Burden of Healthcare-Associated Infections on Mortality Among COVID-19 Hospitalized Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Variables Analyzed
2.3. Statistical Analysis
2.4. Use of GenAI in Writing
3. Results
3.1. Clinical and Demographic Profile of the Cohort
3.2. ROC (Receiver Operating Characteristic) Analysis
3.3. Kaplan–Meier Analysis
3.4. Cox Analysis
4. Discussion
4.1. Types of Infections and Pathogens
4.2. Risk Factors
4.3. Impact on Mortality and Survival
4.4. National and International Context
4.5. Clinical Implications
4.6. Strengths and Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CDC | Centers for Disease Control and Prevention |
| CDI | Clostridium difficile infection |
| CI | Confidence interval |
| ECDC | European Centre for Disease Prevention and Control |
| GI | Gastrointestinal |
| HAIs | Healthcare-Associated Infections |
| HR | Hazard ratios |
| IBM | International Business Machines |
| IBM Corp. | International Business Machines Corporation |
| IIC | Interval interquartile |
| IQR | Interquartile range |
| MDR infections | Multidrug-Resistant Infections |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| NOS | Not Otherwise Specified |
| NY | New York |
| p | p-value |
| PCR | Polymerase Chain Reaction |
| ROC | Receiver Operating Characteristic |
| se | Sensitivity |
| sp | Specificity |
| SPP | Species |
| SPSS | Statistical Package for the Social Sciences |
| SSI | Surgical Site Infection |
| USA | United States of America |
| UTI | Urinal tract infection |
| WHO | World Health Organization |
References
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide; WHO: Geneva, Switzerland, 2011; Available online: https://iris.who.int/server/api/core/bitstreams/1a54fd40-1ff6-4a2f-9741-2573daea8061/content (accessed on 10 September 2025).
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Curtis, S.J.; Barnabas, R.; Cairns, K.A.; Cameron, D.; Coghlan, B.; Jones, R.; Joseph, J.; Kali, A.; Kep, D.; Klintworth, G.; et al. Healthcare-associated infections and antimicrobial use at a major referral hospital in Papua New Guinea: A point prevalence survey. Lancet Reg. Health West Pac. 2024, 48, 101120. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of six healthcare-associated infections on European population health: Estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.Y.; Bloomfield, S.F.; Courvalin, P.; Essack, S.Y.; Gandra, S.; Gerba, C.P.; Rubino, J.R.; Scott, E.A. Reducing antibiotic prescribing and addressing the global problem of antibiotic resistance by targeted hygiene in the home and everyday life settings: A position paper. Am. J. Infect. Control 2020, 48, 1090–1099. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Lazureanu, V.E.; Marinescu, A.R.; Cut, T.G.; Oancea, C.; Pescariu, S.A.; Pop, G.N. Factors Influencing the Evolution of Pulmonary Hypertension in Previously Healthy Subjects Recovering from a SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 5272. [Google Scholar] [CrossRef]
- Molaverdi, G.; Kamal, Z.; Safavi, M.; Shafiee, A.; Mozhgani, S.-H.; Zarei Ghobadi, M.; Goudarzvand, M. Neurological Complications after COVID-19: A Narrative Review. eNeurologicalSci. 2023, 33, 100485. [Google Scholar] [CrossRef]
- Groff, A.; Kavanaugh, M.; Ramgobin, D.; McClafferty, B.; Aggarwal, C.S.; Golamari, R.; Jain, R. Gastrointestinal Manifestations of COVID-19: A Review of What We Know. Ochsner J. 2021, 21, 177–180. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Alqahtani, A.; Alamer, E.; Mir, M.; Alasmari, A.; Alshahrani, M.M.; Asiri, M.; Ahmad, I.; Alhazmi, A.; Algaissi, A. Bacterial coinfections increase mortality of severely ill COVID-19 patients in Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 2424. [Google Scholar] [CrossRef]
- Bentivegna, E.; Alessio, G.; Spuntarelli, V.; Luciani, M.; Santino, I.; Simmaco, M.; Martelletti, P. Impact of COVID-19 prevention measures on risk of health care-associated Clostridium difficile infection. Am. J. Infect. Control 2021, 49, 640–642. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Annual Epidemiological Report for 2018—Healthcare-Associated Infections Acquired in Intensive Care Units; ECDC: Stockholm, Sweden, 2023. [Google Scholar]
- Voinea, C.; Mocanu, E.; Opariuc-Dan, C.; Dantes, E.; Gache, A.-C.; Rugina, S. Global lessons from COVID-19: Regional variations in the management of hospital-acquired infections during and post-pandemic. J. Clin. Med. 2025, 14, 6654. [Google Scholar] [CrossRef] [PubMed]
- National Center for Surveillance and Control of Communicable Diseases (CNSCBT). Annual Report on Healthcare-Associated Infections. 2019. Available online: https://www.cnscbt.ro (accessed on 10 September 2025).
