Surgical Treatment of Spinal Metastases–A Retrospective Single-Center Study of 268 Patients
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
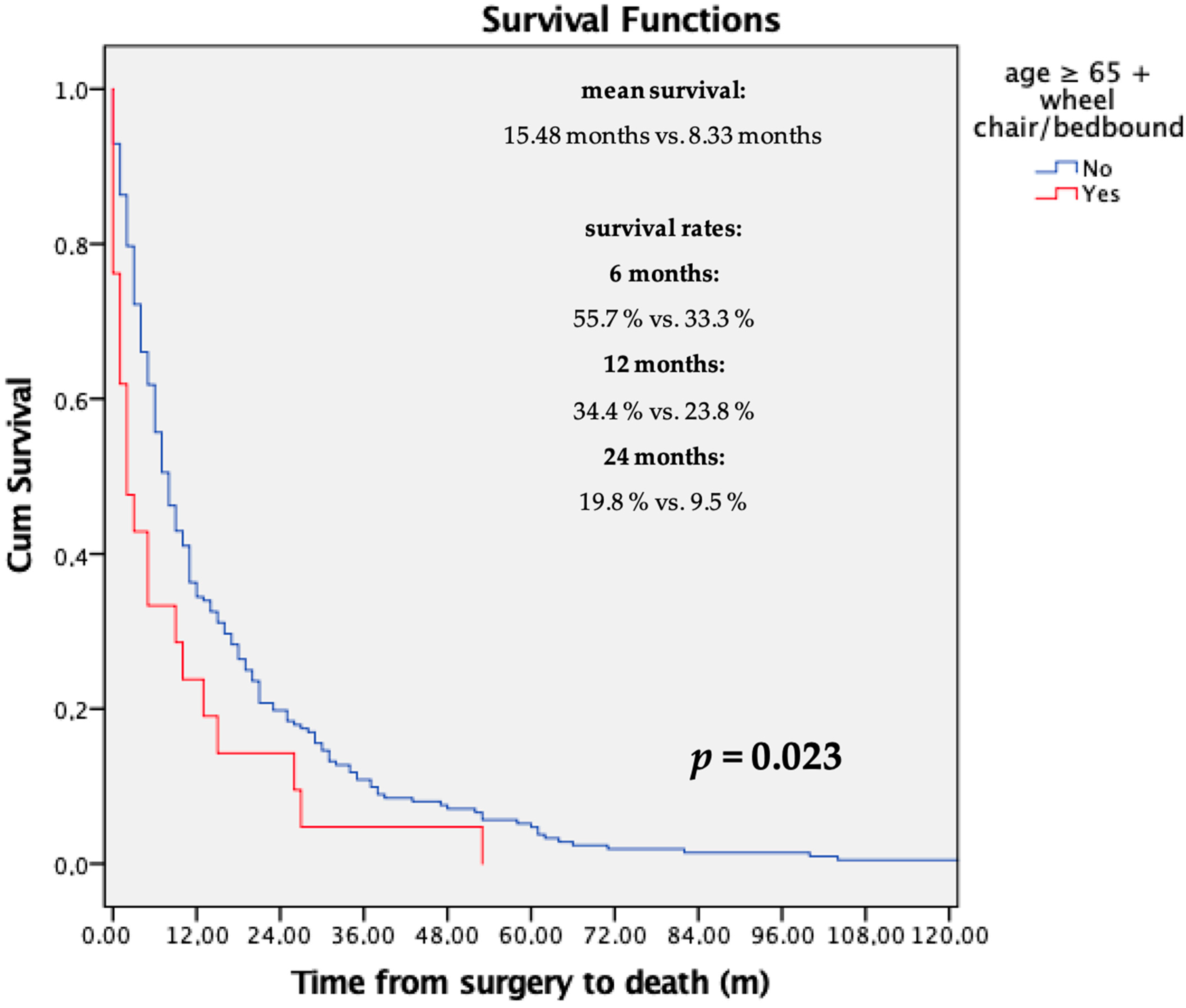
3.1. Survival Analysis
3.2. Analysis of Prognostic Scores
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ratliff, J.K.; Cooper, P.R. Metastatic spine tumors. S. Med. J. 2004, 97, 246–253. [Google Scholar] [CrossRef]
- Harrington, K. Metastatic Tumors of the Spine: Diagnosis and Treatment. J. Am. Acad. Orthop. Surg. 1993, 1, 76–86. [Google Scholar] [CrossRef]
- Silverberg, E.; Boring, C.C.; Squires, T.S. Cancer statistics, 1990. CA Cancer J. Clin. 1990, 40, 9–26. [Google Scholar] [CrossRef]
- Klimo, P., Jr.; Schmidt, M.H. Surgical management of spinal metastases. Oncologist 2004, 9, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Dam-Hieu, P.; Seizeur, R.; Mineo, J.F.; Metges, J.P.; Meriot, P.; Simon, H. Retrospective study of 19 patients with intramedullary spinal cord metastasis. Clin. Neurol. Neurosurg. 2009, 111, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Schaberg, J.; Gainor, B.J. A profile of metastatic carcinoma of the spine. Spine 1985, 10, 19–20. [Google Scholar] [CrossRef]
- Hatrick, N.C.; Lucas, J.D.; Timothy, A.R.; Smith, M.A. The surgical treatment of metastatic disease of the spine. Radiother. Oncol. 2000, 56, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Byrne, T.N. Spinal cord compression from epidural metastases. N. Engl. J. Med. 1992, 327, 614–619. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Welch, W.C. Current surgical management of metastatic spinal disease. Oncology (Williston Park) 2000, 14, 1013–1024; discussion 1024, 1029–1030. [Google Scholar]
- Gilbert, R.W.; Kim, J.H.; Posner, J.B. Epidural spinal cord compression from metastatic tumor: Diagnosis and treatment. Ann. Neurol. 1978, 3, 40–51. [Google Scholar] [CrossRef]
- Potti, A.; Abdel-Raheem, M.; Levitt, R.; Schell, D.A.; Mehdi, S.A. Intramedullary spinal cord metastases (ISCM) and non-small cell lung carcinoma (NSCLC): Clinical patterns, diagnosis and therapeutic considerations. Lung Cancer 2001, 31, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, L.F.; Thanki, A.S.; Spector, H.B. Spinal subdural metastatic adenocarcinoma: Case report and literature review. Neurosurgery 1982, 10, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Jost, G.; Zimmerer, S.; Frank, S.; Cordier, D.; Merlo, A. Intradural spinal metastasis of renal cell cancer. Report of a case and review of 26 published cases. Acta Neurochir. 2009, 151, 815–821; discussion 821. [Google Scholar] [CrossRef]
- Schijns, O.E.; Kurt, E.; Wessels, P.; Luijckx, G.J.; Beuls, E.A. Intramedullary spinal cord metastasis as a first manifestation of a renal cell carcinoma: Report of a case and review of the literature. Clin. Neurol. Neurosurg. 2000, 102, 249–254. [Google Scholar] [CrossRef]
