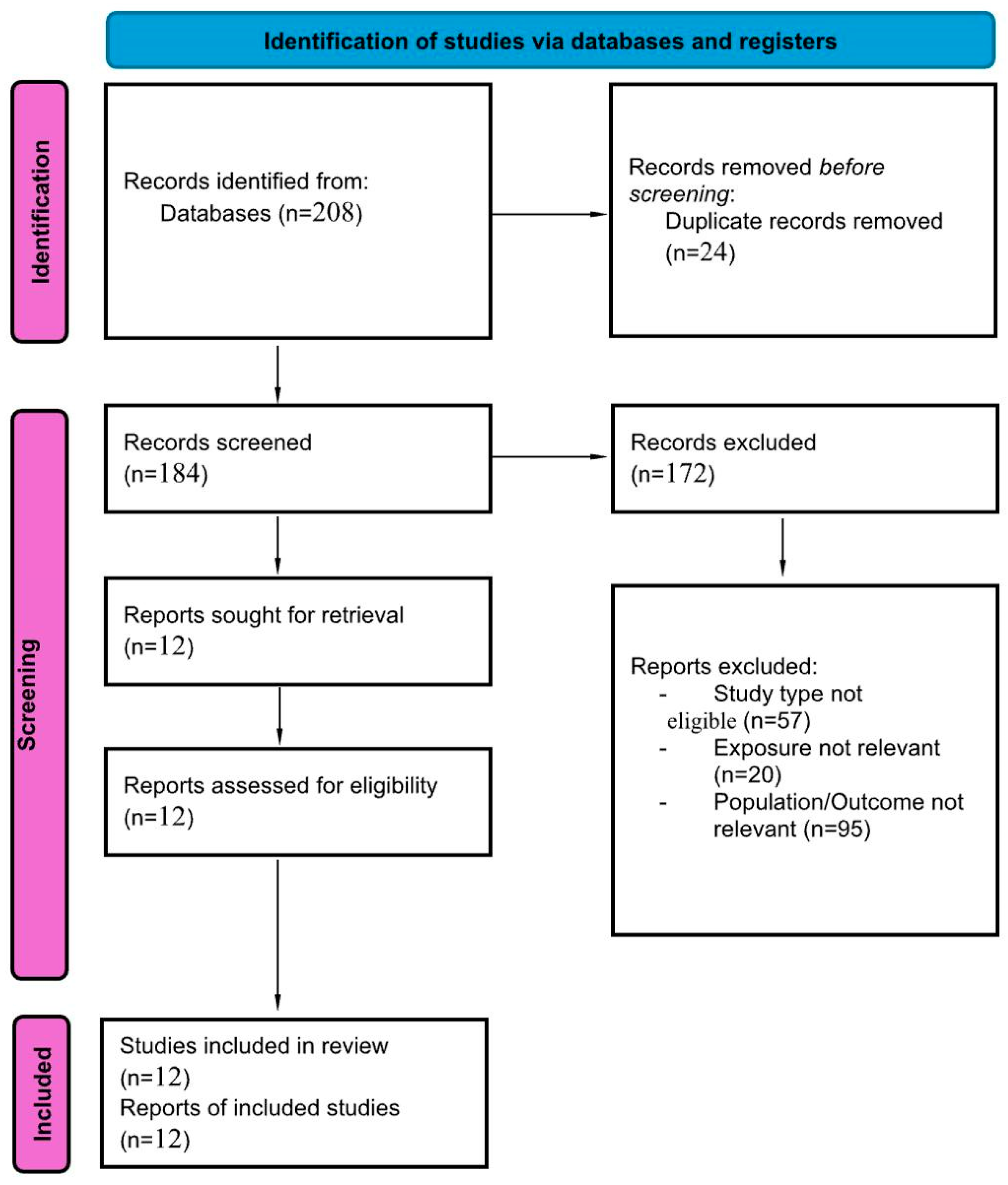
3.1. Search Results and Study Characteristics
3.2. Summary of the Included Studies
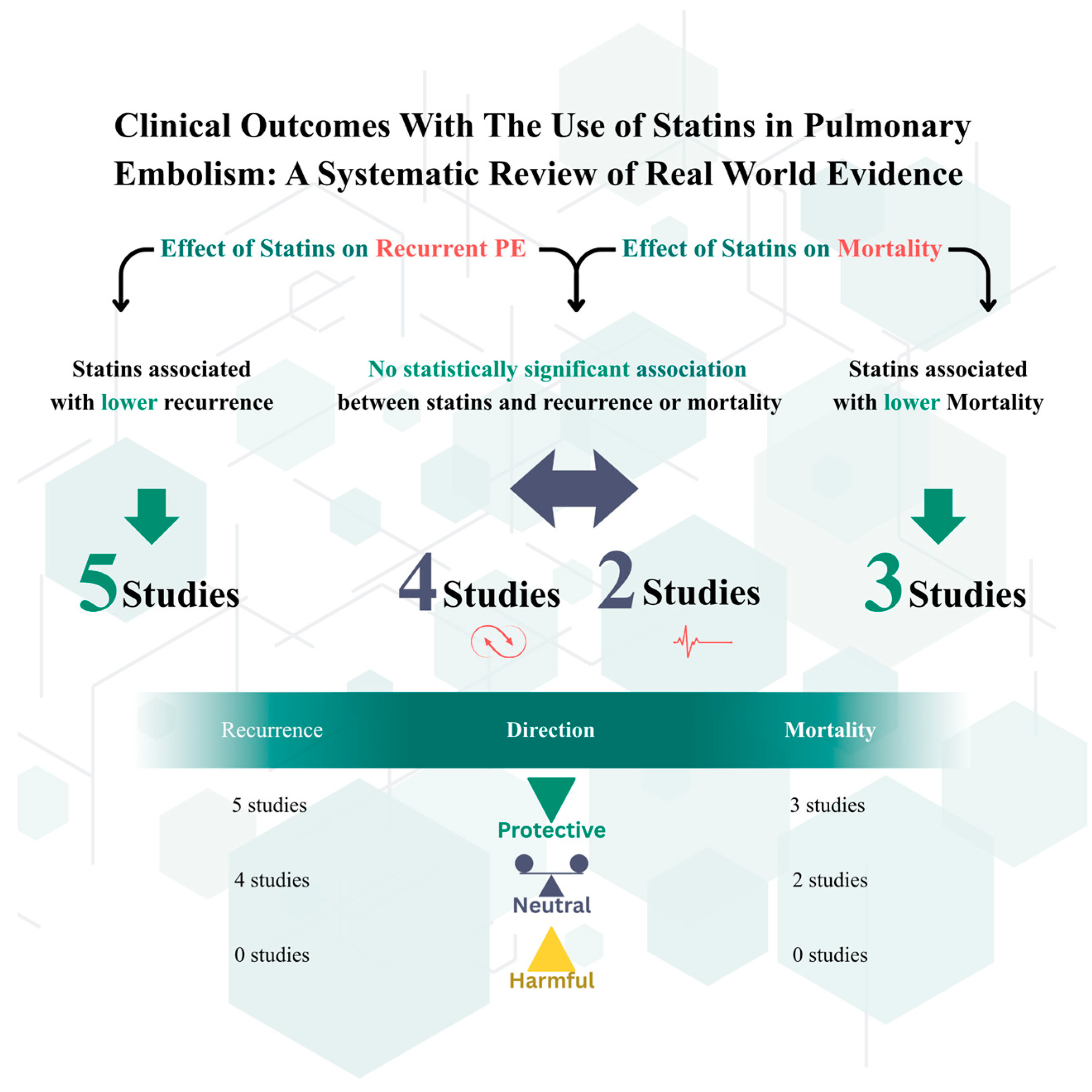
3.3. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | Pulmonary Embolism |
| VTE | Venous Thromboembolism |
| DVT | Deep Vein Thrombosis |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl-Coenzyme A |
| NOS | Newcastle–Ottawa Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| HR | Hazard Ratio |
| aHR | Adjusted Hazard Ratio |
| OR | Odds Ratio |
| IRR | Incidence Rate Ratio |
| CI | Confidence Interval |
| ICD | International Classification of Diseases |
| BMI | Body Mass Index |
| ESC | European Society of Cardiology |
| WHO | World Health Organization |
| RCT | Randomized Controlled Trial |
| RIETE | Registro Informatizado de la Enfermedad TromboEmbólica (Computerized Registry of Patients with Venous Thromboembolism) |
| HOPE-3 | Heart Outcomes Prevention Evaluation-3 Trial |
| JUPITER | Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin |
| CRD | Centre for Reviews and Dissemination (used in PROSPERO IDs, e.g., CRD420251166536) |
| CVD | Cardiovascular Disease |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| PSM | Propensity Score Matching |
| ESC-2019 | 2019 European Society of Cardiology Guidelines for Pulmonary Embolism |
| Eur. Heart J. | European Heart Journal |
| Am. J. Med. | American Journal of Medicine |
| BMJ | British Medical Journal |
| N. Engl. J. Med. | New England Journal of Medicine |
References
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A Major Contributor to Global Disease Burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism. Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Klok, F.A.; van der Hulle, T.; den Exter, P.L.; Lankeit, M.; Huisman, M.V.; Konstantinides, S. The Post-PE Syndrome: A New Concept for Chronic Complications of Pulmonary Embolism. Blood Rev. 2014, 28, 221–226. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ Collaboration. Efficacy and Safety of Statin Therapy in Older People: A Meta-Analysis of Individual Participant Data from 28 Randomised Controlled Trials. Lancet 2019, 393, 407–415. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Liao, J.K.; Laufs, U. Pleiotropic Effects of Statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Statins and Blood Coagulation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Calvieri, C.; Ferro, D.; Pignatelli, P. Statins as Antithrombotic Drugs. Circulation 2013, 127, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Glynn, R.J.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. A Randomized Trial of Rosuvastatin in the Prevention of Venous Thromboembolism. N. Engl. J. Med. 2009, 360, 1851–1861. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Seidu, S.; Khunti, K. Statins and Secondary Prevention of Venous Thromboembolism: Pooled Analysis of Observational Cohort Studies. Eur. Heart J. 2017, 38, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Jing, Z.C. Statin Therapy in Venous Thromboembolism: How Far from Primary and Secondary Prevention? J. Thromb. Haemost. 2022, 20, 1781–1783. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, C.; Muriel, A.; Suriñach Caralt, J.M.; Bikdeli, B.; Jiménez, D.; Lobo, J.L.; Amado, C.; Gil-Díaz, A.; Imbalzano, E.; Monreal, M.; et al. Statin Use and 30-Day Mortality in Patients with Acute Symptomatic Pulmonary Embolism. J. Thromb. Haemost. 2022, 20, 1839–1851. [Google Scholar] [CrossRef]
- Siniscalchi, C.; Bikdeli, B.; Jiménez, D.; Suriñach, J.M.; Demelo-Rodríguez, P.; Moustafa, F.; Gil-Díaz, A.; García-Ortega, A.; Bui, H.M.; Monreal, M.; et al. Statin Use and Mortality in Patients with Deep Vein Thrombosis: Data from the RIETE Registry. Thromb. Res. 2024, 236, 88–96. [Google Scholar] [CrossRef]
- Biere-Rafi, S.; Hutten, B.A.; Squizzato, A.; Ageno, W.; Souverein, P.C.; de Boer, A.; Gerdes, V.E.; Büller, H.R.; Kamphuisen, P.W. Statin Treatment and the Risk of Recurrent Pulmonary Embolism. Eur. Heart J. 2013, 34, 1800–1806. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Andersson, C.; Jensen, T.B.; Gjesing, A.; Schjerning Olsen, A.M.; Malta Hansen, C.; Büller, H.; Torp-Pedersen, C.; Gislason, G.H. Statin Treatment and Risk of Recurrent Venous Thromboembolism: A Nationwide Cohort Study. BMJ Open 2013, 3, e003135. [Google Scholar] [CrossRef]