- European Centre for Disease Prevention and Control. Surveillance of Healthcare-Associated Infections and Prevention Indicators in European Acute Care Hospitals—HAI-Net Annual Report 2022; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu (accessed on 10 September 2025).
- Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Patient Safety Component Manual: Surgical Site Infection (SSI) Event; CDC: Atlanta, GA, USA, 2023; 9p. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf (accessed on 10 September 2025).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.J.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.; Misseri, G.; Catalisano, G.; Marino, C.; Ingoglia, G.; Alessi, M.; Consiglio, E.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Sciurti, A.; Baccolini, V.; Ceparano, M.; Isonne, C.; Migliara, G.; Iera, J.; Alessandri, F.; Ceccarelli, G.; Marzuillo, C.; Tellan, G.; et al. Incidence and predictors of healthcare-associated infections in patients admitted to a temporary ICU during the COVID-19 pandemic Waves: A Two-Year (2021–2023) Retrospective Cohort Study in Rome, Italy. Antibiotics 2024, 13, 842. [Google Scholar] [CrossRef]
- Lewandowski, K.; Rosołowski, M.; Kaniewska, M.; Kucha, P.; Meler, A.; Wierzba, W.; Rydzewska, G. Clostridioides difficile infection in coronavirus disease 2019 (COVID-19): An underestimated problem? Pol. Arch. Intern. Med. 2021, 131, 121–127. [Google Scholar] [CrossRef]
- Khavandegar, A.; Siami, Z.; Rasouli, A.; Nazemi, P.; Gull, A. Healthcare-associated infections and mortality before and after COVID-19: A multicenter comparative analysis. Front. Public Health 2025, 13, 1475221. [Google Scholar] [CrossRef]