- Cook, A.M.; Lau, T.N.; Tomlinson, M.J.; Vaidya, M.; Wakeley, C.J.; Goddard, P. Magnetic resonance imaging of the whole spine in suspected malignant spinal cord compression: Impact on management. Clin. Oncol. (R Coll. Radiol.) 1998, 10, 39–43. [Google Scholar] [CrossRef]
- Constans, J.P.; de Divitiis, E.; Donzelli, R.; Spaziante, R.; Meder, J.F.; Haye, C. Spinal metastases with neurological manifestations. Review of 600 cases. J. Neurosurg. 1983, 59, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Aebi, M. Spinal metastasis in the elderly. Eur. Spine J. 2003, 12 (Suppl. 2), S202–S213. [Google Scholar] [CrossRef]
- Gokaslan, Z.L. Spine surgery for cancer. Curr. Opin. Oncol. 1996, 8, 178–181. [Google Scholar] [CrossRef]
- Gokaslan, Z.L.; York, J.E.; Walsh, G.L.; McCutcheon, I.E.; Lang, F.F.; Putnam, J.B., Jr.; Wildrick, D.M.; Swisher, S.G.; Abi-Said, D.; Sawaya, R. Transthoracic vertebrectomy for metastatic spinal tumors. J. Neurosurg. 1998, 89, 599–609. [Google Scholar] [CrossRef]
- Jackson, R.J.; Loh, S.C.; Gokaslan, Z.L. Metastatic renal cell carcinoma of the spine: Surgical treatment and results. J. Neurosurg. 2001, 94, 18–24. [Google Scholar] [CrossRef]
- Wong, D.A.; Fornasier, V.L.; MacNab, I. Spinal metastases: The obvious, the occult, and the impostors. Spine 1990, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Heary, R.F.; Bono, C.M. Metastatic spinal tumors. Neurosurg. Focus. 2001, 11, e1. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Cancer. Metastatic spinal cord compression. Diagnosis and management of adults at risk of and with metastatic spinal cord compression. In NICE Guidelines CG75; TJ International Ltd.: Cardiff, UK, 2008. [Google Scholar]
- Leithner, A.; Radl, R.; Gruber, G.; Hochegger, M.; Leithner, K.; Welkerling, H.; Rehak, P.; Windhager, R. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur. Spine J. 2008, 17, 1488–1495. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical Strategy for Spinal Metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Toriyama, S.; Kawano, H.; Ohsaka, S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine 1990, 15, 1110–1113. [Google Scholar] [CrossRef]
- Bauer, H.C.; Wedin, R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop. Scand. 1995, 66, 143–146. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Le, H.V.; Marjoua, Y.; Leonard, D.A.; Belmont, P.J.; Bono, C.M.; Harris, M.B. Assessing the utility of a clinical prediction score regarding 30-day morbidity and mortality following metastatic spinal surgery: The New England Spinal Metastasis Score (NESMS). Spine J. 2016, 16, 482–490. [Google Scholar] [CrossRef]
