Age and Witnessed Apneas as Independent Predictors of Obstructive Sleep Apnea After Stroke: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
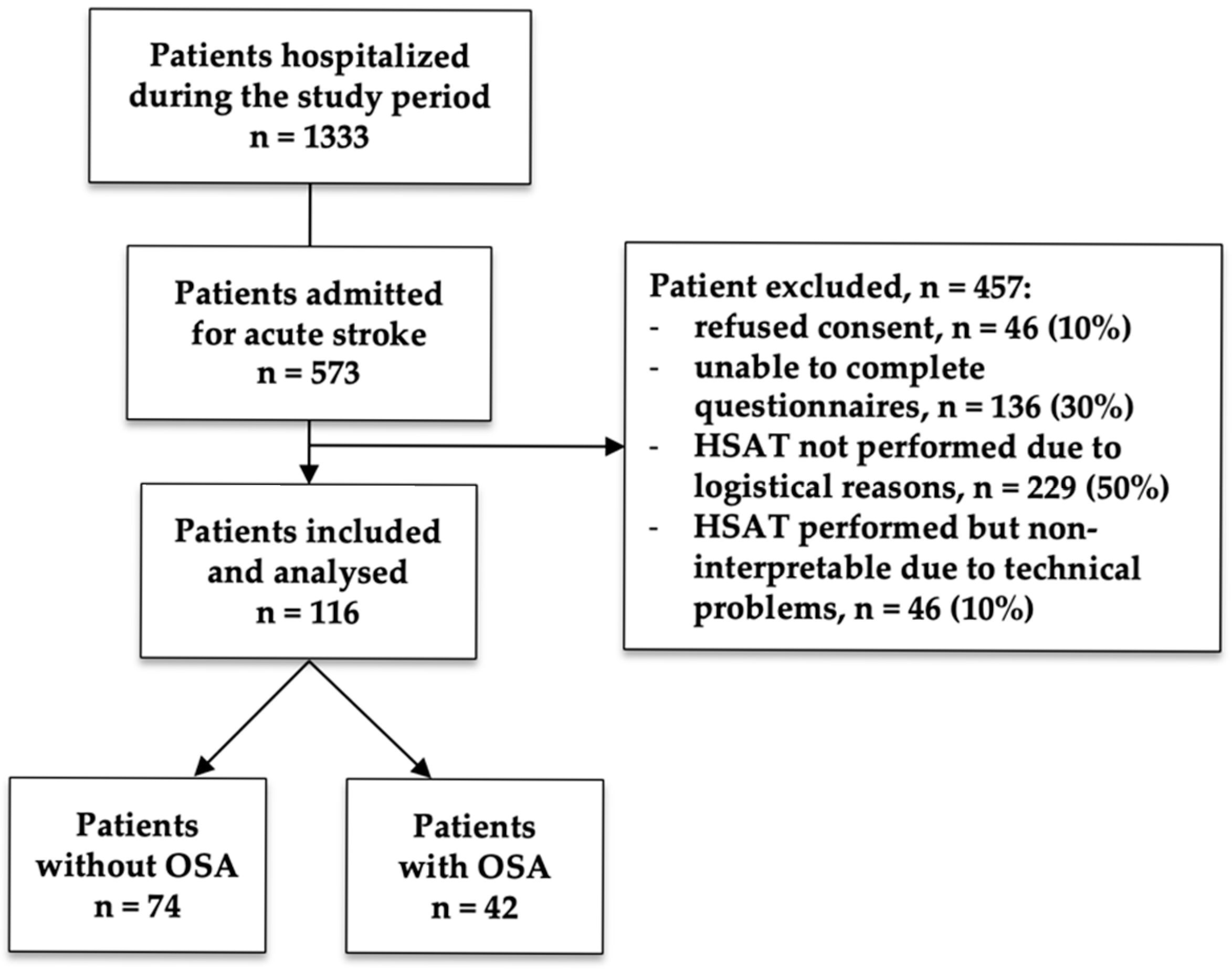
2.1. Study Design and Population
2.2. Clinical Assessment
2.3. Sleep Assessment
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Stroke Characteristics
3.3. Sleep Assessment
3.4. Multivariate Analysis
3.5. ROC Analysis
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Literature
4.3. Clinical Implications
4.4. Limitations
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSA | Obstructive sleep apnea |
| CPAP | Continuous positive airway pressure |
| PSG | Polysomnography |
| HSAT | Home sleep apnea testing |
| BQ | Berlin Questionnaire |
| ESS | Epworth Sleepiness Scale |
| TIA | Transient ischemic attack |
| BMI | Body mass index |
| NIHSS | National Institutes of Health Stroke Scale |
| mRS | Modified Rankin Scale |
| PFO | Patent foramen ovale |
| AF | Atrial fibrillation |
| ESUS | Embolic stroke of undetermined source |
| AASM | American Academy of Sleep Medicine |
| AHI | Apnea–hypopnea index |
| ODI | Oxygen desaturation index |
| T90% | Percentage of total recording time spent with SpO2 < 90% |
| SD | Standard deviation |
| OR | Odds ratio |
| CI | Confidence interval |
| ROC | Receiver operating characteristic |
| AUC | Areas under the curve |
References
- Brown, D.L.; Gibbs, R.; Shi, X.; Case, E.; Chervin, R.; Lisabeth, L.D. Abstract P597: Growing Prevalence of Post-Stroke Sleep-Disordered Breathing. Stroke 2021, 52, AP597. [Google Scholar] [CrossRef]
- Aaronson, J.A.; Hofman, W.F.; Van Bennekom, C.A.M.; Van Bezeij, T.; Van Den Aardweg, J.G.; Groet, E.; Kylstra, W.A.; Schmand, B. Effects of Continuous Positive Airway Pressure on Cognitive and Functional Outcome of Stroke Patients with Obstructive Sleep Apnea: A Randomized Controlled Trial. J. Clin. Sleep Med. 2016, 12, 533–541. [Google Scholar] [CrossRef]
- Johnson, K.G.; Johnson, D.C. Frequency of Sleep Apnea in Stroke and TIA Patients: A Meta-Analysis. J. Clin. Sleep Med. 2010, 6, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, J.A.; Van Bennekom, C.A.M.; Hofman, W.F.; Van Bezeij, T.; Van Den Aardweg, J.G.; Groet, E.; Kylstra, W.A.; Schmand, B. Obstructive Sleep Apnea Is Related to Impaired Cognitive and Functional Status after Stroke. Sleep 2015, 38, 1431–1437. [Google Scholar] [CrossRef]
- Brown, D.L.; Shafie-Khorassani, F.; Kim, S.; Chervin, R.D.; Case, E.; Morgenstern, L.B.; Yadollahi, A.; Tower, S.; Lisabeth, L.D. Sleep-Disordered Breathing Is Associated With Recurrent Ischemic Stroke. Stroke 2019, 50, 571–576. [Google Scholar] [CrossRef]