- Shabana, H.; Abdelwahed, H. Impact of the COVID-19 pandemic on Clostridioides difficile infection rates in a tertiary hospital: A retrospective comparative study. Cureus 2025, 17, e377180. [Google Scholar] [CrossRef]
- Granata, G.; Petrosillo, N.; Al Moghazi, S.; Caraffa, E.; Puro, V.; Tillotson, G.; Cataldo, M.A. The burden of Clostridioides difficile infection in COVID-19 patients: A systematic review and meta-analysis. Anaerobe 2022, 74, 102484. [Google Scholar] [CrossRef] [PubMed]
- Tsankof, A.; Protopapas, A.A.; Mantzana, P.; Protonotariou, E.; Skoura, L.; Protopapas, N.D.; Savopoulos, C.; Mimidis, K. Clostridioides difficile infection in patients with and without COVID-19 during the pandemic: A retrospective cohort study from a tertiary referral hospital. Anaerobe 2024, 88, 102864. [Google Scholar] [CrossRef] [PubMed]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Punjabi Katiyar, C.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York city pandemic surge. Infect. Control Hosp. Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Solís-Huerta, F.; Martinez-Guerra, B.A.; Roman-Montes, C.M.; Tamez-Torres, K.M.; Rajme-Lopez, S.; Ortíz-Conchi, N.; López-García, N.I.; Villalobos-Zapata, G.Y.; Rangel-Cordero, A.; Santiago-Cruz, J.; et al. Risk factors associated with the development of hospital-acquired infections in hospitalized patients with severe COVID-19. Antibiotics 2023, 12, 1108. [Google Scholar] [CrossRef]