- Choi, D.; Crockard, A.; Bunger, C.; Harms, J.; Kawahara, N.; Mazel, C.; Melcher, R.; Tomita, K. Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur. Spine J. 2010, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- De Meue, E.; Smeijers, S.; Langmans, C.; Clement, P.M.; Depreitere, B. Identifying new predictive factors for survival after surgery for spinal metastases: An exploratory in-depth retrospective analysis. Acta Clin. Belg. 2022, 77, 606–615. [Google Scholar] [CrossRef]
- Dripps, R.D.; Lamont, A.; Eckenhoff, J.E. The role of anesthesia in surgical mortality. JAMA 1961, 178, 261–266. [Google Scholar] [CrossRef]
- Frankel, H.L.; Hancock, D.O.; Hyslop, G.; Melzak, J.; Michaelis, L.S.; Ungar, G.H.; Vernon, J.D.; Walsh, J.J. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Spinal Cord 1969, 7, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Karnofsky, D.A.; Bruchenal, J.H. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In Evaluation of Chemotherapeutic Agents; MacLeod, C.M., Ed.; Columbia University Press: New York, NY, USA, 1949; Volume 196. [Google Scholar]
- Newman, W.C.; Bilsky, M.H. Fifty-year history of the evolution of spinal metastatic disease management. J. Surg. Oncol. 2022, 126, 913–920. [Google Scholar] [CrossRef]
- Paulino Pereira, N.R.; Janssen, S.J.; van Dijk, E.; Harris, M.B.; Hornicek, F.J.; Ferrone, M.L.; Schwab, J.H. Development of a Prognostic Survival Algorithm for Patients with Metastatic Spine Disease. J. Bone Jt. Surg. Am. 2016, 98, 1767–1776. [Google Scholar] [CrossRef]
- Crnalic, S.; Hildingsson, C.; Wikström, P.; Bergh, A.; Löfvenberg, R.; Widmark, A. Outcome after surgery for metastatic spinal cord compression in 54 patients with prostate cancer. Acta Orthop. 2012, 83, 80–86. [Google Scholar] [CrossRef]
- Choi, D.; Fox, Z.; Albert, T.; Arts, M.; Balabaud, L.; Bunger, C.; Buchowski, J.M.; Coppes, M.H.; Depreitere, B.; Fehlings, M.G.; et al. Prediction of Quality of Life and Survival After Surgery for Symptomatic Spinal Metastases: A Multicenter Cohort Study to Determine Suitability for Surgical Treatment. Neurosurgery 2015, 77, 698–708; discussion 708. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Nater, A.; Tetreault, L.; Kopjar, B.; Arnold, P.; Dekutoski, M.; Finkelstein, J.; Fisher, C.; France, J.; Gokaslan, Z.; et al. Survival and Clinical Outcomes in Surgically Treated Patients With Metastatic Epidural Spinal Cord Compression: Results of the Prospective Multicenter AOSpine Study. J. Clin. Oncol. 2016, 34, 268–276. [Google Scholar] [CrossRef]