- Schmidt, M.; Cannegieter, S.C.; Johannesdottir, S.A.; Dekkers, O.M.; Horváth-Puhó, E.; Sørensen, H.T. Statin Use and Venous Thromboembolism Recurrence: A Combined Nationwide Cohort and Nested Case-Control Study. J. Thromb. Haemost. 2014, 12, 1207–1215. [Google Scholar] [CrossRef]
- Smith, N.L.; Harrington, L.B.; Blondon, M.; Wiggins, K.L.; Floyd, J.S.; Sitlani, C.M.; McKnight, B.; Larson, E.B.; Rosendaal, F.R.; Heckbert, S.R.; et al. The Association of Statin Therapy with the Risk of Recurrent Venous Thrombosis. J. Thromb. Haemost. 2016, 14, 1384–1392. [Google Scholar] [CrossRef]
- Brækkan, S.K.; Caram-Deelder, C.; Siegerink, B.; van Hylckama Vlieg, A.; le Cessie, S.; Rosendaal, F.R.; Cannegieter, S.C.; Lijfering, W.M. Statin Use and Risk of Recurrent Venous Thrombosis: Results from the MEGA Follow-Up Study. Res. Pract. Thromb. Haemost. 2017, 1, 112–119. [Google Scholar] [CrossRef]
- Hsu, C.; Brahmandam, A.; Brownson, K.E.; Huynh, N.; Reynolds, J.; Lee, A.I.; Fares, W.H.; Ochoa Chaar, C.I. Statin Therapy Associated with Improved Thrombus Resolution in Patients with Deep Vein Thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 169–175.e4. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Sarmiento, E.J.; Kline, J.A. Statin Use Is Associated with Reduced Risk of Recurrence in Patients with Venous Thromboembolism. Am. J. Med. 2020, 133, 930–935.e8. [Google Scholar] [CrossRef]
- Wang, L.; Shu, T.; Wang, W.; Chen, H.; Feng, P.; Xiang, R.; Huang, W. Association of Statin Use and the Risk of Recurrent Pulmonary Embolism in Real-World Chinese Population. Pulm. Circ. 2021, 11, 20458940211035006. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, H.; Harada, K.; Nishimura, Y.; Yamamoto, M.; Nishimura, S.; Yamamoto, M.; Niimura, T.; Osaki, Y.; Vu, Q.T.; Fujii, M.; et al. Global Trends in Mortality Related to Pulmonary Embolism: An Epidemiological Analysis of Data from the WHO Mortality Database (2001–2023). EClinicalMedicine 2025, 86, 103389. [Google Scholar] [CrossRef] [PubMed]
- Gressenberger, P.; Wachmann, B.; Borenich, A.; Pregartner, G.; Moser, L.; Schreiber, N.; Schmid, J.; Kolesnik, E.; Silbernagel, G.; Raggam, R.B.; et al. The Impact of Statins on Pulmonary Embolism Severity: A Retrospective Data Analysis. Res. Pract. Thromb. Haemost. 2025, 9, 102982. [Google Scholar] [CrossRef]