- Trifi, A.; Sellaouti, S.; Mehdi, A.; Messaoud, L.; Seghir, E.; Tlili, B.; Abdellatif, S. Healthcare-associated infections in critical COVID-19 patients: Impact on mortality and predictors. Acute Crit. Care 2023, 38, 425–434. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Augello, M.; Castoldi, R.; Tavelli, A.; Nardo, R.; Sala, V.; Albertini, L.; Lundgren, L.B.; De Benedittis, S.; Borghi, E.; Viganò, O.; et al. Incidence, risk factors and outcomes of healthcare-associated bacterial infections in COVID-19 patients receiving respiratory support: A retrospective cohort study. J. Infect. Public Health. 2025. [CrossRef]
- Langlete, P.; Eriksen-Volle, H.-M.; Hessevik Paulsen, T.; Raastad, R.; Fagernes, M.; Bøås, H.; Himmels, J. Healthcare-Associated COVID-19 Infections and Mortality. J. Hosp. Infect. 2025, 158, 61–68. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Pattabiraman, V.; Konnor, R.Y.; Patel, P.R.; Wong, E.; Xu, S.Y.; Smith, B.; Edwards, J.R.; Dudeck, M.A. The Impact of Coronavirus Disease 2019 (COVID-19) on Healthcare-Associated Infections in 2020: A Summary of Data Reported to the National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2022, 43, 12–25. [Google Scholar] [CrossRef]
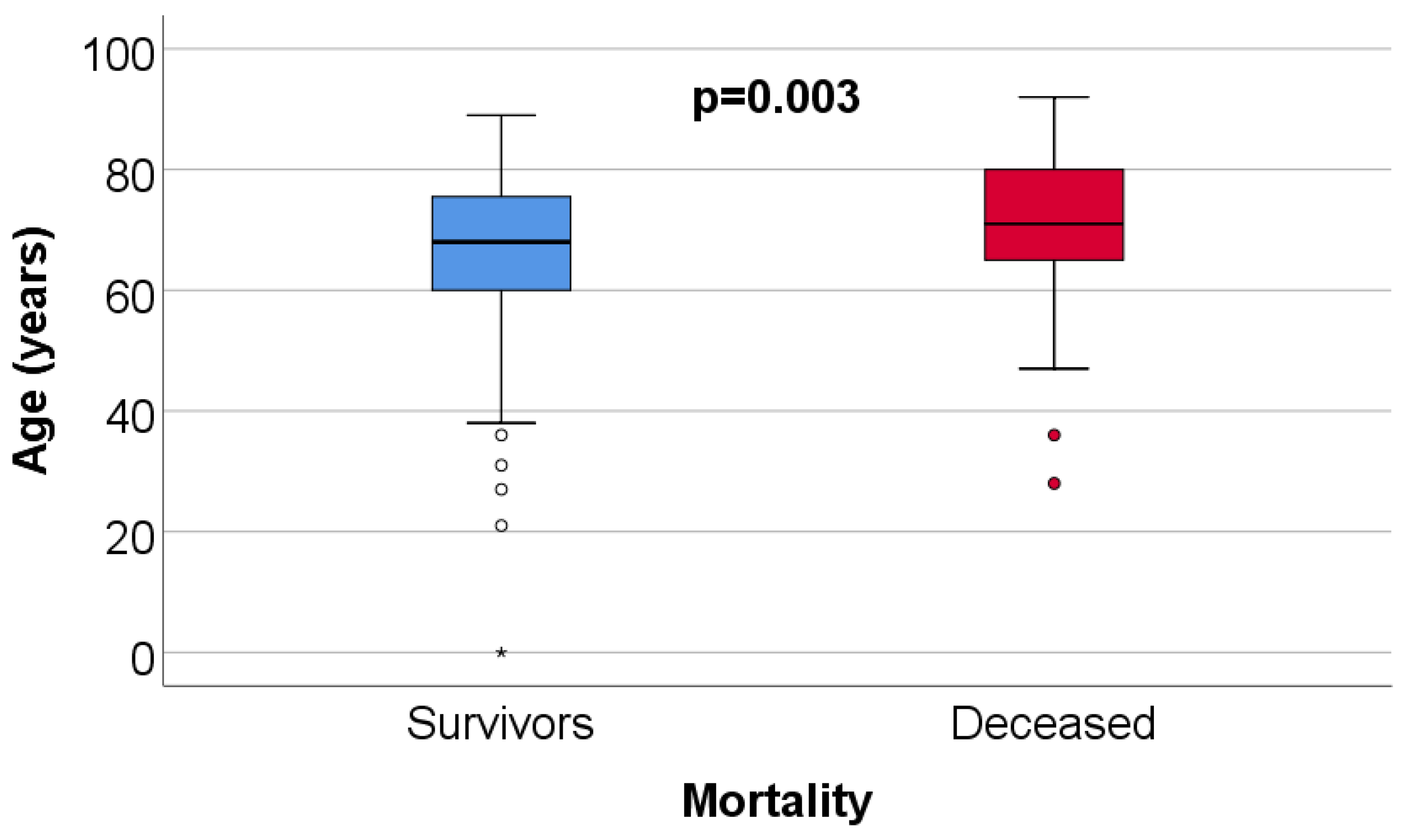
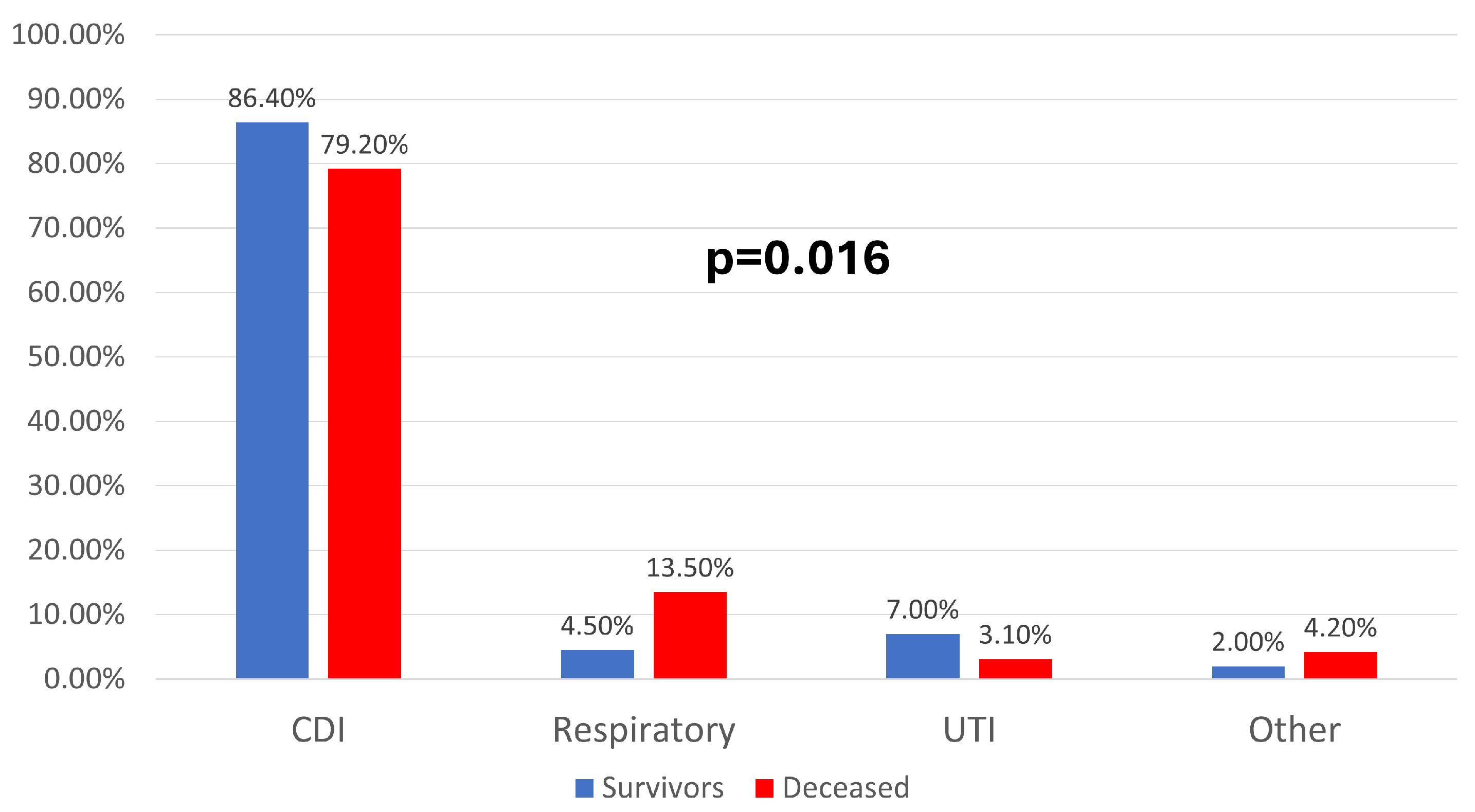
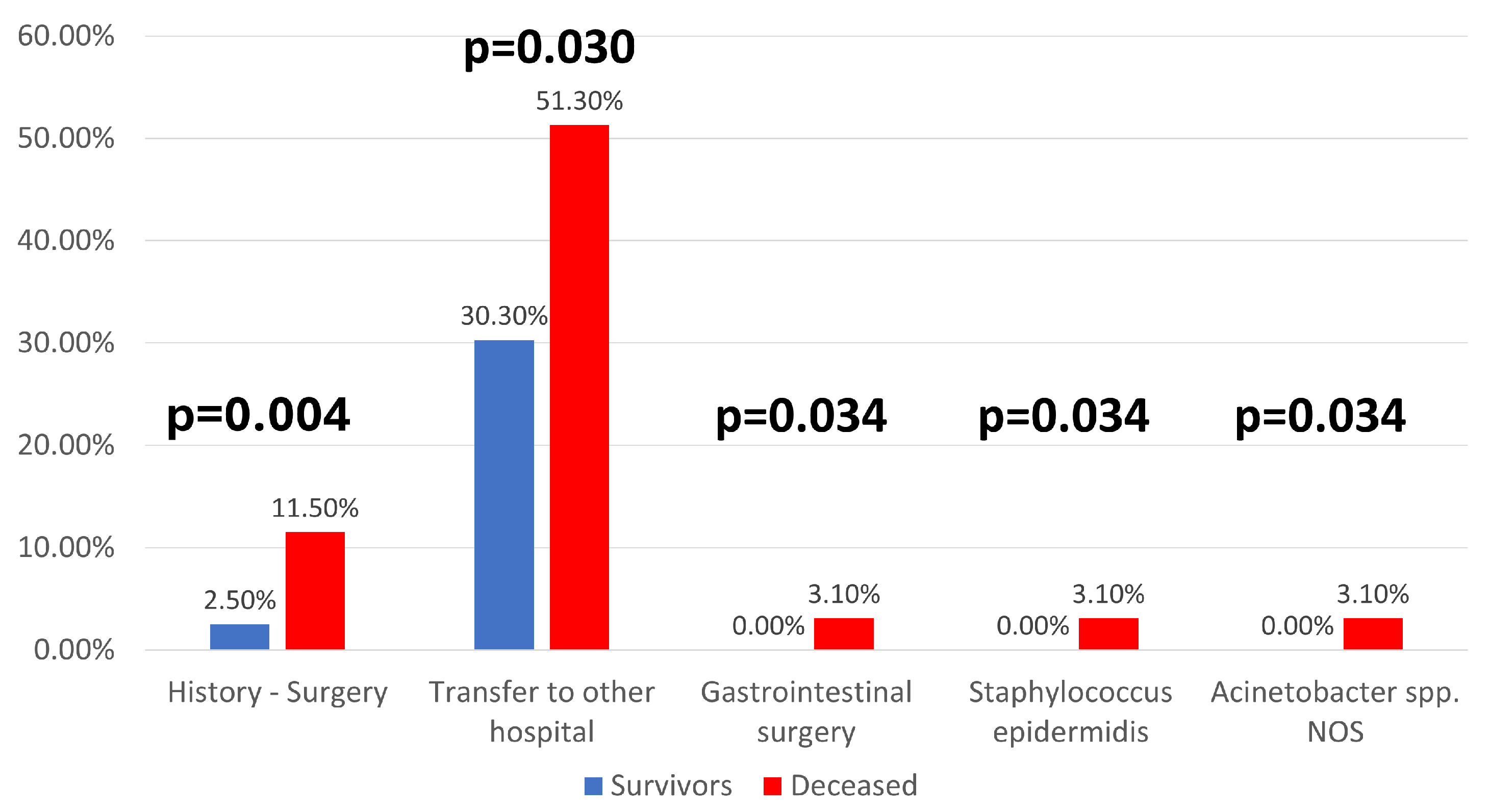
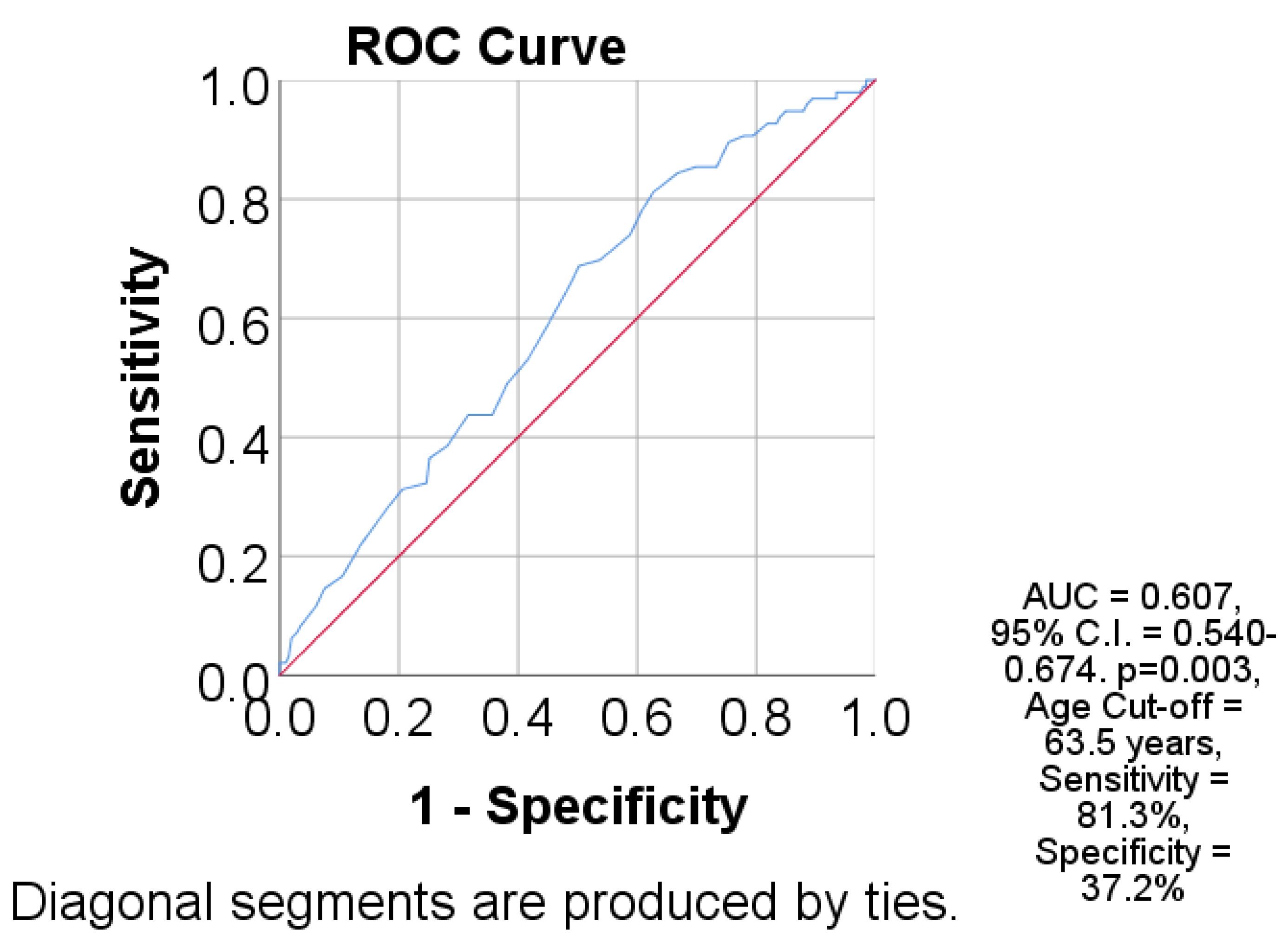
| Parameter (No., %) | Total | Survivors | Deceased | p |
|---|---|---|---|---|
| N | 295 (100%) | 199 (67.5%) | 96 (32.5%) | - |
| Age (Median (IQR)) | 69 (61–77) | 68 (60–76) | 71 (65–80) | 0.003 ** |
