Mucositis and Peri-Implant Disease Treatment with Chitosan and Titanium Brushes: A Systematic Review
Abstract
1. Introduction
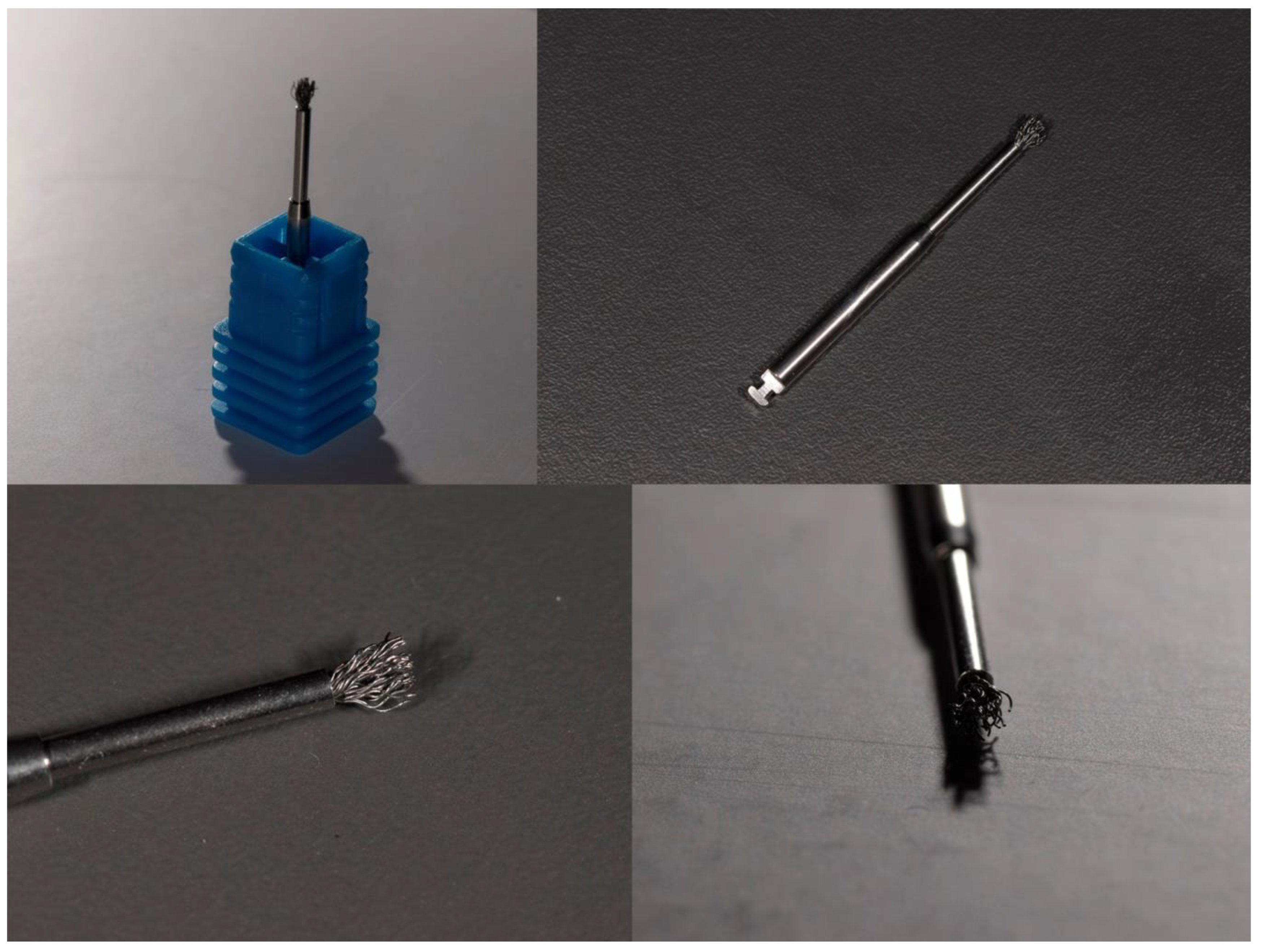
1.1. Titanium Brushes
1.2. Chitosan Brushes
2. Materials and Methods
2.1. PICOS Framework and Research Question
2.2. Inclusion Criteria
2.3. Exclusion Criteria
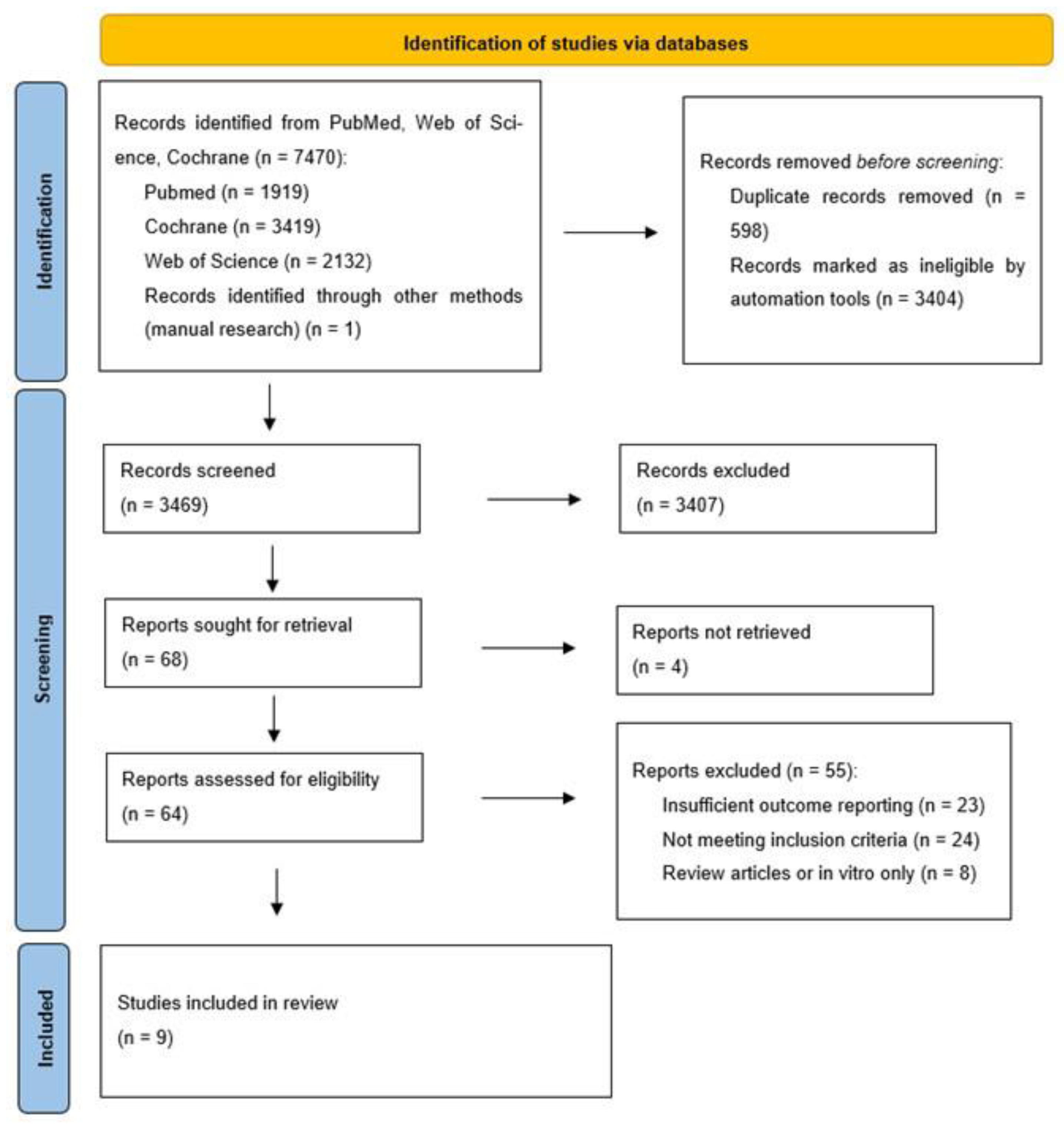
2.4. Database AMD Search Strategy
2.5. Study Selection
2.6. Data Extraction and Handling
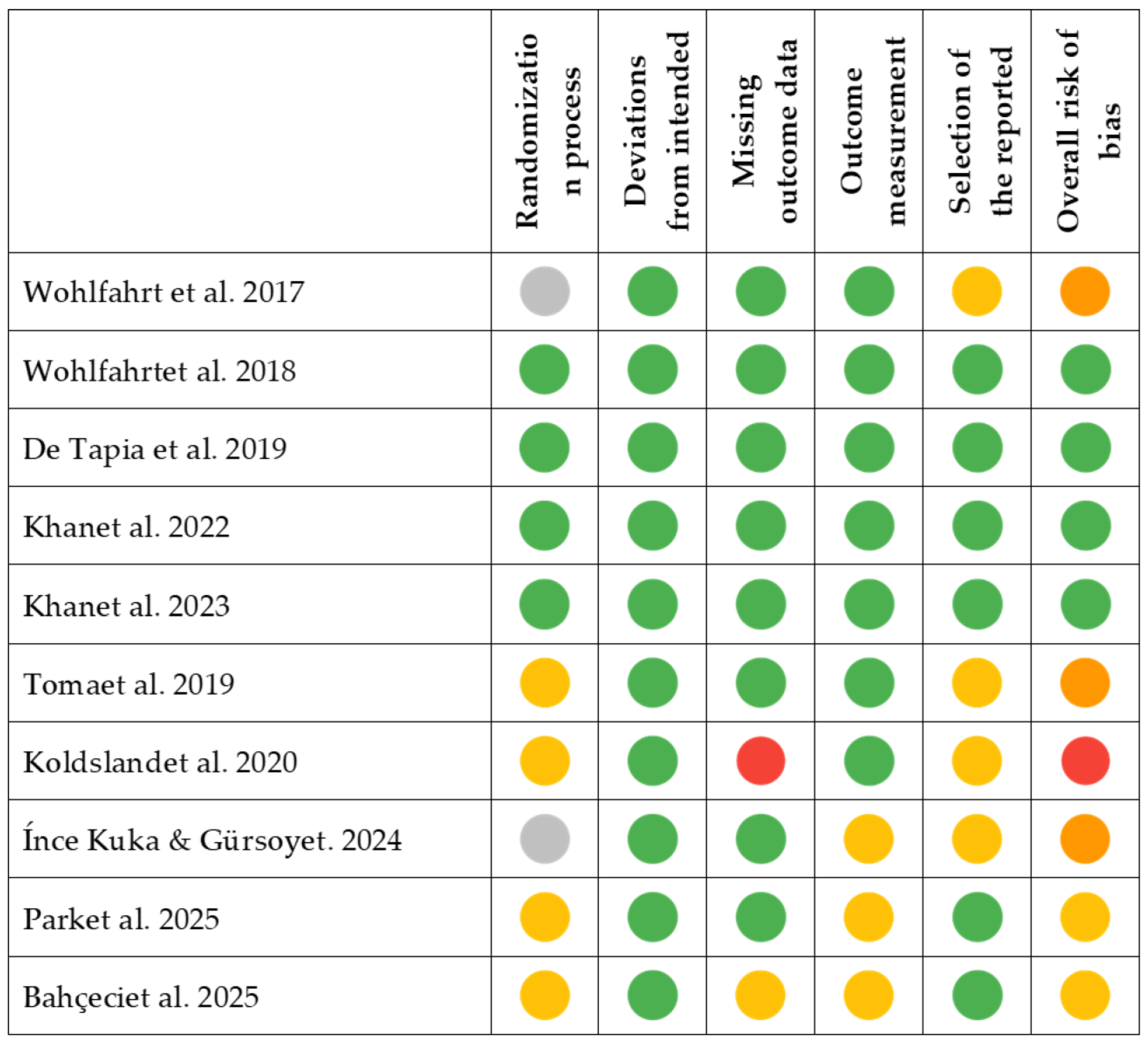
2.7. Risk of Bias Assessment
2.8. Synthesis Methods
2.9. Effect Measures
2.10. Statistical Analysis
2.11. Certainty of Evidence Assesment
3. Results
3.1. Results of Individual Studies
3.2. Results of Syntheses
3.2.1. Non-Surgical Protocols
3.2.2. Surgical Protocols
3.3. Reporting Biases
3.4. Certainty of Evidence
4. Discussion
4.1. Surgical vs. Non-Surgical Comparison
4.2. Reduction in PPD
4.3. Reduction in BoP
4.4. Effectivenes of Antimicrobial Therapies
4.5. Impact of Oral Hygiene
4.6. Limitations and Strengths of the Study
4.7. Future Lines of Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TB | Titanium Brush |
| OCB | Oscillating Chitosan Brush |
| PPD | Probing Pocket Depth |
| BoP | Bleeding on Probing |
| mBoP | Modified Bleeding on Probing |
| TC | Titanium Curettes |
| PC | Plastic Curettes |
| RBL | Radiographic Bone Loss |
| AP | Air-Polishing |
| CAL | Clinical Attachment Level |
| GBR | Guided Bone Regeneration |
| RCT | Randomized Controlled Trial |
| PI | Plaque Index |
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant Health and Disease. A Systematic Review of Current Epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef]
- Romandini, M.; Lima, C.; Pedrinaci, I.; Araoz, A.; Soldini, M.C.; Sanz, M. Prevalence and Risk/Protective Indicators of Peri-implant Diseases: A University-representative Cross-sectional Study. Clin. Oral Implant. Res. 2021, 32, 112–122. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J.; on behalf of Group D of the European Workshop on Periodontology. Peri-implant Diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef]
- Meyer, S.; Giannopoulou, C.; Courvoisier, D.; Schimmel, M.; Müller, F.; Mombelli, A. Experimental Mucositis and Experimental Gingivitis in Persons Aged 70 or over. Clinical and Biological Responses. Clin. Oral Implant. Res. 2017, 28, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Schincaglia, G.P.; Hong, B.Y.; Rosania, A.; Barasz, J.; Thompson, A.; Sobue, T.; Panagakos, F.; Burleson, J.A.; Dongari-Bagtzoglou, A.; Diaz, P.I. Clinical, Immune, and Microbiome Traits of Gingivitis and Peri-Implant Mucositis. J. Dent. Res. 2017, 96, 47–55. [Google Scholar] [CrossRef]
- Salvi, G.E.; Aglietta, M.; Eick, S.; Sculean, A.; Lang, N.P.; Ramseier, C.A. Reversibility of Experimental Peri-implant Mucositis Compared with Experimental Gingivitis in Humans. Clin. Oral Implant. Res. 2012, 23, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Reis, I.N.R.D.; Huamán-Mendoza, A.A.; Ramadan, D.; Honório, H.M.; Naenni, N.; Romito, G.A.; Holzhausen, M.; Pannuti, C.M. The Prevalence of Peri-Implant Mucositis and Peri-Implantitis Based on the World Workshop Criteria: A Systematic Review and Meta-Analysis. J. Dent. 2025, 160, 105914. [Google Scholar] [CrossRef]