- Yusuf, S.; Bosch, J.; Dagenais, G.; Zhu, J.; Xavier, D.; Liu, L.; Pais, P.; López-Jaramillo, P.; Leiter, L.A.; Dans, A.; et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N. Engl. J. Med. 2016, 374, 2021–2031. [Google Scholar] [CrossRef]
| Author/Year | Setting (Country/Registry) | Study Design | Total Number of Participants | Mean/Median Age (Years) | PE/Outcome Defined As | Follow-Up Duration |
|---|---|---|---|---|---|---|
| Biere-Rafi 2013 [16] | Netherlands (PHARMO Record Linkage System) | Retrospective cohort study | 3186 (acute PE 1998–2008) | (mean years ± SD) 61 ± 17 | Recurrent pulmonary embolism confirmed by hospitalization records | Median 1529 days |
| Nguyen 2013 [17] | Denmark (Nationwide Cohort) | Nationwide cohort study | 44,330 patients with VTE | (mean years ± SD) 62 ± 18 years | Hospitalized recurrent VTE (PE ± DVT) | Up to 13 years (1997–2009) |
| Schmidt 2014 [18] | Denmark (National Health Registries) | Combined nationwide cohort and nested case–control study. | 27,862 with first-time VTE | N/A | Recurrent VTE confirmed by discharge registry | Up to 8.5 years (2004–2012 registry window) |
| Smith 2016 [19] | United States (Single health system) | Population-based inception cohort | 2798 incident VTE | N/A | Recurrent VTE (PE or DVT) identified by ICD-9 codes | Up to 8 years (2002–2010 observation). |
| Brækkan 2017 [20] | Netherlands (MEGA follow-up study) | Prospective cohort study | 2547 first VTE | Median 48 (IQR 37–58) | Recurrent VTE confirmed by medical record and imaging | Median 5.7 years |
| Hsu 2021 [21] | USA (Single-center hospital) | Retrospective single-center cohort | 3097 patients with confirmed PE (522 [16.9%] on statins prior to admission) | (mean years ± SD) 69 ± 13 | In-hospital mortality, short-term complications | During hospitalization |
| Stewart 2020 [22] | USA (Indiana statewide records) | Retrospective registry analysis | 192,908 DVT or PE | Mean 67 | Recurrent VTE via hospitalization data | Up to 13 years (2004–2017) |
| Wang 2021 [23] | China | Retrospective cohort study | 365 patients with an ICD-confirmed diagnosis of pulmonary embolism (PE) | Median (IQR) 75.0 (66.0, 81.5) | Recurrent PE | Median 19.2 months (interquartile range: 10.6–26.2 months) |