| Gender (Male) | 150 (50.8%) | 106 (53.3%) | 44 (45.8%) | 0.264 * |
| Environment (Urban) | 224 (75.9%) | 151 (75.9%) | 73 (76%) | 1.000 * |
| HAIs type | ||||
| CDI | 248 (84.1%) | 172 (86.4%) | 76 (79.2%) | 0.016 * |
| Respiratory | 22 (7.5%) | 9 (4.5%) | 13 (13.5%) | |
| UTI | 17 (5.8%) | 14 (7%) | 3 (3.1%) | |
| Other | 8 (2.7%) | 4 (2%) | 4 (4.2%) | |
| History-Surgery | 16 (5.4%) | 5 (2.5%) | 11 (11.5%) | 0.004 * |
| Isolated | 262 (89.1%) | 181 (91%) | 81 (85.3%) | 0.162 * |
| Infected contacts | 56 (19.1%) | 39 (19.7%) | 17 (17.9%) | 0.753 * |
| HAIs classification | ||||
| From the reporting hospital | 180 (61%) | 115 (57.8%) | 65 (67.7%) | 0.326 * |
| From another hospital | 7 (2.4%) | 6 (3%) | 1 (1%) | |
| CDI | 107 (36.3%) | 77 (38.7%) | 30 (31.3%) | |
| Chronic healthcare units | 1 (0.3%) | 1 (0.5%) | 0 (0%) | |
| Immunodepression | 34 (11.5%) | 21 (10.6%) | 13 (13.5%) | 0.443 * |
| Transfer to another hospital | 50 (36.2%) | 30 (30.3%) | 20 (51.3%) | 0.030 * |
| Recurrence | 10 (8.8%) | 8 (9.8%) | 2 (6.5%) | 0.725 * |
| Complications | 50 (16.9%) | 28 (14.1%) | 22 (22.9%) | 0.069 * |
| Admission—Last year | 84 (56.8%) | 55 (51.9%) | 29 (69%) | 0.067 * |
| Last admission | ||||
| Less than 4 weeks ago | 73 (90.1%) | 47 (88.7%) | 26 (92.9%) | 0.843 * |
| 4–12 weeks ago | 3 (3.7%) | 2 (3.8%) | 1 (3.6%) | |
| >12 weeks ago | 5 (6.2%) | 4 (7.5%) | 1 (3.6%) | |
| Admission location | ||||
| CDI reporting hospital | 26 (31%) | 19 (35.2%) | 7 (23.3%) | 0.328 * |
| Other hospital | 58 (69%) | 35 (64.8%) | 23 (76.7%) |
| Parameter (No., %) | Total | Survivors | Deceased | p |
