Body Mass Index and Hemoglobin A1c Correlate with Clinical Needs After COVID-19 Vaccination in the Veterans Affairs System
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| COVID-19 | SARS Coronavirus-19 |
| HbA1c | Hemoglobin A1c |
| HR | Hazard Ratio |
| OR | Odds Ratio |
| US | United States |
| VA | Veterans Affairs |
| VINCI | VA Informatics and Computation Infrastructure |
References
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’dOnnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.; Sucosky, M.; Lange, S.; Gundlapalli, A.; Boehmer, T.; Blanck, H. Body Mass Index and Risk for COVID-19-Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death—United States, March–December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Boppana, A.; Traupman, J.A.; Unson, E.; Maddock, D.A.; Chao, K.; Dobesh, D.P.; Brufsky, A.; Connor, R.I. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J. Med. Virol. 2021, 93, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Heubner, L.; Petrick, P.L.; Güldner, A.; Bartels, L.; Ragaller, M.; Mirus, M.; Rand, A.; Tiebel, O.; Beyer-Westendorf, J.; Rößler, M.; et al. Extreme obesity is a strong predictor for in-hospital mortality and the prevalence of long-COVID in severe COVID-19 patients with acute respiratory distress syndrome. Sci. Rep. 2022, 12, 18418. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef] [PubMed]
- Movahed, M.R.; Khoubyari, R.; Hashemzadeh, M.; Hashemzadeh, M. Obesity is strongly and independently associated with a higher prevalence of pulmonary embolism. Respir. Investig. 2019, 57, 376–379. [Google Scholar] [CrossRef]