- Baima, G.; Citterio, F.; Romandini, M.; Romano, F.; Mariani, G.M.; Buduneli, N.; Aimetti, M. Surface Decontamination Protocols for Surgical Treatment of Peri-implantitis: A Systematic Review with Meta-analysis. Clin. Oral Implant. Res. 2022, 33, 1069–1086. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Eger, M.; Sterer, N.; Liron, T.; Kohavi, D.; Gabet, Y. Scaling of Titanium Implants Entrains Inflammation-Induced Osteolysis. Sci. Rep. 2017, 7, 39612. [Google Scholar] [CrossRef]
- Ravidà, A.; Siqueira, R.; Saleh, I.; Saleh, M.H.A.; Giannobile, A.; Wang, H.L. Lack of Clinical Benefit of Implantoplasty to Improve Implant Survival Rate. J. Dent. Res. 2020, 99, 1348–1355. [Google Scholar] [CrossRef]
- Schlee, M.; Rathe, F.; Brodbeck, U.; Ratka, C.; Weigl, P.; Zipprich, H. Treatment of Peri-Implantitis—Electrolytic Cleaning Versus Mechanical and Electrolytic Cleaning—A Randomized Controlled Clinical Trial—Six-Month Results. JCM 2019, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Albaker, A.M.; ArRejaie, A.S.; Alrabiah, M.; Al-Aali, K.A.; Mokeem, S.; Alasqah, M.N.; Vohra, F.; Abduljabbar, T. Effect of Antimicrobial Photodynamic Therapy in Open Flap Debridement in the Treatment of Peri-Implantitis: A Randomized Controlled Trial. Photodiagnosis Photodyn. Ther. 2018, 23, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ashnagar, S.; Gianfilippo, R.D.; Arnett, M.; Kinney, J.; Wang, H. Laser-assisted Regenerative Surgical Therapy for Peri-implantitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2021, 92, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.A.; Vouros, I.; Menexes, G.; Konstantinidis, A. The Utilization of a Diode Laser in the Surgical Treatment of Peri-Implantitis. A Randomized Clinical Trial. Clin. Oral. Investig. 2015, 19, 1851–1860. [Google Scholar] [CrossRef]
- Isler, S.C.; Unsal, B.; Soysal, F.; Ozcan, G.; Peker, E.; Karaca, I.R. The Effects of Ozone Therapy as an Adjunct to the Surgical Treatment of Peri-Implantitis. J. Periodontal Implant. Sci. 2018, 48, 136. [Google Scholar] [CrossRef]
- Schwarz, F.; Sculean, A.; Romanos, G.; Herten, M.; Horn, N.; Scherbaum, W.; Becker, J. Influence of Different Treatment Approaches on the Removal of Early Plaque Biofilms and the Viability of SAOS2 Osteoblasts Grown on Titanium Implants. Clin. Oral. Investig. 2005, 9, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Carcuac, O.; Derks, J.; Charalampakis, G.; Abrahamsson, I.; Wennström, J.; Berglundh, T. Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Peri-Implantitis: A Randomized Controlled Clinical Trial. J. Dent. Res. 2016, 95, 50–57. [Google Scholar] [CrossRef]
- Hallström, H.; Persson, G.R.; Lindgren, S.; Olofsson, M.; Renvert, S. Systemic Antibiotics and Debridement of Peri-implant Mucositis. A Randomized Clinical Trial. J. Clin. Periodontol. 2012, 39, 574–581. [Google Scholar] [CrossRef]
- Hallström, H.; Persson, G.R.; Lindgren, S.; Renvert, S. Open Flap Debridement of Peri-implantitis with or without Adjunctive Systemic Antibiotics: A Randomized Clinical Trial. J. Clin. Periodontol. 2017, 44, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- De Waal, Y.C.M.; Raghoebar, G.M.; Meijer, H.J.A.; Winkel, E.G.; Van Winkelhoff, A.J. Implant Decontamination with 2% Chlorhexidine during Surgical Peri-implantitis Treatment: A Randomized, Double-blind, Controlled Trial. Clin. Oral Implant. Res. 2015, 26, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Toma, S.; Brecx, M.C.; Lasserre, J.F. Clinical Evaluation of Three Surgical Modalities in the Treatment of Peri-Implantitis: A Randomized Controlled Clinical Trial. JCM 2019, 8, 966. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Schwarz, K.; Becker, J. Impact of Defect Configuration on the Clinical Outcome Following Surgical Regenerative Therapy of Peri-implantitis. J. Clin. Periodontol. 2010, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Wennström, J.L.; Lindhe, J. Long-term Outcome of Surgical Treatment of Peri-implantitis. A 2–11-year Retrospective Study. Clin. Oral Implant. Res. 2018, 29, 404–410. [Google Scholar] [CrossRef]