| Siniscalchi 2022 [14] | International (RIETE Registry) | Prospective registry | 31,169 acute PE | (mean years ± SD) 75 ± 11 74 ± 11 74 ± 11 Low, moderate and high intensity respectively | 30-day all-cause mortality | 30 days |
| Siniscalchi 2024 [15] | International (RIETE Registry) | Prospective registry | 46,440 isolated DVT | (mean years ± SD) 72 ± 12 | 3-month mortality | 3 months |
| Hagiya 2025 [24] | Global (WHO mortality database) | Epidemiological analysis | 1,550,883 | N/A | Global PE-related mortality | 22 years (2001–2023) |
| Gressenberger 2025 [25] | Austria (Univ. Hospital Graz) | Retrospective data analysis | 1590 acute PE | 74 years [IQR, 66–80] | PE severity (ESC 2019 criteria) | 30 days and 2 years |
| Study (Author, Year) | Selection (0–4) | Comparability (0–2) | Outcome (0–3) | Total (0–9) | Quality |
|---|---|---|---|---|---|
| Biere-Rafi, 2013 [16] | ★★★ | ★★ | ★★ | 7/9 | Good |
| Nguyen, 2013 [17] | ★★★★ | ★★ | ★★★ | 9/9 | High |
| Schmidt, 2014 [18] | ★★★★ | ★★ | ★★ | 8/9 | Good |
| Smith, 2016 [19] | ★★★★ | ★★ | ★★ | 8/9 | Good |
| Brækkan, 2017 [20] | ★★★ | ★★ | ★★ | 7/9 | Good |
| Hsu, 2019 [21] | ★★★ | ★★ | ★★ | 7/9 | Good |
| Stewart, 2020 [22] | ★★★ | ★ | ★★ | 6/9 | Fair |
| Wang, 2021 [23] | ★★★ | ★ | ★★ | 6/9 | Fair |
| Siniscalchi, 2022 [14] | ★★★ | ★ | ★★ | 6/9 | Fair |
| Siniscalchi, 2024 [15] | ★★★ | ★ | ★★ | 6/9 | Fair |
| Gressenberger, 2025 [25] | ★★★ | ★ | ★★ | 6/9 | Fair |
| Global Trends, 2025 [24] | ★★ | ★ | ★★ | 5/9 | Fair (ecological, population-level, not patient data) |
| Outcome | Study (Author, Year) | Effect Estimate (95% CI) | p-Value |
|---|---|---|---|
| Recurrent VTE (PE/DVT) | Biere-Rafi (2013) [16] | aHR 0.50 (0.36–0.70) | |
| Nguyen (2013) [17] | HR 0.74 (0.68–0.79) | ||
| Schmidt (2014) [18] | aHR 0.72 (0.59–0.88) | ||
| Smith (2016) [19] | HR 0.74 (0.59–0.94) | ||
| Smith (2016, no-CVD) [19] | HR 0.62 (0.45–0.85) | ||
| Smith (2016, with CVD) [19] | HR 1.10 (0.70–1.70) | ||
| Brækkan (2017) [20] | HR 0.78 (0.46–1.31) | ||
| Stewart (2020) [22] | OR 0.66 (0.64–0.69) | ||
| Stewart (2020, PSM) [22] | OR 0.75 (0.72–0.79) | ||
| Wang (2021) [23] | OR 1.06 (0.52–2.09) | ||
| Wang (2021, PSM) [23] | OR 0.49 (0.19–1.26) | (p = 0.138) | |
| Siniscalchi (2022) * [14] | 198 events—effect estimate not reported | ||