- Yang, J.; Hu, J.; Zhu, C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Kassir, R. Risk of COVID-19 for patients with obesity. Obes. Rev. 2020, 21, e13034. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Locke, E.; Green, P.; Berry, K.; O’hAre, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Dominitz, J.A.; Fan, V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10131 US Veterans With SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022310. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Kjölhede, E.A.; Rawshani, A.; Sattar, N.; Eeg-Olofsson, K.; Adiels, M.; Ludvigsson, J.; Lindh, M.; Gisslén, M.; Hagberg, E.; et al. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: A nationwide retrospective cohort study. Lancet Reg. Health Eur. 2021, 4, 100105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, Z.; Zhu, F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res. Clin. Pract. 2020, 164, 108214. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N. Metabolic disorders, COVID-19 and vaccine-breakthrough infections. Nat. Rev. Endocrinol. 2022, 18, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Verde, L.; Vetrani, C.; Savastano, S.; Colao, A. The “identikit” of subject with obesity and COVID-19 vaccine breakthrough. EXCLI J. 2022, 21, 687–694. [Google Scholar] [PubMed]
- Juthani, P.V.; Gupta, A.; Borges, K.A.; Price, C.C.; Lee, A.I.; Won, C.H.; Chun, H.J. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect. Dis. 2021, 21, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Bajema, K.; Dahl, N.; Evener, S.; Prill, M.; Rodriguez-Barradas, M.; Marconi, V.; Beenhouwer, D.; Holodniy, M.; Lucero-Obusan, C.; Brown, S.; et al. Comparative Effectiveness and Antibody Responses to Moderna and Pfizer-BioNTech COVID-19 Vaccines among Hospitalized Veterans—Five Veterans Affairs Medical Centers, United States, 1 February–30 September, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Cohn, B.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2022, 375, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Puskarich, M.A.