Relationships Among Non-Functional Occlusal Habits, Temporomandibular Disorder Symptoms, and Skeletal Morphology in Patients with Dentofacial Deformities
Abstract
1. Introduction
2. Patients and Methods
2.1. Inclusion Criteria
Exclusion Criteria
- a history of systemic abnormalities, facial trauma, juvenile idiopathic arthritis, or idiopathic condylar resorption
- congenital anomalies
- pituitary adenoma
- unwillingness to provide informed consent
2.2. Methods
2.2.1. Questionnaire on Non-Functional Occlusal Habits and TMD Symptoms
- Presence of non-functional occlusal habits (during waking hours only; sleep-related behaviors were not considered):
- ○
- unilateral chewing
- ○
- bruxism
- ○
- clenching
- History of TMD symptoms, scored as follows:
- ○
- limited mouth opening (2 points)
- ○
- TMJ pain (2 points)
- ○
- joint sounds (1 point).
2.2.2. Evaluation of Skeletal Morphology
2.2.3. Statistical Analysis
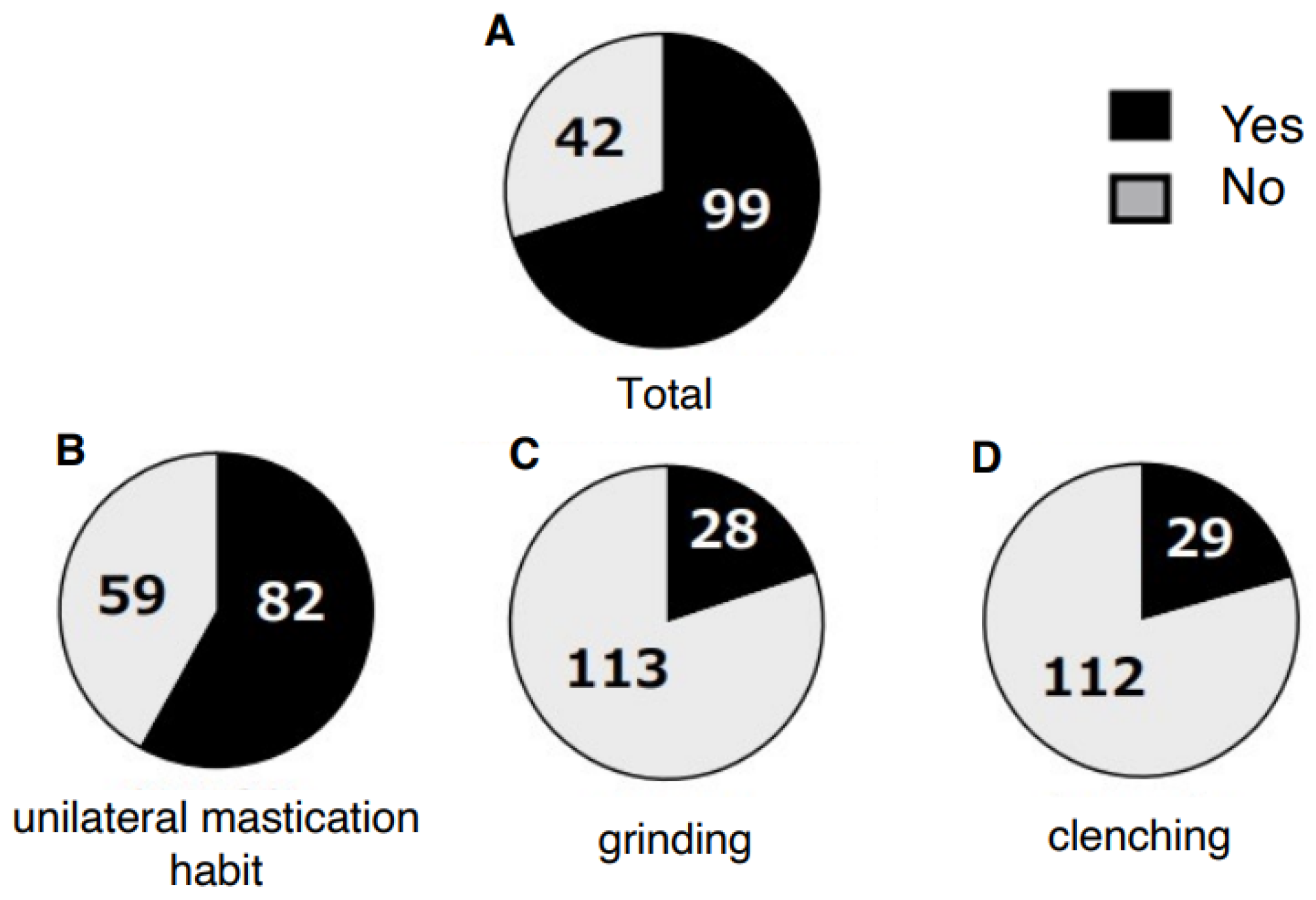
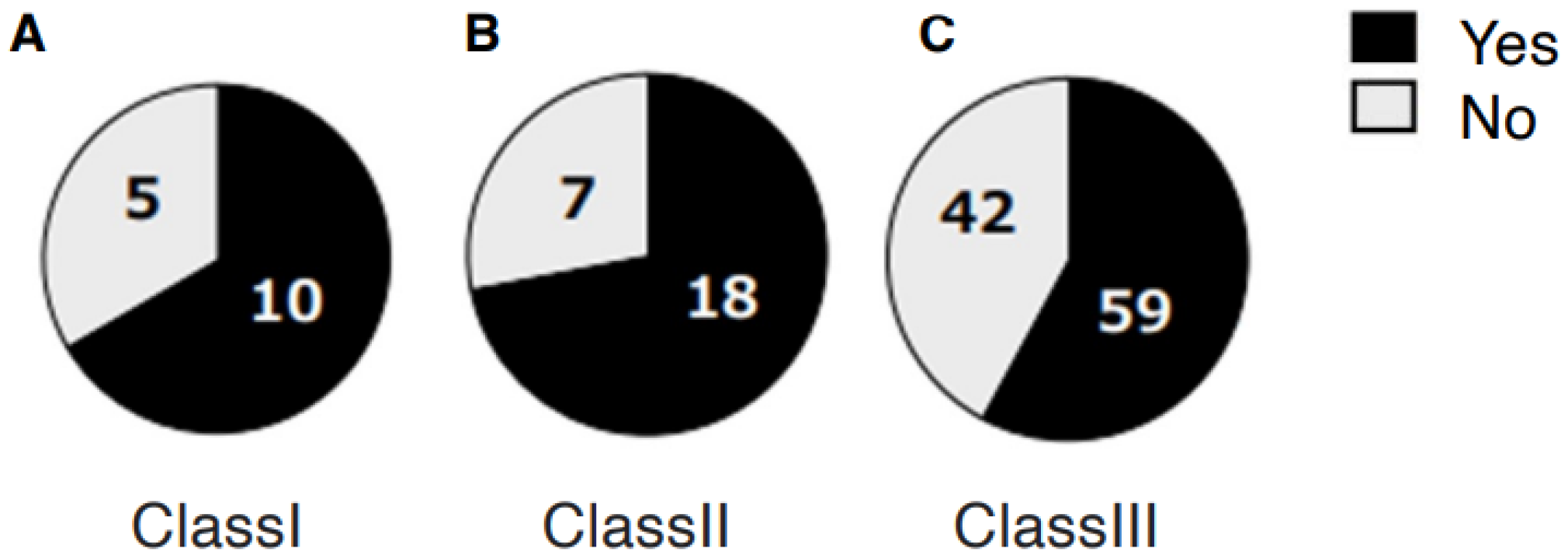
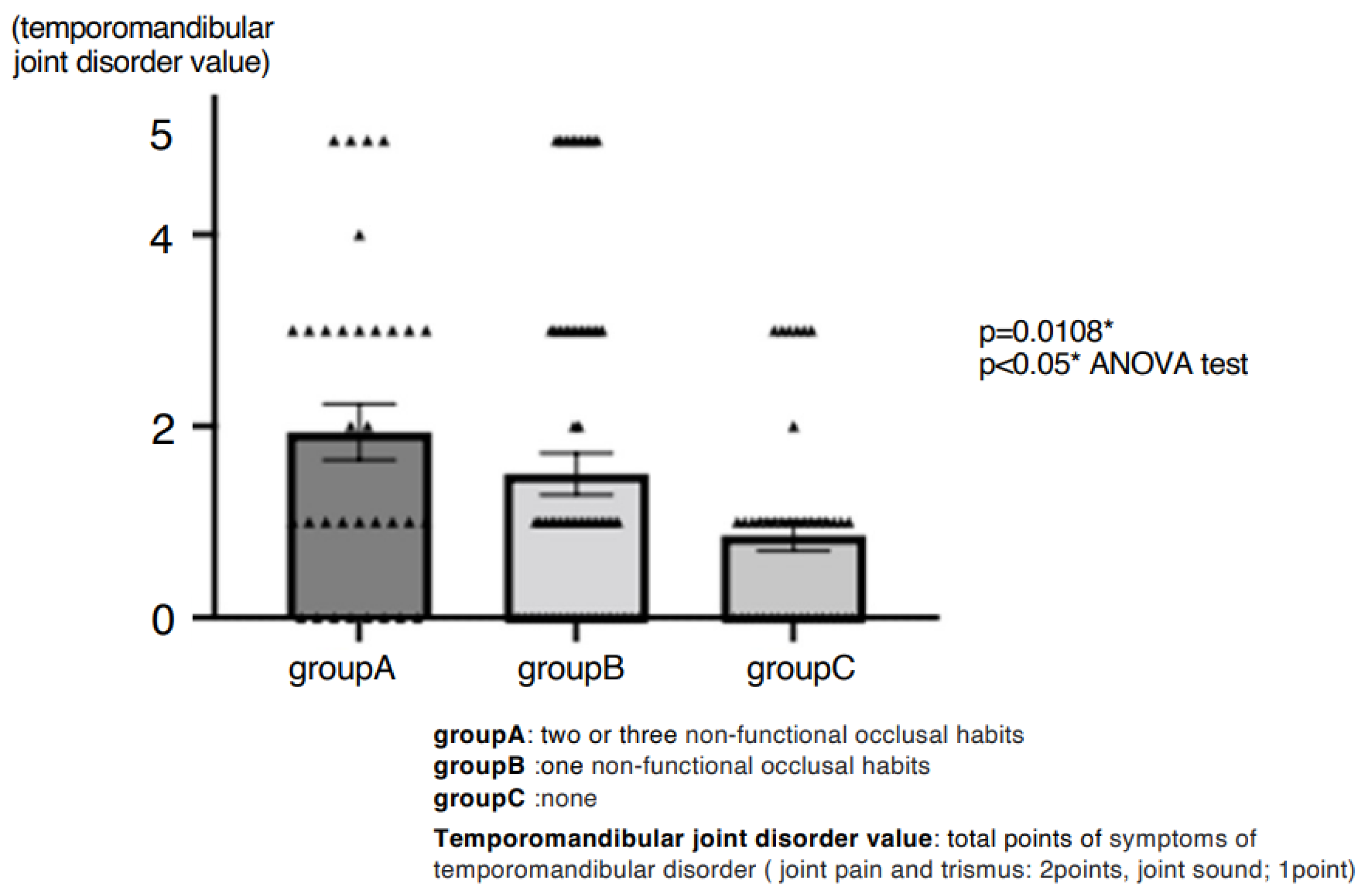
3. Results
4. Discussion
4.1. Prevalence and Impact of Parafunctional Habits
4.2. Unilateral Chewing and Mandibular Asymmetry
4.3. Skeletal Morphology, Disk Displacement, and Joint Pathology
4.4. Psychosocial and Neurophysiological Considerations
4.5. Clinical Implications and Treatment Outcomes
4.6. Limitations
4.7. Future Perspectives
4.8. Summary of the Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurup, S.; Perez-Pino, A.; Litt, M. The association between temporomandibular disorders signs and symptoms, bruxism, and health variables: A cross-sectional study. Cranio 2024, 43, 1083–1091. [Google Scholar] [CrossRef]
- Yalçın Yeler, D.; Yılmaz, N.; Koraltan, M.; Aydın, E. A survey on the potential relationships between TMD, possible sleep bruxism, unilateral chewing, and occlusal factors in Turkish university students. Cranio 2017, 35, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.X.; Zhu, Y.M.; He, L.T.; Gu, Y.; Liang, Z.G.; Zheng, C.S. Investigation of related risk factors of temporomandibular disorders in 109 patients. Shanghai J. Stomatol. 2017, 26, 213–216. (In Chinese) [Google Scholar]
