Multicenter Evaluation of Different Anti-Xa Assays and Diluted Russell’s Viper Venom Time in Ex Vivo Plasma Samples from Patients Treated with Rivaroxaban or Apixaban
Abstract
1. Introduction
2. Materials and Methods
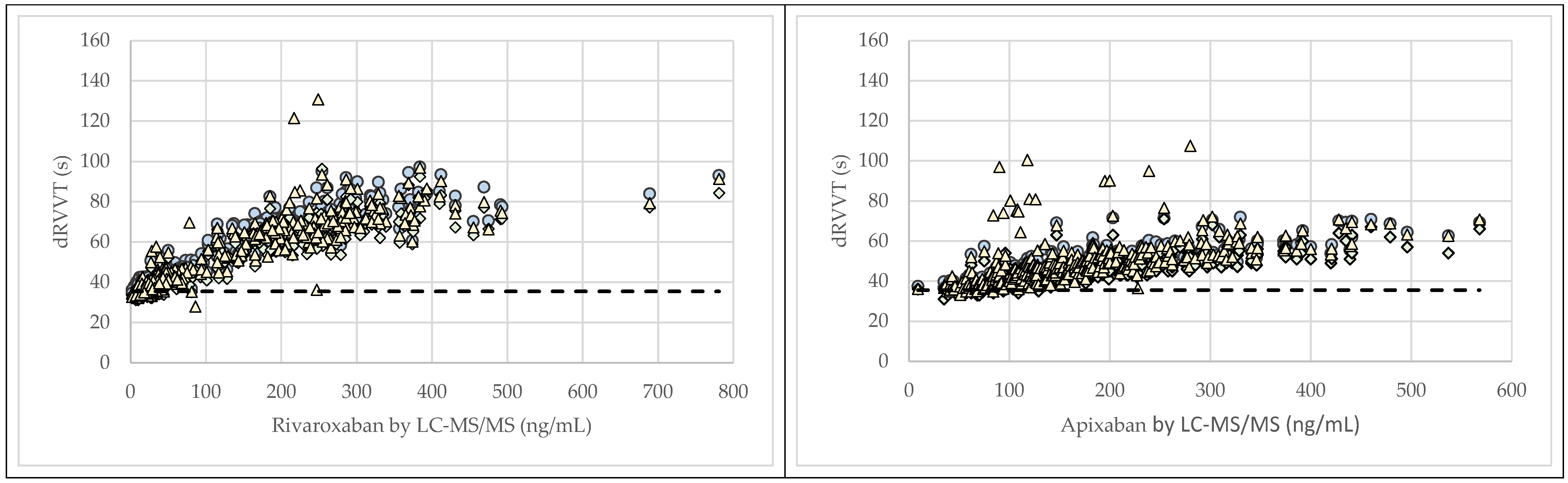
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APTT | activated partial thromboplastin time |
| DXI | direct factor X inhibitors |
| dRVVT | diluted Russell’s viper venom time |
| LC-MS/MS | liquid chromatography with tandem mass spectrometry |
References
- Cohen, A.T.; Hamilton, M.; Mitchell, S.A.; Phatak, H.; Liu, X.; Bird, A.; Tushabe, D.; Batson, S. Comparison of the Novel Oral Anticoagulants Apixaban, Dabigatran, Edoxaban, and Rivaroxaban in the Initial and Long-Term Treatment and Prevention of Venous Thromboembolism: Systematic Review and Network Meta-Analysis. PLoS ONE 2015, 10, e0144856. [Google Scholar] [CrossRef] [PubMed]
- Douxfils, J.; Pochet, L.; Lessire, S.; Vancraeynest, C.; Dogné, J.M.; Mullier, F. Mass spectrometry in the therapeutic drug monitoring of direct oral anticoagulants. Useful or useless? TrAC Trends Anal. Chem. 2016, 84, 41–50. [Google Scholar] [CrossRef]
- Douxfils, J.; Mullier, F.; Loosen, C.; Chatelain, C.; Chatelain, B.; Dogne, J.M. Assessment of the impact of rivaroxaban on coagulation assays: Laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb. Res. 2012, 130, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Gouin-Thibault, I.; Flaujac, C.; Delavenne, X.; Quenet, S.; Horellou, M.H.; Laporte, S.; Siguret, V.; Lecompte, T. Assessment of apixaban plasma levels by laboratory tests: Suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb. Haemost. 2014, 111, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.E.; Song, Y.; Shenker, A.; Wang, J.; Barrett, Y.C.; Schuster, A.; Harris, S.I.; LaCreta, F. Effects of Age and Sex on the Single-Dose Pharmacokinetics and Pharmacodynamics of Apixaban. Clin. Pharmacokinet. 