- Nater, A.; Tetreault, L.A.; Kopjar, B.; Arnold, P.M.; Dekutoski, M.B.; Finkelstein, J.A.; Fisher, C.G.; France, J.C.; Gokaslan, Z.L.; Rhines, L.D.; et al. Predictive factors of survival in a surgical series of metastatic epidural spinal cord compression and complete external validation of 8 multivariate models of survival in a prospective North American multicenter study. Cancer 2018, 124, 3536–3550. [Google Scholar] [CrossRef] [PubMed]
- Karhade, A.V.; Thio, Q.; Ogink, P.T.; Bono, C.M.; Ferrone, M.L.; Oh, K.S.; Saylor, P.J.; Schoenfeld, A.J.; Shin, J.H.; Harris, M.B.; et al. Predicting 90-Day and 1-Year Mortality in Spinal Metastatic Disease: Development and Internal Validation. Neurosurgery 2019, 85, E671–E681. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Pavlou, M.; Omar, R.; Arts, M.; Balabaud, L.; Buchowski, J.M.; Bunger, C.; Chung, C.K.; Coppes, M.H.; Depreitere, B.; et al. A novel risk calculator to predict outcome after surgery for symptomatic spinal metastases; use of a large prospective patient database to personalise surgical management. Eur. J. Cancer 2019, 107, 28–36. [Google Scholar] [CrossRef]
- Czigléczki, G.; Mezei, T.; Pollner, P.; Horváth, A.; Banczerowski, P. Prognostic Factors of Surgical Complications and Overall Survival of Patients with Metastatic Spinal Tumor. World Neurosurg. 2018, 113, e20–e28. [Google Scholar] [CrossRef]
- Lau, D.; Leach, M.R.; Than, K.D.; Ziewacz, J.; La Marca, F.; Park, P. Independent predictors of complication following surgery for spinal metastasis. Eur. Spine J. 2013, 22, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Lad, S.P.; Santarelli, J.; Boakye, M. National inpatient complications and outcomes after surgery for spinal metastasis from 1993–2002. Cancer 2007, 110, 625–630. [Google Scholar] [CrossRef]
- Luksanapruksa, P.; Buchowski, J.M.; Zebala, L.P.; Kepler, C.K.; Singhatanadgige, W.; Bumpass, D.B. Perioperative Complications of Spinal Metastases Surgery. Clin. Spine Surg. 2017, 30, 4–13. [Google Scholar] [CrossRef]
- Amelot, A.; Balabaud, L.; Choi, D.; Fox, Z.; Crockard, H.A.; Albert, T.; Arts, C.M.; Buchowski, J.M.; Bunger, C.; Chung, C.K.; et al. Surgery for metastatic spine tumors in the elderly. Advanced age is not a contraindication to surgery! Spine J. 2017, 17, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Fox, Z.; Albert, T.; Arts, M.; Balabaud, L.; Bunger, C.; Buchowski, J.M.; Coppes, M.H.; Depreitere, B.; Fehlings, M.G.; et al. Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. Br. J. Neurosurg. 2016, 30, 337–344. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Ferrone, M.L.; Blucher, J.A.; Agaronnik, N.; Nguyen, L.; Tobert, D.G.; Balboni, T.A.; Schwab, J.H.; Shin, J.H.; Sciubba, D.M.; et al. Prospective comparison of the accuracy of the New England Spinal Metastasis Score (NESMS) to legacy scoring systems in prognosticating outcomes following treatment of spinal metastases. Spine J. Off. J. N. Am. Spine Soc. 2022, 22, 39–48. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Goodwin, C.R.; Heravi, A.; Kim, R.; Abu-Bonsrah, N.; Sankey, E.; Kerekes, D.; De la Garza Ramos, R.; Schwab, J.; Sciubba, D.M. Predicting survival for metastatic spine disease: A comparison of nine scoring systems. Spine J. Off. J. N. Am. Spine Soc. 2018, 18, 1804–1814. [Google Scholar] [CrossRef]