- Zieliński, G.; Pająk-Zielińska, B.; Pająk, A.; Wójcicki, M.; Litko-Rola, M.; Ginszt, M. Global co-occurrence of bruxism and temporomandibular disorders: A meta-regression analysis. Dent. Med. Probl. 2025, 62, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.V.; Kumar, M.P.; Sravanthi, D.; Mohsin, A.H.; Anuhya, V. Bruxism: A literature review. J. Int. Oral Health 2014, 6, 105–109. [Google Scholar] [PubMed]
- Molina, O.F.; dos Santos, J.; Mazzetto, M.; Nelson, S.; Nowlin, T.; Mainieri, E.T. Oral jaw behaviors in TMD and bruxism: A comparison study by severity of bruxism. Cranio 2001, 19, 114–122. [Google Scholar] [CrossRef]
- Leketas, M.; Šaferis, V.; Kubilius, R.; Cervino, G.; Bramanti, E.; Cicciù, M. Oral behaviors and parafunctions: Comparison of temporomandibular dysfunction patients and controls. J. Craniofac Surg. 2017, 28, 1933–1938. [Google Scholar] [CrossRef]
- Fernandes, G.; Franco-Micheloni, A.L.; Siqueira, J.T.; Gonçalves, D.A.; Camparis, C.M. Parafunctional habits are associated cumulatively to painful temporomandibular disorders in adolescents. Braz. Oral Res. 2016, 30, S1806-83242016000100214. [Google Scholar] [CrossRef]
- Jiménez-Silva, A.; Peña-Durán, C.; Tobar-Reyes, J.; Frugone-Zambra, R. Sleep and awake bruxism in adults and its relationship with temporomandibular disorders: A systematic review from 2003 to 2014. Acta Odontol Scand. 2017, 75, 36–58. [Google Scholar] [CrossRef]
- Manfredini, D.; Cantini, E.; Romagnoli, M.; Bosco, M. Prevalence of bruxism in patients with different research diagnostic criteria for temporomandibular disorders (RDC/TMD) diagnoses. Cranio 2003, 21, 279–285. [Google Scholar] [CrossRef]
- Reissmann, D.R.; John, M.T.; Aigner, A.; Schön, G.; Sierwald, I.; Schiffman, E.L. Interaction between awake and sleep bruxism is associated with increased presence of painful temporomandibular disorder. J. Oral Facial Pain Headache 2017, 31, 299–305. [Google Scholar] [CrossRef]
- Ooi, K.; Inoue, N.; Matsushita, K.; Mikoya, T.; Minowa, K.; Kawashiri, S.; Tei, K. Relations between anterior disc displacement and maxillomandibular morphology in skeletal anterior open bite with changes to the mandibular condyle. Br. J. Oral Maxillofac. Surg. 2020, 58, 1084–1090. [Google Scholar] [CrossRef]