- Dharmakulaseelan, L.; Black, S.E.; Swartz, R.H.; Murray, B.J.; Boulos, M.I. Sex Differences in Obstructive Sleep Apnea after Stroke. Can. J. Neurol. Sci. 2024, 51, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Boulos, M.I.; Dharmakulaseelan, L.; Brown, D.L.; Swartz, R.H. Trials in Sleep Apnea and Stroke: Learning From the Past to Direct Future Approaches. Stroke 2021, 52, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP Questionnaire: A Tool to Screen Patients for Obstructive Sleep Apnea. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Sico, J.J.; Yaggi, H.K.; Ofner, S.; Concato, J.; Austin, C.; Ferguson, J.; Qin, L.; Tobias, L.; Taylor, S.; Vaz Fragoso, C.A.; et al. Development, Validation, and Assessment of an Ischemic Stroke or Transient Ischemic Attack-Specific Prediction Tool for Obstructive Sleep Apnea. J. Stroke Cerebrovasc. Dis. 2017, 26, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Boulos, M.I.; Wan, A.; Im, J.; Elias, S.; Frankul, F.; Atalla, M.; Black, S.E.; Basile, V.S.; Sundaram, A.; Hopyan, J.J.; et al. Identifying Obstructive Sleep Apnea after Stroke/TIA: Evaluating Four Simple Screening Tools. Sleep Med. 2016, 21, 133–139. [Google Scholar] [CrossRef]
- Camilo, M.R.; Sander, H.H.; Eckeli, A.L.; Fernandes, R.M.F.; Dos Santos-Pontelli, T.E.G.; Leite, J.P.; Pontes-Neto, O.M. SOS Score: An Optimized Score to Screen Acute Stroke Patients for Obstructive Sleep Apnea. Sleep Med. 2014, 15, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Šiarnik, P.; Klobučníková, K.; Kollár, B.; Pirošová, M.; Malík, M.; Turčáni, P.; Sýkora, M. Sleep Apnea Prediction in Acute Ischemic Stroke (SLAPS Score): A Derivation Study. Sleep Med. 2021, 77, 23–28. [Google Scholar] [CrossRef]
- Pezzella, F.R.; Picconi, O.; De Luca, A.; Lyden, P.D.; Fiorelli, M. Development of the Italian Version of the National Institutes of Health Stroke Scale: It-NIHSS. Stroke 2009, 40, 2557–2559. [Google Scholar] [CrossRef]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver Agreement for the Assessment of Handicap in Stroke Patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Hart, R.G.; Diener, H.-C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J.; Cryptogenic Stroke/ESUS International Working Group. Embolic Strokes of Undetermined Source: The Case for a New Clinical Construct. Lancet Neurol. 2014, 13, 429–438. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 3; American Academy of Sleep Medicine: Westchester, IL, USA, 2023; ISBN 978-0-9706137-2-1. [Google Scholar]
- International Classification of Sleep Disorders ICSD-3-Text Revision, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2023; ISBN 978-0-9657220-9-4.