- Koo, K.-T.; Khoury, F.; Keeve, P.L.; Schwarz, F.; Ramanauskaite, A.; Sculean, A.; Romanos, G. Implant Surface Decontamination by Surgical Treatment of Periimplantitis: A Literature Review. Implant. Dent. 2019, 28, 173–176. [Google Scholar] [CrossRef]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of Alternative or Adjunctive Measures to Conventional Treatment of Peri-Implant Mucositis and Peri-Implantitis: A Systematic Review and Meta-Analysis. Int. J. Implant. Dent. 2015, 1, 22. [Google Scholar] [CrossRef]
- De Tapia, B.; Valles, C.; Ribeiro-Amaral, T.; Mor, C.; Herrera, D.; Sanz, M.; Nart, J. The Adjunctive Effect of a Titanium Brush in Implant Surface Decontamination at Peri-implantitis Surgical Regenerative Interventions: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2019, 46, 586–596. [Google Scholar] [CrossRef]
- John, G.; Becker, J.; Schwarz, F. Rotating Titanium Brush for Plaque Removal from Rough Titanium Surfaces—An in Vitro Study. Clin. Oral Implant. Res. 2014, 25, 838–842. [Google Scholar] [CrossRef]
- Khan, S.N.; Koldsland, O.C.; Roos-Jansåker, A.; Wohlfahrt, J.C.; Verket, A.; Mdala, I.; Magnusson, A.; Salvesen, E.; Hjortsjö, C. Non-surgical Treatment of Mild to Moderate Peri-implantitis Using an Oscillating Chitosan Brush or a Titanium Curette—A Randomized Multicentre Controlled Clinical Trial. Clin. Oral Implant. Res. 2022, 33, 1254–1264. [Google Scholar] [CrossRef]
- Viganò, P.; Alccayhuaman, K.A.A.; Sakuma, S.; Amari, Y.; Bengazi, F.; Botticelli, D. Use of TiBrush for Surface Decontamination at Peri-implantitis Sites in Dogs: Radiographic and Histological Outcomes. J. Investig. Clin. Dent. 2019, 10, e12378. [Google Scholar] [CrossRef]
- Toma, S.; Lasserre, J.; Brecx, M.C.; Nyssen-Behets, C. In Vitro Evaluation of Peri-implantitis Treatment Modalities on Saos-2osteoblasts. Clin. Oral Implant. Res. 2016, 27, 1085–1092. [Google Scholar] [CrossRef]
- Brunello, G.; Becker, K.; Rauch, N.; Schwarz, F.; Becker, J. The Effect of NiTi Brush, Polishing Brush, and Chemical Agent on the Dental Implant Surface Morphology and Cytocompatibility. Clin. Implant. Dent. Relat. Res. 2025, 27, e13417. [Google Scholar] [CrossRef]
- Jordan, A.; Smojver, I.; Budimir, A.; Gabrić, D.; Vuletić, M. Evaluation of Different Procedures for Titanium Dental Implant Surface Decontamination—In Vitro Study. Bioengineering 2024, 11, 326. [Google Scholar] [CrossRef]
- Villa, O.; Lyngstadaas, S.P.; Monjo, M.; Satué, M.; Rønold, H.J.; Petzold, C.; Wohlfahrt, J.C. Suture Materials Affect Peri-Implant Bone Healing and Implant Osseointegration. J. Oral Sci. 2015, 57, 219–227. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Wohlfahrt, J.C.; Evensen, B.J.; Zeza, B.; Jansson, H.; Pilloni, A.; Roos-Jansåker, A.M.; Di Tanna, G.L.; Aass, A.M.; Klepp, M.; Koldsland, O.C. A Novel Non-Surgical Method for Mild Peri-Implantitis- a Multicenter Consecutive Case Series. Int. J. Implant. Dent. 2017, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.I.; Enersen, M.; Kristoffersen, A.K.; Wennerberg, A.; Bunæs, D.F.; Lie, S.A.; Leknes, K.N. Antimicrobial Effects of Three Different Treatment Modalities on Dental Implant Surfaces. J. Oral Implantol. 2017, 43, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wohlfahrt, J.C.; Aass, A.M.; Koldsland, O.C. Treatment of Peri-implant Mucositis with a Chitosan Brush—A Pilot Randomized Clinical Trial. Int. J. Dent. Hyg. 2019, 17, 170–176. [Google Scholar] [CrossRef]