- Serafim, I.; Rode, S.; Lopes, S.; Oliveira, W.; Pinho, S.; Silva, E.; Winocur, E.; Meira ECruz, M. Impact of bruxism on craniomandibular morphology: A cone-beam computed tomographic study. Cranio 2025, 43, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Firmani, M.; Reyes, M.; Becerra, N.; Flores, G.; Weitzman, M.; Espinosa, P. Bruxismo de sueño en niños y adolescents (Sleep bruxism in children and adolescents). Rev. Chil. Pediatr. 2015, 86, 373–379. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Plaza, S.P.; Reimpell, A.; Silva, J.; Montoya, D. Relationship between skeletal Class II and Class III malocclusions with vertical skeletal pattern. Dent. Press J. Orthod. 2019, 24, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Pająk, A.; Wójcicki, M. Global Prevalence of Sleep Bruxism and Awake Bruxism in Pediatric and Adult Populations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4259. [Google Scholar] [CrossRef]
- Pontes, L.D.S.; Prietsch, S.O.M. Sleep bruxism: Population based study in people with 18 years or more in the city of Rio Grande, Brazil. Rev. Bras. Epidemiol. 2019, 22, e190038. [Google Scholar] [CrossRef]
- Widmalm, S.E.; Christiansen, R.L.; Gunn, S.M. Oral parafunctions as temporomandibular disorder risk factors in children. Cranio. 1995, 13, 242–246. [Google Scholar] [CrossRef]
- Bruguiere, F.; Sciote, J.J.; Roland-Billecart, T.; Raoul, G.; Machuron, F.; Ferri, J.; Nicot, R. Pre-operative parafunctional or dysfunctional oral habits are associated with the temporomandibular disorders after orthognathic surgery: An observational cohort study. J. Oral Rehabil. 2019, 46, 321–329. [Google Scholar] [CrossRef]
- Casazza, E.; Ballester, B.; Siaud, B.; Philip-Alliez, C.; Raskin, A. Relationship between bruxism and mandibular bone modifications based on medical imaging: A scoping review. BMC Oral Health 2023, 23, 483. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, W.; Wang, J.; Wang, S.; Li, Y.; Jing, H.; Li, Z.; Li, X.; Liang, M.; Wang, Y. A Comparative Study of Temporomandibular Joints in Adults with Definite Sleep Bruxism on Magnetic Resonance Imaging and Cone-Beam Computer Tomography Images. J. Clin. Med. 2023, 12, 2570. [Google Scholar] [CrossRef]