|---|---|---|---|---|
| CDI—Antibiotic treatment | 214 (72.5%) | 145 (72.9%) | 69 (71.9%) | 0.890 * |
| No. Treatment sessions | ||||
| One session | 92 (43%) | 68 (46.9%) | 24 (34.8%) | 0.106 * |
| >1 session | 122 (57%) | 77 (53.1%) | 45 (65.2%) | |
| No. Antibiotics | ||||
| One | 125 (58.4%) | 90 (62.1%) | 35 (50.7%) | 0.138 * |
| Combination | 89 (41.6%) | 55 (37.9%) | 34 (49.3%) | |
| Last 3 months—Treatment | 73 (24.7%) | 53 (26.6%) | 20 (20.8%) | 0.315 * |
| No. Treatment sessions | ||||
| One session | 35 (47.9%) | 24 (45.3%) | 11 (55%) | 0.600 * |
| >1 session | 38 (52.1%) | 29 (54.7%) | 9 (45%) | |
| No. Antibiotics | ||||
| One | 43 (58.9%) | 33 (62.3%) | 10 (50%) | 0.426 * |
| Combination | 30 (41.1%) | 20 (37.7%) | 10 (50%) | |
| Immunosuppressants | 28 (9.5%) | 18 (9%) | 10 (10.4%) | 0.678 * |
| Gastric antisecretory agents | 113 (38.3%) | 77 (38.7%) | 36 (37.5%) | 0.899 * |
| Gastrointestinal surgery | 3 (1%) | 0 (0%) | 3 (3.1%) | 0.034 * |
| Chemotherapy | 3 (1%) | 1 (0.5%) | 2 (2.1%) | 0.248 * |
| Contact with the CDI case | 15 (5.1%) | 11 (5.5%) | 4 (4.2%) | 0.780 * |
| A/B toxin | 248 (84.1%) | 172 (86.4%) | 76 (79.2%) | 0.127 * |
| PCR gene detection | 5 (1.7%) | 4 (2%) | 1 (1%) | 1.000 * |
| Official HAIs-CDI source | ||||
| Reporting hospital | 239 (96%) | 167 (96.5%) | 72 (94.7%) | 0.641 * |
| Other hospital | 8 (3.2%) | 4 (2.3%) | 4 (5.3%) | |
| Outpatient unit | 1 (0.4%) | 1 (0.6%) | 0 (0%) | |
| Chronic healthcare unit | 1 (0.4%) | 1 (0.6%) | 0 (0%) | |
| Identified microorganism | ||||
| Clostridium difficile | 249 (84.4%) | 173 (86.9%) | 76 (79.2%) | 0.090 * |