2015, 54, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Studt, J.D.; Alberio, L.; Angelillo-Scherrer, A.; Asmis, L.M.; Fontana, P.; Korte, W.; Mendez, A.; Schmid, P.; Stricker, H.; Tsakiris, D.A.; et al. Accuracy and consistency of anti-Xa activity measurement for determination of rivaroxaban plasma levels. J. Thromb. Haemost. 2017, 15, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Al-Aieshy, F.; Malmstrom, R.E.; Antovic, J.; Pohanka, A.; Ronquist-Nii, Y.; Berndtsson, M.; Al-Khalili, F.; Skeppholm, M. Clinical evaluation of laboratory methods to monitor exposure of rivaroxaban at trough and peak in patients with atrial fibrillation. Eur. J. Clin. Pharmacol. 2016, 72, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Yang, H.; Barrett, Y.; Mohan, P.; Wang, J.; Wallentin, L.; Alexander, J.H. Chromogenic laboratory assays to measure the factor Xa-inhibiting properties of apixaban--an oral, direct and selective factor Xa inhibitor. J. Thromb. Thrombolysis 2011, 32, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Topic, E.; Nikolac, N.; Panteghini, M.; Theodorsson, E.; Salvagno, G.L.; Miler, M.; Simundic, A.M.; Infusino, I.; Nordin, G.; Westgard, S. How to assess the quality of your analytical method? Clin. Chem. Lab. Med. 2015, 53, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Celap, I.; Margetic, S.; Brcic, M.; Mihic, R. Analytical verification and comparison of chromogenic assays for dabigatran, rivaroxaban and apixaban determination on BCSXP and STA Compact Max analysers. Biochem. Med. 2020, 30, 010706. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Steiner, T.; Caso, V.; Wahlgren, N.; ESO-KSU Session Participants. Recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 13–15 November 2016. Eur. Stroke J. 2017, 2, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Ageno, W.; Chan, N.C.; Crowther, M.; Verhamme, P.; Weitz, J.I.; Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Exner, T.; Ellwood, L.; Rubie, J.; Barancewicz, A. Testing for new oral anticoagulants with LA-resistant Russells viper venom reagents. An in vitro study. Thromb. Haemost. 