- Sato, F.; Kino, K.; Sugisaki, M.; Haketa, T.; Amemori, Y.; Ishikawa, T.; Shibuya, T.; Amagasa, T.; Shibuya, T.; Tanabe, H.; et al. Teeth contacting habit as a contributing factor to chronic pain in patients with temporomandibular disorders. J. Med. Dent. Sci. 2006, 53, 103–109. [Google Scholar] [PubMed]
- Ma, H.; Zheng, T.; Shao, B.; Liu, Z. Evaluation of the effect of unilateral mastication on the morphology of temporomandibular joint from the perspective of dynamic joint space. J. Oral Rehabil. 2024, 51, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Diernberger, S.; Bernhardt, O.; Schwahn, C.; Kordass, B. Self-reported chewing side preference and its associations with occlusal, temporomandibular and prosthodontic factors: Results from the population-based Study of Health in Pomerania (SHIP-0). J Oral Rehabil. 2008, 35, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, Z.; Wang, H. Research progress in effect of chewing-side preference on temporomandibular joint and its relationship with temporomandibular disorders. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023, 52, 386–397. [Google Scholar] [CrossRef]
- Zheng, C.; Shu, J.; Shao, B.; Liu, Z. Temporomandibular joint stress analysis of patients with different mandibular deformities during unilateral molar occlusion. Comput. Methods Biomech. Biomed. Engin. 2024, 28, 2162–2169. [Google Scholar] [CrossRef]
- Otake, Y.; Nogami, S.; Ezoe, Y.; Sugai, Y.; Okuyama, K.; Saito, S.; Takeda, Y.; Yamauchi, K. Clinical and radiographic features of temporomandibular joint in patients with facial asymmetry. Oral Maxillofac. Surg. 2025, 29, 98. [Google Scholar] [CrossRef]
- Reinhardt, R.; Tremel, T.; Wehrbein, H.; Reinhardt, W. The unilateral chewing phenomenon, occlusion, and TMD. Cranio 2006, 24, 166–170. [Google Scholar] [CrossRef]
- Su, N.; Liu, Y.; Yang, X.; Shen, J.; Wang, H. Association of malocclusion, self-reported bruxism and chewing-side preference with oral health-related quality of life in patients with temporomandibular joint osteoarthritis. Int. Dent. J. 2018, 68, 97–104. [Google Scholar] [CrossRef]