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J.; et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. 2023, 23, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Adatorwovor, R.; Davarpanah, M.A.; Mansoori, Y.; Hajiani, M.; Azodi, F.; Sefidbakht, S.; Davoudi, S.; Rezaei, F.; Mohammadmoradi, S.; et al. A Randomized Trial of Sitagliptin and Spironolactone with Combination Therapy in Hospitalized Adults With COVID-19. J. Endocr. Soc. 2022, 6, bvac017. [Google Scholar] [CrossRef] [PubMed]
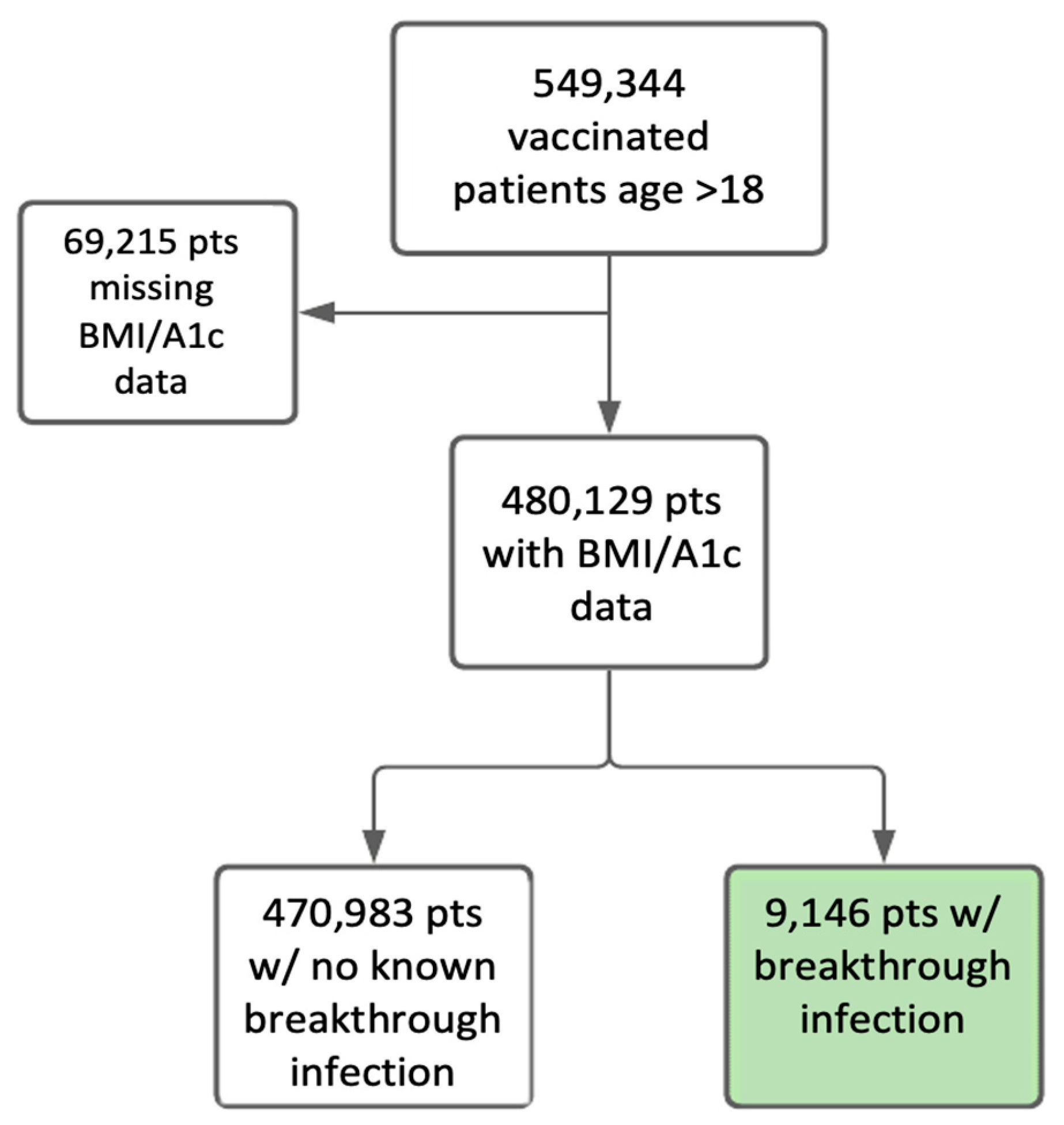
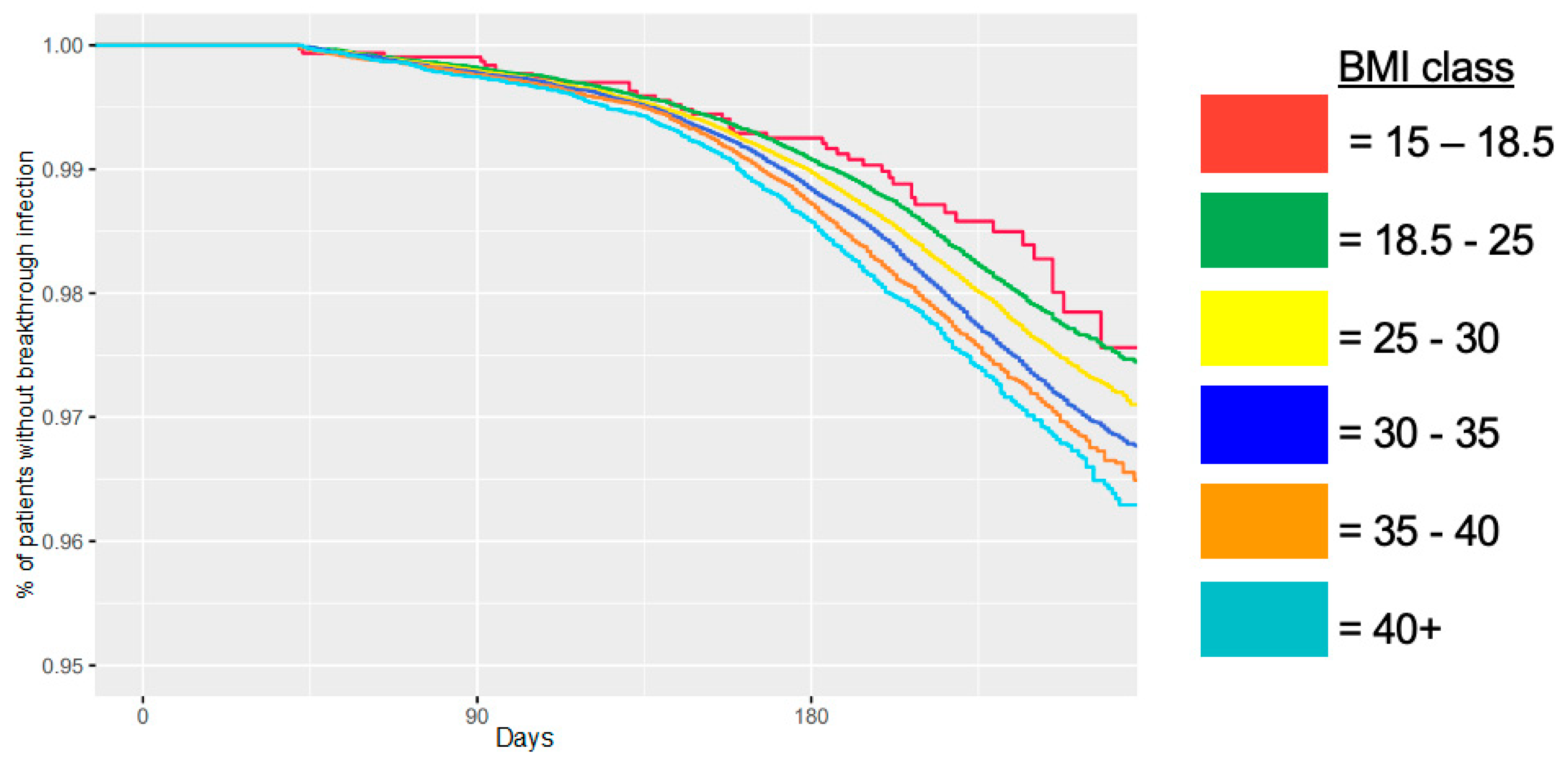
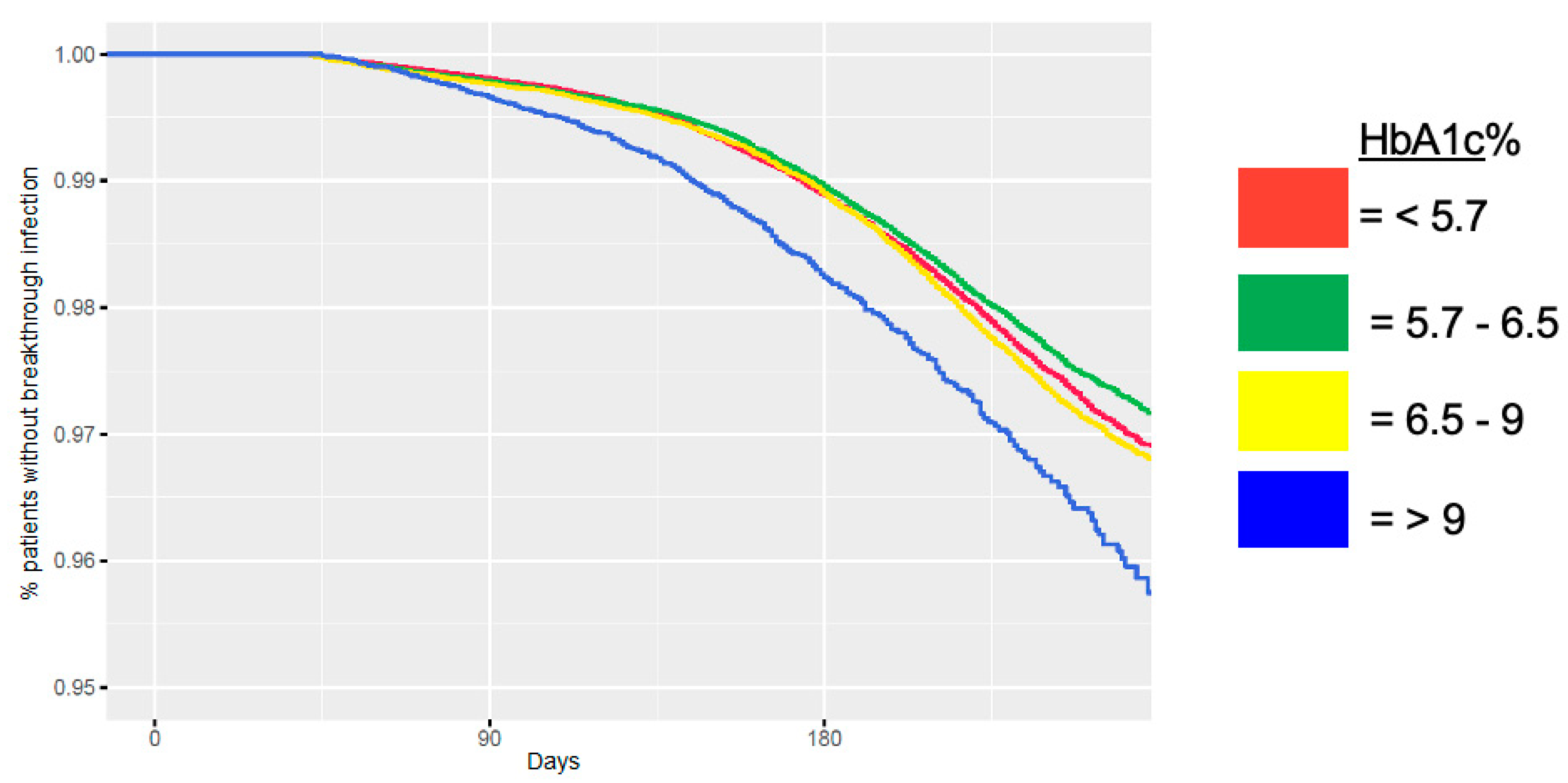
| Characteristic | Category | N (% of Total) | No Breakthrough Infection N (% in Category) | Breakthrough Infection N (% in Category) | |||
|---|---|---|---|---|---|---|---|
| Total | Vaccinated patients | 549,344 (100.0%) | 440,868 (80.2%) | 4090 (0.75%) | |||
| Race | White | 357,929 (65.2%) | 292,544 (66.4%) | 2628 (64.2%) | |||
| Black or African American | 123,230 (22.3%) | 94,261 (21.4%) | 951 (23.7%) | ||||
| Multi-race | 22,012 (4.0%) | 17,245 (3.9%) | 167 (4.1%) | ||||
| Asian | 6627 (1.2%) | 5361 (1.2%) | 429 (0.7%) | ||||
| Other | 8526 (1.6%) | 6733 (1.2%) | 60 (0.01%) | ||||
| Missing | 32,020 (5.8%) | 24,724 (5.6%) | 255 (6.2%) | ||||
| Ethnicity | Not Hispanic or Latino | 437,453 | (79.6%) | 354,718 | (80.4%) | 3173 | (77.6%) |
| Multi-ethnic | 47,764 | (8.7%) | 36,506 | (8.3%) | 394 | (9.6%) | |
| Hispanic or Latino | 42,646 | (7.8%) | 33,516 | (7.6%) | 362 | (8.9%) | |
| Missing | 21,481 | (3.9%) | 16,128 | (3.7%) | 161 | (3.9%) | |
| Sex | M | 490,892 | (89.4%) | 397,764 | (90.2%) | 3665 | (89.6%) |
| F | 58,452 | (10.6%) | 43,104 | (9.8%) | 425 | (10.4%) | |
| Age (years) | Median | 67 | 69 | 65 | |||
| Mean (SD) | 64.5 | (14.3) | 66.1 | (13.3) | 63.2 | (13.9) | |
| Variable | Category | N | Value | % of Total | No Breakthrough Infection N (% in Category) | Breakthrough Infection N (% in Category) | ||