| Pseudomonas aeruginosa | 7 (2.4%) | 7 (3.5%) | 0 (0%) | 0.100 * |
| Escherichia coli | 6 (2%) | 5 (2.5%) | 1 (1%) | 0.668 * |
| Klebsiella spp. NOS | 2 (0.7%) | 1 (0.5%) | 1 (1%) | 0.546 * |
| Klebsiella oxytoca | 1 (0.3%) | 1 (0.5%) | 0 (0%) | 1.000 * |
| Klebsiella pneumonia | 6 (2%) | 2 (1%) | 4 (4.2%) | 0.090 * |
| Staphylococcus epidermidis | 3 (1%) | 0 (0%) | 3 (3.1%) | 0.034 * |
| Staphylococcus aureus | 3 (1%) | 3 (1.5%) | 0 (0%) | 0.553 * |
| Enterococcus spp. NOS | 5 (1.7%) | 3 (1.5%) | 2 (2.1%) | 0.662 * |
| Acinetobacter spp. NOS | 3 (1%) | 0 (0%) | 3 (3.1%) | 0.034 * |
| Acinetobacter baumannii | 9 (3.1%) | 6 (3%) | 3 (3.1%) | 1.000 * |
| Candida krusei | 2 (0.7%) | 1 (0.5%) | 1 (1%) | 0.546 * |
| Hafnia spp. | 1 (0.3%) | 1 (0.5%) | 0 (0%) | 1.000 * |
| Parameter | AUC (95% C.I.) | Std. Error | p | Cut-Off | Se | Sp |
|---|---|---|---|---|---|---|
| Age | 0.607 (0.540–0.674) | 0.034 | 0.003 | 63.5 | 81.3 | 37.2 |
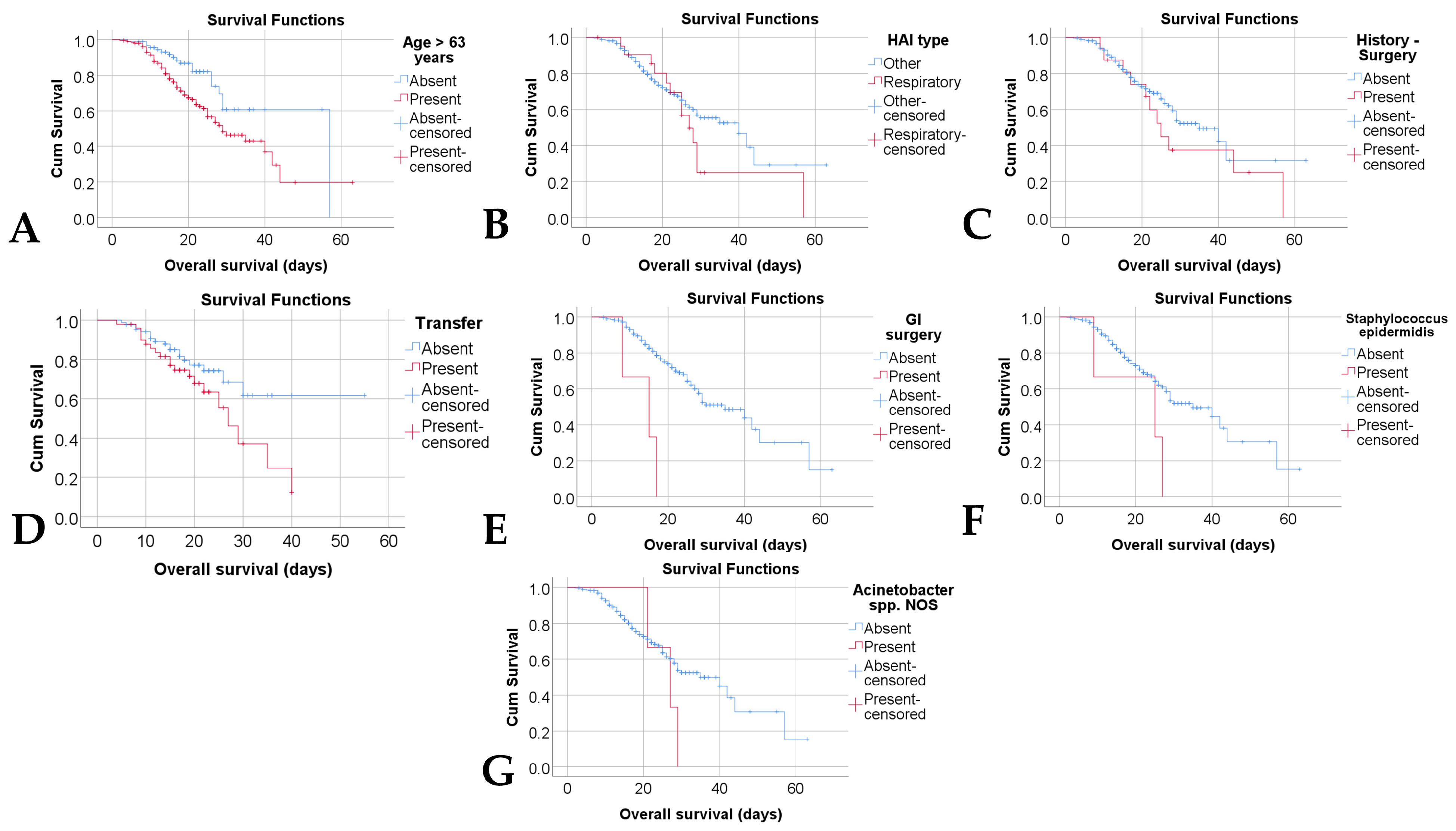
| Age > 63 Years | Mean | 95% C.I. | p * |
|---|---|---|---|
| Absent | 43.22 | 37.33–49.11 | 0.005 |
| Present | 33.21 | 28.21–38.21 | |
| HAIs type | |||
| Other | 37.33 | 32.14–42.52 | 0.251 |
| Respiratory | 31.38 | 23.13–39.62 | |
| History—Surgery | |||
| Absent | 37.31 | 31.89–42.73 | 0.276 |
| Present | 31.77 | 22.25–41.30 | |
| Transfer | |||
| Absent | 41.10 | 35.26–46.94 | 0.060 |
| Present | 26.44 | 22.22–30.66 | |
| GI surgery | |||
| Absent | 36.14 | 31.82–40.45 | <0.001 |
| Present | 13.33 | 7.98–18.68 | |
| Staphylococcus epidermidis | |||