- Cassidy, J.T.; Baker, J.F.; Lenehan, B. The Role of Prognostic Scoring Systems in Assessing Surgical Candidacy for Patients With Vertebral Metastasis: A Narrative Review. Glob. Spine J. 2018, 8, 638–651. [Google Scholar] [CrossRef]
- Amelot, A.; Cristini, J.; Salaud, C.; Moles, A.; Hamel, O.; Moreau, P.; Bord, E.; Buffenoir, K. Overall Survival in Spine Myeloma Metastases: Difficulties in Predicting With Prognostic Scores. Spine 2017, 42, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Carrwik, C.; Olerud, C.; Robinson, Y. Predictive Scores Underestimate Survival of Patients With Metastatic Spine Disease: A Retrospective Study of 315 Patients in Sweden. Spine 2020, 45, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Ulmar, B.; Reichel, H.; Catalkaya, S.; Naumann, U.; Schmidt, R.; Gerstner, S.; Huch, K. Evaluation and modification of the Tomita score in 217 patients with vertebral metastases. Onkologie 2007, 30, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.-H.; Park, S.-J.; Chung, S.-S.; Kim, E.-S.; Eoh, W.; Lee, C.-S. Analysis of the predictive role and new proposal for surgical strategies based on the modified Tomita and Tokuhashi scoring systems for spinal metastasis. World J. Surg. Oncol. 2014, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Orenday-Barraza, J.M.; Cavagnaro, M.J.; Avila, M.J.; Strouse, I.M.; Dowell, A.; Kisana, H.; Khan, N.; Ravinsky, R.; Baaj, A.A. 10-Year Trends in the Surgical Management of Patients with Spinal Metastases: A Scoping Review. World Neurosurg. 2022, 157, 170–186.e173. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Haag, E.; Joerger, A.K.; Jost, P.; Combs, S.E.; Wostrack, M.; Gempt, J.; Meyer, B. Comprehensive surgical treatment strategy for spinal metastases. Sci. Rep. 2021, 11, 7988. [Google Scholar] [CrossRef]
- Barzilai, O.; Fisher, C.G.; Bilsky, M.H. State of the Art Treatment of Spinal Metastatic Disease. Neurosurgery 2018, 82, 757–769. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.; Chang, S.Y.; Kim, H.; Chang, B.S. Treatment Strategy for Impending Instability in Spinal Metastases. Clin. Orthop. Surg. 2020, 12, 337–342. [Google Scholar] [CrossRef]

| Primary Tumor | Number of Patients (%) | Survival in Months (95% CI) | 75 Percentile [Months] |
|---|---|---|---|
| Renal | 72 (26.9%) | 16.19 (12.20–20.18) | 26.00 |
| Thyroid | 11 (4.1%) | 20.90 (6.05–35.75) | 35.50 |