- Khan, S.N.; Koldsland, O.C.; Roos-Jansåker, A.; Wohlfahrt, J.C.; Verket, A.; Mdala, I.; Magnusson, A.; Salvesen, E.; Hjortsjö, C. Non-surgical Treatment of Mild to Moderate Peri-implantitis with an Oscillating Chitosan Brush or a Titanium Curette—12-month Follow-up of a Multicenter Randomized Clinical Trial. Clin. Oral Implant. Res. 2023, 34, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Bahçeci, K.; Gültekin, B.A.; Yalçın, S. Efficacy of an Oscillating Chitosan Brush Versus an Air Abrasive Device in the Management of Peri-Implant Mucositis: A Randomized Clinical Trial. JFB 2025, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Koldsland, O.C.; Aass, A.M. Supportive Treatment Following Peri-implantitis Surgery: An RCT Using Titanium Curettes or Chitosan Brushes. J. Clin. Periodontol. 2020, 47, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- İnce Kuka, G.; Gürsoy, H. Reconstructive Surgical Treatment of Peri-Implantitis with Use of a Chitosan Brush for Decontamination- Case Series with 1-Year Follow-Up. Int. J. Implant. Dent. 2024, 10, 60. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Kim, D.; Sanz-Martin, I.; Sanz-Sanchez, I.; Derks, J.; Cha, J. Implantoplasty vs. Rotating Titanium Brushes in the Surgical Treatment of Peri-Implantitis: A 1-Year Randomised Controlled Clinical Trial. J. Clin. Periodontol. 2025, 0, 1–12. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennström, J.L.; Berglundh, T. Risk for Recurrence of Disease Following Surgical Therapy of Peri-implantitis—A Prospective Longitudinal Study. Clin. Oral Implant. Res. 2020, 31, 1072–1077. [Google Scholar] [CrossRef]
- Cosgarea, R.; Roccuzzo, A.; Jepsen, K.; Sculean, A.; Jepsen, S.; Salvi, G.E. Efficacy of Mechanical/Physical Approaches for Implant Surface Decontamination in Non-surgical Submarginal Instrumentation of Peri-implantitis. A Systematic Review. J. Clin. Periodontol. 2023, 50, 188–211. [Google Scholar] [CrossRef]
- Cha, J.K.; Lee, J.S.; Kim, C.S. Surgical Therapy of Peri-Implantitis with Local Minocycline: A 6-Month Randomized Controlled Clinical Trial. J. Dent. Res. 2019, 98, 288–295. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Fretwurst, T.; Schwarz, F. Efficacy of Alternative or Adjunctive Measures to Conventional Non-Surgical and Surgical Treatment of Peri-Implant Mucositis and Peri-Implantitis: A Systematic Review and Meta-Analysis. Int. J. Implant. Dent. 2021, 7, 112. [Google Scholar] [CrossRef]
- Verket, A.; Koldsland, O.C.; Bunæs, D.; Lie, S.A.; Romandini, M. Non-surgical Therapy of Peri-implant Mucositis—Mechanical/Physical Approaches: A Systematic Review. J. Clin. Periodontol. 2023, 50, 135–145. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Layton, D.M.; Roccuzzo, A.; Heitz-Mayfield, L.J. Clinical Outcomes of Peri-implantitis Treatment and Supportive Care: A Systematic Review. Clin. Oral Implant. Res. 2018, 29, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, A.; Shapira, L.; Limones, A.; Martin, C. The Efficacy of Implant Surface Decontamination Using Chemicals during Surgical Treatment of Peri--implantitis: A Systematic Review and META—ANALYSIS. J. Clin. Periodontol. 