|---|---|---|---|---|---|---|---|---|
| Body Mass Index (BMI) | Missing data | 33,698 | 6.1% | 26,066 | (5.9%) | 520 | (8.2%) | |
| Available data | 518,958 | 93.9% | 414,868 | (94.1%) | 5802 | (91.8%) | ||
| Median | 29.6 | 29.7 | 30.5 | |||||
| Mean (SD) | 30.3 | (6.1) | 30.4 | (6.0) | 31.2 * | (6.2) | ||
| Variable | Class | Variable | N% of Total | Value | No Breakthrough Infection N (% in Category) | Breakthrough Infection N (% in Category) | |||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c (%) | Missing | 45,127 | (8.2%) | 33,981 | (7.7%) | 380 | (9.3%) | ||
| Available data | 504,217 | (91.8%) | 406,887 | (92.3%) | 3710 | (90.7%) | |||
| Median | 5.8 | 5.8 | 5.9 | ||||||
| Mean (SD) | 6.18 (1.27) | 6.20 | (1.24) | 6.34 * | (1.5) | ||||
| Model Without BMI or HbA1c | Model with BMI | Model with HbA1c | Model with BMI and HbA1c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | HR Change per Unit Change | p-Value | Coefficient | HR Change per Unit Change | p-Value | Coefficient | HR Change per Unit Change | p-Value | Coefficient | HR Change per Unit Change | p-Value | |
| Female | −0.182 | 0.834 | 3.49 × 10−6 | −0.183 | 0.833 | 3.04 × 10−6 | −0.165 | 0.848 | 0.000 | −0.168 | 0.845 | 1.72 × 10−1 |
| Age | −0.007 | 9.93 × 10−1 | 3.70 × 10−15 | −0.006 | 9.95 × 10−1 | 3.55 × 10−10 | −0.008 | 9.92 × 10−1 | 6.06 × 10−19 | −0.007 | 9.94 × 10−1 | 2.78 × 10−13 |
| BMI | 0.017 | 1.017 | 1.16 × 10−23 | 0.01 | 1.015 | 1.89 × 10−16 | ||||||
| HbA1c | 0.07 | 1.077 | 6.07 × 10−22 | 0.06 | 1.063 | 2.83 × 10−14 | ||||||
| Model Without BMI or A1c | Model with BMI | Model with A1c | Model with BMI and A1c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | OR Change per Unit Change | p-Value | Coefficient | OR Change per Unit Change | p-Value | Coefficient | OR Change per Unit Change | p-Value | Coefficient | OR Change per Unit Change | p-Value | |