| Mamma | 37 (13.8%) | 25.56 (15.60–35.53) | 34.00 |
| Lung | 39 (14.6%) | 10.11 (5.22–15.00) | 9.25 |
| Uterus/Cervix | 9 (3.4%) | 10.78 (3.30–18.25) | 19.50 |
| Colon | 11 (4.1%) | 11.50 (4.80–18.20) | 20.75 |
| Liver | 7 (2.6%) | 8.42 (0–18.09) | 18.00 |
| Gastric | 2 (0.7%) | 1.00 (0–2.96) | N/A |
| Myeloma | 5 (1.9%) | 23.00 (23.00–23.00) | 23.00 |
| Prostate | 12 (4.5%) | 13.58 (8.55–18.62) | 20.75 |
| Other known tumors | 56 (20.9) | 12.61 (3.25–22.00) | 11.00 |
| Unknown primary tumor | 7 (2.6%) | 2.40 (0–5.35) | 5.50 |
| Location | Number of Patients (%) |
|---|---|
| Extraspinal bone metastases | 85 (31.9%) |
| Lung metastases | 68 (25.4%) |
| Liver metastases | 33 (12.3%) |
| Other abdominal metastases | 43 (16.0%) |
| Brain metastases | 7 (2.7%) |
| Variable | β-Value (95% CI) | p-Value |
|---|---|---|
| Sex | 1.323 | 0.280 |
| (0.796–2.198) | ||
| Body mass index (kg/m2) | 0.942 | 0.055 |
| (0.887–1.001) | ||
| Primary tumor | 1.052 | 0.277 |
| (0.960–1.152) | ||
| Age at surgery | 1.025 | 0.041 * |
| (1.001–1.049) | ||
| Reason for admission | 0.972 | 0.782 |
| (7.95–1.188) | ||
| Preoperative extraspinal bone metastases | 1.949 | 0.047 * |
| (1.010–3.760) | ||
| Mobility | 1.207 | 0.013 * |
| (1.040–1.401) | ||
| Pre-/postoperative chemotherapy | 1.312 | 0.362 |
| (0.732–2.351) | ||
| Radiation of the spine preoperatively | 0.717 | 0.413 |
| (0.324–1.589) | ||
| Preoperative radiation | 1.248 | 0.591 |
| (0.556–2.802) | ||
| Postoperative radiation | 0.695 | 0.154 |
| (0.422–1.904) | ||
| Albumin level | 1.182 | 0.539 |
| (0.693–2.015) | ||
| CRP-value > 5 mg/dL | 3.015 | 0.001 * |
| (1.586–5.731) | ||
| Decreased hemoglobin level preoperatively | 0.877 | 0.649 |
| (0.499–1.542) | ||
| Perioperative complications | 1.123 | 0.665 |
| (0.663–1.713) |
| Variable | 1990s | 2000s | 2010s | p-Value (90s vs. 00s) | p-Value (90s vs. 10s) | p-Value (00s vs. 10s) |
|---|---|---|---|---|---|---|
| Survival | 18 | 16.6 | 8.1 | 0.809 | <0.001 * | 0.002 * |
| (months) | (12.7–23.3) | (11.8–21.3) | (5.3–10.8) | |||
| ASA | 2.64 | 2.38 | 2.69 | 0.002 * | 0.348 | <0.001 * |
| −0.48 | −0.56 | −0.57 | ||||
| Complication rate | 33.30% | 30.40% | 24.70% | 0.677 | 0.334 | 0.612 |
| Revision rate | 10.40% | 16.50% | 24.70% | 0.239 | 0.01 * | 0.1 |
| Dead ≤ 3 Months (n = 268) | |||
|---|---|---|---|
| Yes (n = 71; 26%) | No (n = 197; 74%) | ||
| Variable | Mean (SD) | Mean (SD) | p-Value |
| Karnofsky Index | 0.00 (16.90) | 75.43 (16.86) | 0.006 * |
| Bauer Score | 2.23 (0.91) | 2.77 (0.91) | <0.001 * |
| New England Spinal Metastasis Score | 1.46 (1.23) | 2.31 (0.95) | <0.001 * |
| Tokuhashi Score | 8.49 (2.52) | 10.36 (2.6) | <0.001 * |
| Tomita Score | 5.66 (2.24) | 4.53 (1.9) | <0.001 * |