2023, 50, 336–358. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.; Mombelli, A. The Therapy of Peri-Implantitis: A Systematic Review. Int. J. Oral. Maxillofac. Implant. 2014, 29, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Fons-Badal, C.; Labaig-Rueda, C.; Agustín-Panadero, R.; Solá-Ruiz, M.F.; Roig-Vanaclocha, A.; Fernández-Estevan, L.; Fons-Font, A. Retrospective Study of the Association between Peri-Implantitis and Keratinized Mucosa. Appl. Sci. 2022, 12, 6980. [Google Scholar] [CrossRef]
- Checchi, V.; Racca, F.; Bencivenni, D.; Lo Bianco, L. Role of Dental Implant Homecare in Mucositis and Peri-Implantitis Prevention: A Literature Overview. TODENTJ 2019, 13, 470–477. [Google Scholar] [CrossRef]
- Mojaver, S.; Zad, A.; Sarmiento, H.; Fiorellini, J.P. Efficacy of Supportive Peri-Implant Therapy in the Management of Peri-Implant Mucositis and Peri-Implantitis. J. Am. Dent. Assoc. 2025, S0002-8177(25)00497-0. [Google Scholar] [CrossRef]
- Costa, F.O.; Costa, A.M.; Ferreira, S.D.; Lima, R.P.E.; Pereira, G.H.M.; Cyrino, R.M.; Oliveira, A.M.S.D.; Oliveira, P.A.D.; Cota, L.O.M. Long-term Impact of Patients’ Compliance to Peri-implant Maintenance Therapy on the Incidence of Peri-implant Diseases: An 11-year Prospective Follow-up Clinical Study. Clin. Implant. Dent. Relat. Res. 2023, 25, 303–312. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Database | Date | Search Strategy |
|---|---|---|
| Medline (via PUBMED) | 9 November 2025 | (“dental implants”) AND (“periimplantitis” OR “peri implantitis” OR “peri-implantitis” OR “implant disease” OR “peri implant disease” OR “peri-implant disease” OR “mucositis” OR “titanium brush” OR “TiBrush” OR “Chitosan brush” OR “Labrida” OR “Labrida Bioclean” OR “therapy” OR “treatment” OR “decontamination” OR “debridement” OR “surface decontamination” OR “pocket depth reduction” OR “bleeding on probing” OR “BoP”). |
| Web of Science | 9 November 2025 | TS = (“dental implants”) AND TS = (“periimplantitis” OR “peri implantitis” OR “peri-implantitis” OR “implant disease” OR “peri implant disease” OR “peri-implant disease” OR “mucositis” OR “titanium brush” OR “TiBrush” OR “chitosan brush” OR “Labrida” OR “Labrida Bioclean” OR “therapy” OR “treatment” OR “decontamination” OR “debridement” OR “surface decontamination” OR “pocket depth reduction” OR “bleeding on probing” OR “BoP”) |
| Cochrane | 9 November 2025 | (“dental implants”) AND (“periimplantitis” OR “peri implantitis” OR “peri-implantitis” OR “implant disease” OR “peri implant disease” OR “peri-implant disease” OR “mucositis” OR “titanium brush” OR “TiBrush” OR “chitosan brush” OR “Labrida” OR “Labrida Bioclean” OR “therapy” OR “treatment” OR “decontamination” OR “debridement” OR “surface decontamination” OR “pocket depth reduction” OR “bleeding on probing” OR “BoP”) |
| Study | Study Design | Sample Size | Diagnosis (Clinical Signs) | Intervention | Material | Combination | Follow-Up | Results | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Implants | with Chemical Agents | with Other Techniques | |||||||
| Wohlfahrt, 2017 [37] | Prospective, non-randomized case series | 63 | 63 | Mild peri-implantitis defined as: 1–2 mm bone loss, ≥4 mm PPD, and bleeding on probing | Debridement with OCB at baseline and 3 months | LABRIDA BioClean™ (Straumann, Basel Switzerland) | Not specified. Saline used during debridement. | Not combined with other techniques | 2 weeks, 4 weeks, 3 months, 6 months | Significant reduction in BoP and PPD, except between weeks 2 and 4. |