| Female | −0.450 | 0.637 | 4.84 × 10−3 | −0.457 | 0.633 | 4.28 × 10−3 | −0.402 | 0.669 | 1.22 × 10−2 | −0.408 | 0.665 | 1.10 × 10−2 |
| Age | 0.060 | 1.062 | 4.42 × 10−87 | 0.062 | 1.064 | 2.22 × 10−87 | 0.060 | 1.062 | 1.15 × 10−85 | 0.062 | 1.064 | 3.08 × 10−84 |
| BMI | 0.017 | 1.017 | 0.001 | 0.010 | 1.010 | 0.053 | ||||||
| HbA1c | 0.1468 | 1.1581 | 1.37 × 10−13 | 0.1393 | 1.1495 | 6.47 × 10−12 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendse, J.; Jordan, G.; Wang, B.; Tenner, C.; Dorcely, B.; Ulrich, R.J.; Zhang, K.; Felson, S.; Jay, M.; Alemán, J.O. Body Mass Index and Hemoglobin A1c Correlate with Clinical Needs After COVID-19 Vaccination in the Veterans Affairs System. J. Clin. Med. 2025, 14, 8271. https://doi.org/10.3390/jcm14238271
Pendse J, Jordan G, Wang B, Tenner C, Dorcely B, Ulrich RJ, Zhang K, Felson S, Jay M, Alemán JO. Body Mass Index and Hemoglobin A1c Correlate with Clinical Needs After COVID-19 Vaccination in the Veterans Affairs System. Journal of Clinical Medicine. 2025; 14(23):8271. https://doi.org/10.3390/jcm14238271
Chicago/Turabian StylePendse, Jay, Gabriela Jordan, Binhuan Wang, Craig Tenner, Brenda Dorcely, Robert J. Ulrich, Kevin Zhang, Sabrina Felson, Melanie Jay, and José O. Alemán. 2025. "Body Mass Index and Hemoglobin A1c Correlate with Clinical Needs After COVID-19 Vaccination in the Veterans Affairs System" Journal of Clinical Medicine 14, no. 23: 8271. https://doi.org/10.3390/jcm14238271
APA StylePendse, J., Jordan, G., Wang, B., Tenner, C., Dorcely, B., Ulrich, R. J., Zhang, K., Felson, S., Jay, M., & Alemán, J. O. (2025). Body Mass Index and Hemoglobin A1c Correlate with Clinical Needs After COVID-19 Vaccination in the Veterans Affairs System. Journal of Clinical Medicine, 14(23), 8271. https://doi.org/10.3390/jcm14238271