- Commisso, M.S.; Martínez-Reina, J.; Mayo, J. A study of the temporomandibular joint during bruxism. Int. J. Oral Sci. 2014, 6, 116–123. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, X.M.; Wang, M.Q.; Widmalm, S.E. Asymmetric muscle function in patients with developmental mandibular asymmetry. J. Oral Rehabil. 2008, 35, 27–36. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yonemitsu, I.; Takei, M.; Kure-Hattori, I.; Ono, T. The imbalance of masticatory muscle activity affects the asymmetric growth of condylar cartilage and subchondral bone in rats. Arch. Oral Biol. 2016, 63, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sella-Tunis, T.; Pokhojaev, A.; Sarig, R.; O’Higgins, P.; May, H. Human mandibular shape is associated with masticatory muscle force. Sci. Rep. 2018, 8, 6042. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhu, P.; Lin, Y.; Wan, S.; Shu, X.; Xu, Y.; Zheng, Y. Mandibular asymmetry: A three-dimensional quantification of bilateral condyles. Head Face Med. 2013, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.S.; Li, Z.H. Cone beam CT imaging findings in patients with temporomandibular joint disorder syndrome and unilateral chewing. Shanghai J. Stomatol. 2022, 31, 653–656. (In Chinese) [Google Scholar]
- De Carli, E.; Lagou, A.; Kiliaridis, S.; Denes, B.J. Mandibular condyle changes in rats with unilateral masticatory function. Orthod. Craniofac Res. 2023, 26, 37–45. [Google Scholar] [CrossRef]
- Manfredini, D.; Segù, M.; Arveda, N.; Lombardo, L.; Siciliani, G.; Rossi, A.; Guarda-Nardini, L. Temporomandibular joint disorders in patients with different facial morphology: A systematic review of the literature. J. Oral Maxillofac. Surg. 2016, 74, 29–46. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, T.W.; Ahn, S.J.; Lee, S.J.; Donatelli, R.E. The relationship between temporomandibular joint disk displacement and mandibular asymmetry in skeletal Class III patients. Angle Orthod. 2011, 81, 624–631. [Google Scholar] [CrossRef]
- Chou, S.T.; Wang, J.L.; Chen, S.C.; Pan, C.Y.; Chen, C.M.; Tseng, Y.C. Correlation between facial asymmetry of skeletal class III jaw relationship and morphology of the temporomandibular joint: A cone beam computed tomography study. J. Dent. Sci. 2023, 18, 1031–1041. [Google Scholar] [CrossRef]