| Wohlfahrt, 2018 [41] | Multicenter RCT, split-mouth design | 11 (13 enrolled, 2 excluded) | 24 (12 test, 12 control) | Peri-implant mucositis: ≥4 mm probing depth and bleeding on probing, with no bone loss on X-rays | Debridement with OCB (test) or TC (control) at baseline and 3 months | LABRIDA BioClean™ (test) (Straumann, Basel Switzerland) or TC (control) | Not specified | Not combined with other techniques | 2 weeks, 4 weeks, 6 months | Significant reduction in BoP in both groups, with greater improvement in the test group between weeks 2 and 4. |
| Khan, 2022 [30] | Multicenter RCT | 38 (39 enrolled, 1 excluded) | 39 (22 test, 17 control) | Mild to moderate peri-implantitis: 2–4 mm bone loss on X-rays, BoP ≥ 2, and probing depth ≥ 4 mm | Debridement with OCB (test) or TC (control) | LABRIDA BioClean™ (test) (Straumann, Basel Switzerland) or TC (control) | Not specified. Saline used during debridement. | Not combined with other techniques | 4 weeks, 3 months, 6 months | No statistically significant differences between groups. Significant reductions in PPD and BoP in both groups. |
| Khan, 2023 [42] | Multicenter RCT | 31 (39 enrolled, 8 excluded) | 39 (22 test, 17 control) | Mild to moderate peri-implantitis: 2–4 mm bone loss on X-rays, BoP ≥ 2, and probing depth ≥ 4 mm | Debridement with OCB (test) or TC (control) | LABRIDA BioClean™ (test) (Straumann, Basel Switzerland) or titanium curettes (control) | Not specified. Saline used during debridement. | Not combined with other techniques | 4 weeks, 3 months, 6 months, 12 months | Significant reductions in PPD and BoP at 12 months in both groups, with no statistically significant differences between them. |
| Bahçeci, 2025 [43] | RCT | 50 (58 enrolled, 8 dropouts) | 103 (53 test, 50 control) | Peri-implant mucositis: bleeding and/or suppuration on probing with PPD ≥ 4 mm and no radiographic bone loss beyond the first implant thread | Debridement with OCB (test) or Air-abrasive device using glycine powder (control) | LABRIDA BioClean™ (test) (Straumann, Basel Switzerland) or EMS Airflow® (control) (EMS dental, Nyon, Suisse) | No adjunctive chemical therapy. | Not combined with other techniques. | 6 months | Both groups achieved significant reductions in PPD, BoP, and PI vs. baseline. At 24 weeks, BoP improvements were similar; chitosan brush showed slightly faster and greater reductions in PPD and plaque (p < 0.05). No adverse events reported. |
| Study | Study Design | Sample Size | Diagnosis (Clinical Signs) | Intervention | Material | Combination | Follow-Up | Results | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Implants | with Chemical Agents | with Other Techniques | |||||||
| De Tapia, 2019 [28] | Multicenter RCT | 54 (18 test, 36 control) | 54 (18 test, 36 control) | Moderate peri-implantitis: 2–4 mm bone loss, BoP ≥ 2, and probing depth ≥ 5 mm | Flap surgery with ultrasonic debridement. Test group used TB; control used PC and hydrogen peroxide irrigation | TB (test group) or PC (control group) | Hydrogen peroxide in both groups | Bone grafting with alloplastic material in both groups | 12 months | Significant reduction in PPD and radiographic bone level in the test group compared to the control group. |