| Absent | 36.29 | 31.93–40.65 | 0.047 |
| Present | 20.33 | 9.16–31.49 | |
| Acinetobacter spp. NOS | |||
| Absent | 36.24 | 31.95–40.72 | 0.211 |
| Present | 25.66 | 20.95–30.37 |
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age > 63 years | 2.051 (1.228–3.426) | 0.006 | 1.988 (1.188–3.327) | 0.009 |
| GI surgery | 6.599 (2.063–21.103) | 0.001 | 5.557 (1.731–17.845) | 0.004 |
| Staphylococcus epidermidis | 3.014 (0.949–9.574) | 0.061 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voinea, C.; Mocanu, E.; Dantes, E.; Jurja, S.; Neculai, A.-M.; Craciun, A.; Rugina, S. Burden of Healthcare-Associated Infections on Mortality Among COVID-19 Hospitalized Patients. J. Clin. Med. 2025, 14, 8279. https://doi.org/10.3390/jcm14238279
Voinea C, Mocanu E, Dantes E, Jurja S, Neculai A-M, Craciun A, Rugina S. Burden of Healthcare-Associated Infections on Mortality Among COVID-19 Hospitalized Patients. Journal of Clinical Medicine. 2025; 14(23):8279. https://doi.org/10.3390/jcm14238279
Chicago/Turabian StyleVoinea, Corina, Elena Mocanu, Elena Dantes, Sanda Jurja, Ana-Maria Neculai, Aurora Craciun, and Sorin Rugina. 2025. "Burden of Healthcare-Associated Infections on Mortality Among COVID-19 Hospitalized Patients" Journal of Clinical Medicine 14, no. 23: 8279. https://doi.org/10.3390/jcm14238279
APA StyleVoinea, C., Mocanu, E., Dantes, E., Jurja, S., Neculai, A.-M., Craciun, A., & Rugina, S. (2025). Burden of Healthcare-Associated Infections on Mortality Among COVID-19 Hospitalized Patients. Journal of Clinical Medicine, 14(23), 8279. https://doi.org/10.3390/jcm14238279