- Ooi, K.; Inoue, N.; Matsushita, K.; Yamaguchi, H.; Mikoya, T.; Minowa, K.; Kawashiri, S.; Nishikata, S.; Tei, K. Incidence of anterior disc displacement without reduction of the temporomandibular joint in patients with dentofacial deformity. Int. J. Oral Maxillofac. Surg. 2018, 47, 505–510. [Google Scholar] [CrossRef]
- Ooi, K.; Yura, S.; Inoue, N.; Totsuka, Y. Factors related to the incidence of anterior disc displacement without reduction and bony changes of the temporomandibular joint in patients with anterior open bite. Oral Maxillofac. Surg. 2014, 18, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Zemowski, M.; Yushchenko, Y.; Wieczorek, A. The Impact of Parafunctional Habits on Temporomandibular Disorders in Medical Students. J. Clin. Med. 2025, 14, 5301. [Google Scholar] [CrossRef] [PubMed]
- Dharmadhikari, S.; Romito, L.M.; Dzemidzic, M.; Dydak, U.; Xu, J.; Bodkin, C.L.; Manchanda, S.; Byrd, K.E. GABA and glutamate levels in occlusal splint-wearing males with possible bruxism. Arch. Oral Biol. 2015, 60, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Marpaung, C.; Yap, A.U.; Hanin, I.; Fitryanur, A. Psychological distress and well-being: Their association with temporomandibular disorder symptoms. Cranio 2024, 42, 285–291. [Google Scholar] [CrossRef]
- Staniszewski, K.; Lygre, H.; Bifulco, E.; Kvinnsland, S.; Willassen, L.; Helgeland, E.; Berge, T.; Rosén, A. Temporomandibular Disorders Related to Stress and HPA-Axis Regulation. Pain Res. Manag. 2018, 2018, 7020751. [Google Scholar] [CrossRef]
- Tosato Jde, P.; Caria, P.H.; Gomes, C.A.; Berzin, F.; Politti, F.; Gonzalez Tde, O.; Biasotto-Gonzalez, D.A. Correlation of stress and muscle activity of patients with different degrees of temporomandibular disorder. J. Phys. Ther. Sci. 2015, 27, 1227–1231. [Google Scholar] [CrossRef]
- Toh, A.Q.J.; Leung, Y.Y. The effect of orthognathic surgery on temporomandibular disorder. J. Craniomaxillofac Surg. 2022, 50, 218–224. [Google Scholar] [CrossRef]
- Belengeanu, V.; Marian, D.; Hosszu, T.; Ogodescu, A.S.; Belengeanu, A.D.; Samoilă, C.; Freiman, P.; Lile, I.E. A comprehensive evaluation of an OFDI syndrome from child to teenager. Rom. J. Morphol. Embryol. 2019, 60, 697–706. [Google Scholar]