| Toma, 2019 [23] | Randomized clinical trial | 30 | 30 (10 per group) | Peri-implantitis with ≥2 mm bone loss and probing depth ≥ 5 mm | Flap surgery with surface decontamination using titanium brushes (test), plastic curettes, or air polishing (control) | TB (test group), PC, or Perio-Flow® (control group) (EMS dental, Nyon, Suisse) | None | No adjunctive therapies | 12 months | All groups showed reductions in PPD and improvements in CAL. Titanium brushes showed greater bone preservation compared to plastic curettes. |
| Koldsland, 2020 [44] | Randomized controlled trial | 142 | 142 | Peri-implantitis with ≥3 mm probing depth, positive BoP, and RBL | Post-surgical maintenance with either OCB (test) or TC (control) | LABRIDA BioClean™ (test group) (Straumann, Basel Switzerland) or TC (control group) | Not specified | None | 6, 12, and 18 months | Both groups demonstrated stable outcomes with no statistically significant differences in PPD or BoP between groups. |
| İnce Kuka & Gürsoy, 2024 [45] | Prospective clinical case series | 9 | 11 | Peri-implantitis: PPD ≥ 5 mm with BoP and radiographic bone loss ≥ 2 mm compared with baseline | Open-flap debridement with OCB for implant surface decontamination combined with GBR | LABRIDA BioClean™, Cerabone®, Jason® (Straumann, Basel Switzerland) | Saline irrigation only; no chemical decontaminants or antibiotics reported | GBR with xenograft and collagen membrane | 12 months | Significant clinical and radiographic improvement: PPD 7.3 → 3.8 mm (p < 0.001); BoP 96.9% → 15.6% (p = 0.001); RBL 5.5 → 1.4 mm (p = 0.010). 100% implant survival, no complications. Authors conclude that GBR + chitosan brush is effective and safe for complex peri-implant defects. |
| Park, 2025 [46] | RCT | 30 | 15 (test), 15 (control) | Peri-implantitis: PPD ≥ 5 mm with bleeding/suppuration and radiographic bone loss ≥ 2 mm relative to the most coronal intraosseous contact | TB or implantoplasty with carbide burs and polishing | Dentium, Neobiotech®, Seoul, Korea | Saline irrigation only; no systemic or local antimicrobials; identical postoperative maintenance protocol in both groups | No combination with other techniques | 12 months | PPD reduced by 3.6 mm (brush) vs. 3.3 mm (implantoplasty); RBL stable in brush group (0.0 mm) vs. −0.7 mm in control. 80% of implants achieved pockets ≤ 5 mm. Surgery was faster with the brush (3.0 min vs. 5.5 min, p < 0.01). No adverse events reported. |
| Study | Sample (Implants) | Intervention | Initial | Final | Follow-Up | Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean PPD | Mean BI | Mean RBL | Mean PPD | Mean BI | Mean RBL | |||||
| Wohlfahrt, 2017 [37] | 63 | OCB | 5.15 (4.97; 5.32) | 1.86 (1.78; 1.93) | Not specified | 4.35 (3.93; 4.77) | 0.76 (0.53; 0.99) | Not reported | 24 weeks | Significant reductions in PPD and mBoP |
| Wohlfahrt, 2018 [41] | 24 | OCB (Test) vs. TC (Control) | Test: 4.27 ± 1.36 mm Control: 4.29 ± 1.50 mm | Test: 1.54 ± 0.78 Control: 1.35 ± 0.85 | Not specified | Test: 4.09 ± 1.68 mm Control: 3.95 ± 1.27 mm | Test: 0.70 ± 0.70 Control: 0.74 ± 0.80 | Not reported | 24 weeks | No significant reductions in BoP between groups |
| Khan, 2022 [30] | 39 | OCB (Test) vs. TC (Control) | OCB: 5.3 ± 0.16 mm TC: 5.5 ± 0.29 mm | OCB: 2.33 ± 0.48 TC: 2.24 ± 0.44 | OCB: 2.43 ± 0.51 mm TC: 2.58 ± 0.58 mm | OCB: 4.1 ± 1.2 mm TC: 4.2 ± 1.3 mm | OCB: 1.2 ± 0.8 TC: 1.3 ± 0.9 | OCB: 2.4 ± 0.5 mm TC: 2.6 ± 0.6 mm | 24 weeks | No significant differences between groups |