| GSTSG Score | 20.1 (10.39) | 16.34 (10.7) | 0.001 * |
| Dead 4–6 Months (n = 197) | |||
|---|---|---|---|
| Yes (n = 37; 19%) | No (n = 160; 81%) | ||
| Variable | Mean (SD) | Mean (SD) | p-Value |
| Karnofsky Index | 80.27(15.18) | 74.31 (17.07) | 0.014 * |
| Bauer Score | 2.62 (1.01) | 2.81 (0.89) | 0.272 |
| New England Spinal Metastasis Score | 1.94 (1.12) | 2.38 (0.91) | 0.110 |
| Tokuhashi Score | 10.16 (2.59) | 10.40 (2.61) | 0.513 |
| Tomita Score | 5.03 (1.92) | 4.41 (1.93) | 0.048 * |
| GSTSG Score | 30.83 (15.78) | 28.53 (15.26) | 0.350 |
| Dead 7–12 Months (n = 160) | |||
|---|---|---|---|
| Yes (n = 47; 29%) | No (n = 113; 71%) | ||
| Variable | Mean (SD) | Mean (SD) | p-Value |
| Karnofsky Index | 71.70 (17.59) | 75.40 (16.80) | 0.175 |
| Bauer Score | 2.41 (0.91) | 2.96 (0.83) | <0.001 * |
| New England Spinal Metastasis Score | 1.91 (0.97) | 2.54 (0.83) | 0.003 * |
| Tokuhashi Score | 9.89 (2.69) | 10.58 (2.56) | 0.159 |
| Tomita Score | 5.02 (2.21) | 4.18 (1.77) | 0.016 * |
| GSTSG Score | 51.53 (17.82) | 39.43 (17.17) | <0.001 * |
| Dead 13–24 Months (n = 113) | |||
|---|---|---|---|
| yes (n = 34; 30%) | no (n = 79; 70%) | ||
| Variable | Mean (SD) | Mean (SD) | p-Value |
| Karnofsky Index | 77.94 (16.29) | 74.30 (17.00) | 0.243 |
| Bauer Score | 2.88 (0.95) | 3.00 (0.78) | 0.707 |
| New England Spinal Metastasis Score | 2.37(0.89) | 2.59 (0.81) | 0.199 |
| Tokuhashi Score | 10.79 (2.41) | 10.48 (2.63) | 0.614 |
| Tomita Score | 4.56 (2.3) | 4.01 (1.46) | 0.526 |
| GSTSG Score | 56.71 (17.60) | 53.10 (18.43) | 0.168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, B.; Stihsen, C.; Grohs, J.G.; Rienmüller, A.; Funovics, P.; Krepler, P.; Windhager, R. Surgical Treatment of Spinal Metastases–A Retrospective Single-Center Study of 268 Patients. J. Clin. Med. 2025, 14, 8308. https://doi.org/10.3390/jcm14238308
Springer B, Stihsen C, Grohs JG, Rienmüller A, Funovics P, Krepler P, Windhager R. Surgical Treatment of Spinal Metastases–A Retrospective Single-Center Study of 268 Patients. Journal of Clinical Medicine. 2025; 14(23):8308. https://doi.org/10.3390/jcm14238308
Chicago/Turabian StyleSpringer, Bernhard, Christoph Stihsen, Josef G. Grohs, Anna Rienmüller, Philipp Funovics, Petra Krepler, and Reinhard Windhager. 2025. "Surgical Treatment of Spinal Metastases–A Retrospective Single-Center Study of 268 Patients" Journal of Clinical Medicine 14, no. 23: 8308. https://doi.org/10.3390/jcm14238308
APA StyleSpringer, B., Stihsen, C., Grohs, J. G., Rienmüller, A., Funovics, P., Krepler, P., & Windhager, R. (2025). Surgical Treatment of Spinal Metastases–A Retrospective Single-Center Study of 268 Patients. Journal of Clinical Medicine, 14(23), 8308. https://doi.org/10.3390/jcm14238308