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- Yang, H.; Lu, S.; Yang, L. Clinical Prediction Models for the Early Diagnosis of Obstructive Sleep Apnea in Stroke Patients: A Systematic Review. Syst. Rev. 2024, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Dharmakulaseelan, L.; Boulos, M.I. Sleep Apnea and Stroke. Chest 2024, 166, 857–866. [Google Scholar] [CrossRef] [PubMed]

| All (n = 116) | AHI < 15 (n = 74) | AHI ≥ 15 (n = 42) | p-Value | |
|---|---|---|---|---|
| Age, years | 73 ± 13 | 71 ± 14 | 75 ± 10 | 0.145 |
| Female | 50 (43) | 34 (46) | 16 (38) | 0.441 |
| Body Mass Index, kg/m2 | 27 ± 5 | 26 ± 5 | 28 ± 4 | 0.093 |
| Smoking | 29 (25) | 17 (23) | 12 (29) | 0.512 |
| Hypertension | 82 (71) | 50 (68) | 32 (76) | 0.398 |
| Diabetes | 27 (23) | 17 (23) | 10 (24) | 1.000 |
| Dyslipidemia | 85 (73) | 52 (70) | 33 (79) | 0.387 |
| Atrial Fibrillation | 28 (24) | 19 (26) | 9 (21) | 0.658 |
| Coronary Artery Disease | 11 (9) | 7 (10) | 4 (10) | 1.000 |
| Heart Failure | 6 (5) | 4 (5) | 2 (5) | 1.000 |
| Chronic Kidney Disease | 4 (3) | 2 (3) | 2 (5) | 0.620 |
| All (n = 116) | AHI < 15 (n = 74) | AHI ≥ 15 (n = 42) | p-Value | |
|---|---|---|---|---|
| Recurrent Stroke | 16 (14) | 8 (11) | 8 (19) | 0.296 |
| Wake-up Stroke | 35 (30) | 24 (32) | 11 (26) | 0.533 |
| Transient Ischemic Attack | 6 (5) | 4 (5) | 2 (5) | 1.000 |
| Stroke in Young Patients | 18 (16) | 13 (18) | 5 (12) | 0.594 |
| Patent Foramen Ovale | 4 (3) | 4 (5) | 0 (0) | 0.296 |
| Extracranial Atherosclerosis | 67 (58) | 40 (54) | 27 (64) | 0.335 |
| Congruent * Extracranial Atherosclerosis | 24 (21) | 12 (16) | 12 (29) | 0.151 |
| Intracranial Stenosis | 7 (6) | 4 (5) | 3 (7) | 0.701 |
| Congruent * Intracranial Stenosis | 5 (4) | 3 (4) | 2 (5) | 1.000 |
| Embolic Stroke of Undetermined Source | 23 (20) | 14 (19) | 9 (21) | 0.814 |
| NIHSS (admission) | 2.9 ± 2.7 | 2.5 ± 2.2 | 3.6 ± 3.3 | 0.048 |
| NIHSS (discharge) | 1.4 ± 2.0 | 1.2 ± 1.5 | 1.8 ± 2.7 | 0.124 |
| mRS (pre-Stroke) | 0.4 ± 1.1 | 0.5 ± 1.3 | 0.3 ± 0.9 | 0.441 |
| mRS (admission) | 1.1 ± 1.1 | 1.1 ± 1.5 | 1.1 ± 1.4 | 0.992 |
| Hospitalization Length, days | 10.5 ± 10.2 | 9 ± 6 | 13 ± 15 | 0.070 |
| All (n = 116) | AHI < 15 (n = 74) | AHI ≥ 15 (n = 42) | p-Value | |
|---|---|---|---|---|
| Sleep Study: | ||||
| -Total recording time, min | 539 ± 102 | 533 ± 107 | 548 ± 93 | - |