| Khan, 2023 [42] | 39 | OCB (Test) vs. TC (Control) | OCB: 5.2 ± 1.3 mm TC: 5.1 ± 1.2 mm | OCB: 2.1 ± 0.9 TC: 2.0 ± 1.0 | OCB: 2.5 ± 0.6 mm TC: 2.4 ± 0.5 mm | OCB: 4.1 ± 1.2 mm TC: 4.2 ± 1.3 mm | OCB: 1.2 ± 0.8 TC: 1.3 ± 0.9 | OCB: 2.6 ± 0.5 mm TC: 2.5 ± 0.6 mm | 48 weeks | No significant differences between groups |
| Tapia, 2019 [28] | 30 | TB vs. PC with H2O2 | Test: 6.17 ± 0.98 mm Control: 6.16 ± 1.27 mm | 100% (both groups) | Not specified | Test: 4.15 ± 0.84 mm Control: 3.91 ± 0.93 mm | Not reported | Test: 2.51 ± 1.21 mm Control: 0.73 ± 1.26 mm | 48 weeks | Significant reductions in PPD and increases in RBL for test group |
| Toma, 2019 [23] | 70 | TB vs. PC vs. Air-polishing | PC: 5.8 ± 0.8 mm AP: 6.2 ± 0.9 mm TB: 6.0 ± 0.9 mm | PC: 90% AP: 85%, TB: 88% | Not specified | PC: 4.5 ± 1.1 mm AP: 4.7 ± 1.2 mm TB: 4.6 ± 1.1 mm | PC: 45% AP: 40% TB: 38% | PC: 4.3 ± 1.0 mm AP: 4.4 ± 1.1 mm TB: 4.4 ± 1.0 mm | 48 weeks | Less bone loss with titanium brush compared to plastic curettes |
| Koldsland, 2020 [44] | 135 | OCB (Test) vs. TC (Control) | Test: 5.3 ± 1.4 mm Control: 5.1 ± 1.2 mm | Test: 90.1 ± 4.0% Control:89.5 ± 4.1% | Test: 5.1 ± 1.9 mm Control: 5.0 ± 2.1 mm | Test: 4.4 ± 1.8 mm Control: 4.9 ± 2.1 mm | Test: 85.7 ± 4.4% Control: 84.8 ± 4.4% | Test: 4.4 ± 1.8 mm Control: 4.9 ± 2.1 mm | 72 weeks | No significant improvements in either group |
| Ínce Kuka & Gürsoy, 2024 [45] | 11 | OCB combined with GBR | 7.3 ± 0.8 | 96.9% | 5.5 ± 1.4 | 3.8 ± 0.7 | 15.6% | +4.1 mm | 48 weeks | Radiographic bone regeneration evident at 12 mo; 100% implant survival. |
| Bahçeci, 2025 [43] | 103 | OCB (test) vs. AP (control) | Test: 3.81 ± 0.68 Control: 3.74 ± 0.71 | Test: 77.4% Control: 78.8% | Not reported | Test: 2.65 ± 0.55 Control: 2.81 ± 0.59 | Test: 26.9% Control: 29.6% | Not reported | 24 weeks | Comparable BoP reductions at 6 mo; both effective in inflammation control. |
| Park, 2025 [46] | 30 | TB (test) vs. Implantoplasty (control) | Test: 7.0 ± 1.4 Control: 7.2 ± 1.3 | Test: 100% Control: 100% | Not reported | Test: 3.4 ± 1.1 Control: 3.9 ± 1.0 | Test: 20% Control: 25% | Test: Δ0.0 mm (stable) Control: −0.7 mm | 48 weeks | Both groups showed marked PPD reduction; brush group slightly better numerically. Radiographic bone maintained in brush group; slight loss in implantoplasty group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pappolla Sessa, C.; Pappolla Sessa, A.; Martín-Vacas, A.; Docampo-Vázquez, C.; Aragoneses, J.M. Mucositis and Peri-Implant Disease Treatment with Chitosan and Titanium Brushes: A Systematic Review. J. Clin. Med. 2025, 14, 8306. https://doi.org/10.3390/jcm14238306
Pappolla Sessa C, Pappolla Sessa A, Martín-Vacas A, Docampo-Vázquez C, Aragoneses JM. Mucositis and Peri-Implant Disease Treatment with Chitosan and Titanium Brushes: A Systematic Review. Journal of Clinical Medicine. 2025; 14(23):8306. https://doi.org/10.3390/jcm14238306
Chicago/Turabian StylePappolla Sessa, Cristian, Adrián Pappolla Sessa, Andrea Martín-Vacas, Cristian Docampo-Vázquez, and Juan Manuel Aragoneses. 2025. "Mucositis and Peri-Implant Disease Treatment with Chitosan and Titanium Brushes: A Systematic Review" Journal of Clinical Medicine 14, no. 23: 8306. https://doi.org/10.3390/jcm14238306
APA StylePappolla Sessa, C., Pappolla Sessa, A., Martín-Vacas, A., Docampo-Vázquez, C., & Aragoneses, J. M. (2025). Mucositis and Peri-Implant Disease Treatment with Chitosan and Titanium Brushes: A Systematic Review. Journal of Clinical Medicine, 14(23), 8306. https://doi.org/10.3390/jcm14238306