| Open Bite/Asymmetry | Total | ||||
|---|---|---|---|---|---|
| (−/−) | (+/−) | (−/+) | (+/+) | ||
| Skeletal class I | 0 | 7 | 10 | 2 | 15 |
| Skeletal class II | 12 | 8 | 6 | 1 | 25 |
| Skeletal class III | 48 | 23 | 41 | 11 | 101 |
| Class of Non-Functional Occlusal Habits | Skeletal Morphology of Dentofacial Deformity | |||||
|---|---|---|---|---|---|---|
| Class III | Class II | Class I | Open Bite | Asymmetry | ||
| NFOH group | Group A | 22 | 6 | 5 | 13 | 14 |
| Group B | 46 | 12 | 8 | 16 | 32 | |
| non-NFOH group | Group C | 33 | 7 | 2 | 9 | 11 |
| p value | 0.2338 | 0.8294 | 0.1405 | 0.3358 | 0.0249 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukagawa, Y.; Ooi, K.; Nishino, S.; Sasajima, Y.; Ueki, K.; Jokaji, R.; Nakade, Y.; Okita, H.; Yahata, T.; Kawashiri, S. Relationships Among Non-Functional Occlusal Habits, Temporomandibular Disorder Symptoms, and Skeletal Morphology in Patients with Dentofacial Deformities. J. Clin. Med. 2025, 14, 8330. https://doi.org/10.3390/jcm14238330
Fukagawa Y, Ooi K, Nishino S, Sasajima Y, Ueki K, Jokaji R, Nakade Y, Okita H, Yahata T, Kawashiri S. Relationships Among Non-Functional Occlusal Habits, Temporomandibular Disorder Symptoms, and Skeletal Morphology in Patients with Dentofacial Deformities. Journal of Clinical Medicine. 2025; 14(23):8330. https://doi.org/10.3390/jcm14238330
Chicago/Turabian StyleFukagawa, Yurie, Kazuhiro Ooi, Sae Nishino, Yutaka Sasajima, Kosuke Ueki, Rei Jokaji, Yusuke Nakade, Hirokazu Okita, Tetsutaro Yahata, and Shuichi Kawashiri. 2025. "Relationships Among Non-Functional Occlusal Habits, Temporomandibular Disorder Symptoms, and Skeletal Morphology in Patients with Dentofacial Deformities" Journal of Clinical Medicine 14, no. 23: 8330. https://doi.org/10.3390/jcm14238330
APA StyleFukagawa, Y., Ooi, K., Nishino, S., Sasajima, Y., Ueki, K., Jokaji, R., Nakade, Y., Okita, H., Yahata, T., & Kawashiri, S. (2025). Relationships Among Non-Functional Occlusal Habits, Temporomandibular Disorder Symptoms, and Skeletal Morphology in Patients with Dentofacial Deformities. Journal of Clinical Medicine, 14(23), 8330. https://doi.org/10.3390/jcm14238330