| Siniscalchi (2024) [15] | 1.7% vs. 1.6%) | (p = 0.49) | |
| Recurrent DVT | Schmidt (2014) [18] | aHR 0.64 (0.49–0.84) | |
| Brækkan (2017) [20] | HR 0.66 (0.29–1.48) | ||
| Siniscalchi (2024) [15] | 0.91% vs. 0.89%) | (p = 0.81) | |
| Hsu (2019) [21] | 15% vs. 17% (NS) | ||
| Recurrent PE | Biere-Rafi (2013) [16] | aHR 0.50 (0.36–0.70) | |
| Schmidt (2014) [18] | aHR 0.82 (0.62–1.09) | ||
| Wang (2021) [23] | OR 1.06 (0.52–2.09) | ||
| Siniscalchi (2024) [15] | 0.77% vs. 0.70% | (p = 0.005) | |
| 30-Day/Short-Term Mortality | Siniscalchi (2022) [14] | OR 0.65 (0.56–0.76) | p < 0.001 |
| Siniscalchi (2022, Fatal PE) [14] | OR 0.42 (0.28–0.62) | ||
| Siniscalchi (2022, Low statin) [14] | OR 0.51 (0.34–0.77) | ||
| Siniscalchi (2022, Moderate) [14] | OR 0.68 (0.57–0.81) | ||
| Siniscalchi (2022, High) [14] | OR 0.68 (0.51–0.92) | ||
| Gressenberger (2025) [25] | HR 0.85 (0.42–1.72) | ||
| Hsu (2019) [21] | OR 0.79 (0.49–1.27) | ||
| Siniscalchi (2024) [15] | aHR 0.77 (0.69–0.86) | ||
| All-Cause/Long-Term Mortality | Biere-Rafi (2013) [16] | aHR 0.53 (0.41–0.69) | |
| Hagiya (2025) [24] | 3.49 → 2.42 deaths/100,000 (2001–2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshahrani, W.A.; Alshehri, A.M.; Al Yami, M.S. Clinical Outcomes Associated with Statin Use in Pulmonary Embolism: A Systematic Review of Observational Studies. J. Clin. Med. 2025, 14, 8333. https://doi.org/10.3390/jcm14238333
Alshahrani WA, Alshehri AM, Al Yami MS. Clinical Outcomes Associated with Statin Use in Pulmonary Embolism: A Systematic Review of Observational Studies. Journal of Clinical Medicine. 2025; 14(23):8333. https://doi.org/10.3390/jcm14238333
Chicago/Turabian StyleAlshahrani, Walaa A., Abdulmajeed M. Alshehri, and Majed S. Al Yami. 2025. "Clinical Outcomes Associated with Statin Use in Pulmonary Embolism: A Systematic Review of Observational Studies" Journal of Clinical Medicine 14, no. 23: 8333. https://doi.org/10.3390/jcm14238333
APA StyleAlshahrani, W. A., Alshehri, A. M., & Al Yami, M. S. (2025). Clinical Outcomes Associated with Statin Use in Pulmonary Embolism: A Systematic Review of Observational Studies. Journal of Clinical Medicine, 14(23), 8333. https://doi.org/10.3390/jcm14238333