| -% supine | 58 ± 31 | 55 ± 31 | 63 ± 30 | - |
| -AHI | 17 ± 17 | 6 ± 4 | 35 ± 15 | - |
| -ODI | 18 ± 18 | 7 ± 5 | 37 ± 15 | - |
| -Mean SpO2, % | 93 ± 3 | 93 ± 3 | 92 ± 2 | - |
| -Mean Desaturation Amplitude, % | 4.8 ± 3.1 | 4.1 ± 1.5 | 6.1 ± 4.6 | - |
| -T90%, % | 13 ± 21 | 6 ± 17 | 23 ± 22 | - |
| -Mean heart rate, bpm | 66 ± 11 | 65 ± 12 | 67 ± 9 | - |
| Symptoms: | ||||
| -Snoring | 66 (57) | 42 (57) | 24 (57) | 0.826 |
| -Witnessed Apneas | 9 (8) | 3 (4) | 6 (14) | 0.065 |
| -Unrefreshing Sleep | 20 (17) | 11 (15) | 9 (21) | 0.433 |
| -Fatigue | 32 (28) | 17 (23) | 15 (36) | 0.118 |
| -Sleepiness behind the wheel | 8 (7) | 6 (8) | 2 (5) | 0.711 |
| Questionnaires: | ||||
| -ESS Score | 5.7 ± 3.8 | 5.1 ± 3.7 | 6.7 ± 3.9 | 0.047 |
| -ESS > 10 | 11 (9) | 5 (7) | 6 (14) | 0.203 |
| -Positive Berlin Questionnaire | 48 (41) | 27 (36) | 21 (50) | 0.098 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age/10 | 1.64 | 1.03–2.61 | 0.036 |
| Witnessed Apneas | 6.20 | 1.31–29.22 | 0.021 |
| Sex | 0.37 | 0.13–1.07 | 0.066 |
| Body Mass Index | 1.09 | 0.98–1.21 | 0.098 |
| Hospitalization Length | 1.06 | 1.00–1.14 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figorilli, M.; Melis, M.; Cualbu, C.; Frongia, G.; Arippa, F.; Redolfi, S.; Puligheddu, M. Age and Witnessed Apneas as Independent Predictors of Obstructive Sleep Apnea After Stroke: A Prospective Cohort Study. J. Clin. Med. 2025, 14, 8332. https://doi.org/10.3390/jcm14238332
Figorilli M, Melis M, Cualbu C, Frongia G, Arippa F, Redolfi S, Puligheddu M. Age and Witnessed Apneas as Independent Predictors of Obstructive Sleep Apnea After Stroke: A Prospective Cohort Study. Journal of Clinical Medicine. 2025; 14(23):8332. https://doi.org/10.3390/jcm14238332
Chicago/Turabian StyleFigorilli, Michela, Marta Melis, Chiara Cualbu, Giulia Frongia, Federico Arippa, Stefania Redolfi, and Monica Puligheddu. 2025. "Age and Witnessed Apneas as Independent Predictors of Obstructive Sleep Apnea After Stroke: A Prospective Cohort Study" Journal of Clinical Medicine 14, no. 23: 8332. https://doi.org/10.3390/jcm14238332
APA StyleFigorilli, M., Melis, M., Cualbu, C., Frongia, G., Arippa, F., Redolfi, S., & Puligheddu, M. (2025). Age and Witnessed Apneas as Independent Predictors of Obstructive Sleep Apnea After Stroke: A Prospective Cohort Study. Journal of Clinical Medicine, 14(23), 8332. https://doi.org/10.3390/jcm14238332