2013, 109, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Mavri, A.; Vene, N.; Bozic-Mijovski, M.; Miklic, M.; Soderblom, L.; Pohanka, A.; Malmstrom, R.E.; Antovic, J. Apixaban concentration variability and relation to clinical outcomes in real-life patients with atrial fibrillation. Sci. Rep. 2021, 11, 13908. [Google Scholar] [CrossRef] [PubMed]
- Miklic, M.; Mavri, A.; Vene, N.; Soderblom, L.; Bozic-Mijovski, M.; Pohanka, A.; Antovic, J.; Malmstrom, R.E. Intra- and inter- individual rivaroxaban concentrations and potential bleeding risk in patients with atrial fibrillation. Eur. J. Clin. Pharmacol. 2019, 75, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Skeppholm, M.; Al-Aieshy, F.; Berndtsson, M.; Al-Khalili, F.; Ronquist-Nii, Y.; Soderblom, L.; Ostlund, A.Y.; Pohanka, A.; Antovic, J.; Malmstrom, R.E. Clinical evaluation of laboratory methods to monitor apixaban treatment in patients with atrial fibrillation. Thromb. Res. 2015, 136, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Božič, M.; Malovrh, P. Anti-Xa assay for measurement of plasma rivaroxaban is not affected by the addition of exogenous antithrombin. Thromb. Res. 2014, 133, 585. [Google Scholar] [CrossRef]
- Cini, M.; Legnani, C.; Padrini, R.; Cosmi, B.; Dellanoce, C.; De Rosa, G.; Marcucci, R.; Pengo, V.; Poli, D.; Testa, S.; et al. DOAC plasma levels measured by chromogenic anti-Xa assays and HPLC-UV in apixaban- and rivaroxaban-treated patients from the START-Register. Int. J. Lab. Hematol. 2020, 42, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.C.; Adcock Funk, D.M.; Taylor, J.M.; Francart, S.J.; Hawes, E.M.; Friedman, K.D.; Moll, S. Comparison of anti-Xa and dilute Russell viper venom time assays in quantifying drug levels in patients on therapeutic doses of rivaroxaban. Arch. Pathol. Lab. Med. 2014, 138, 1680–1684. [Google Scholar] [CrossRef] [PubMed]
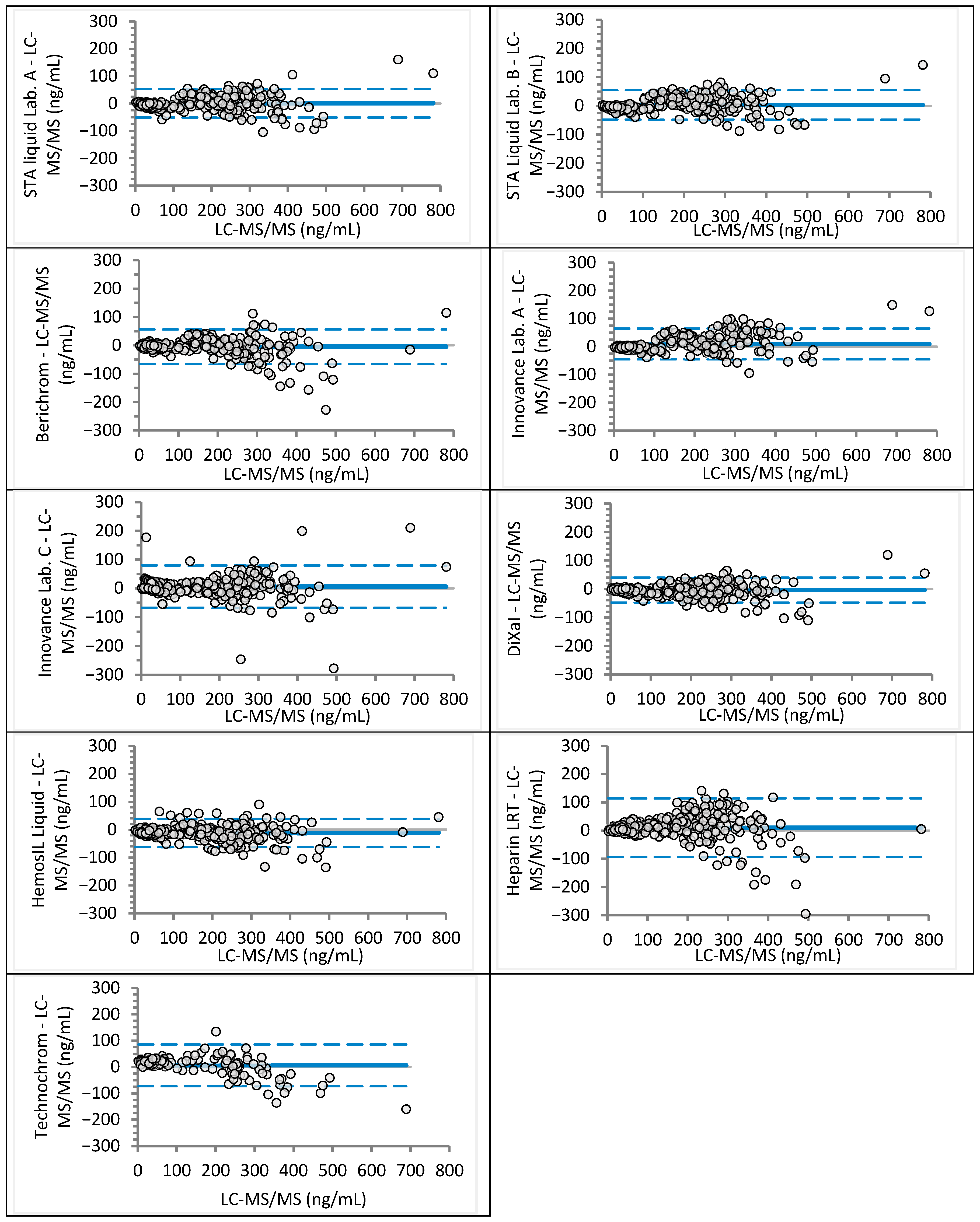
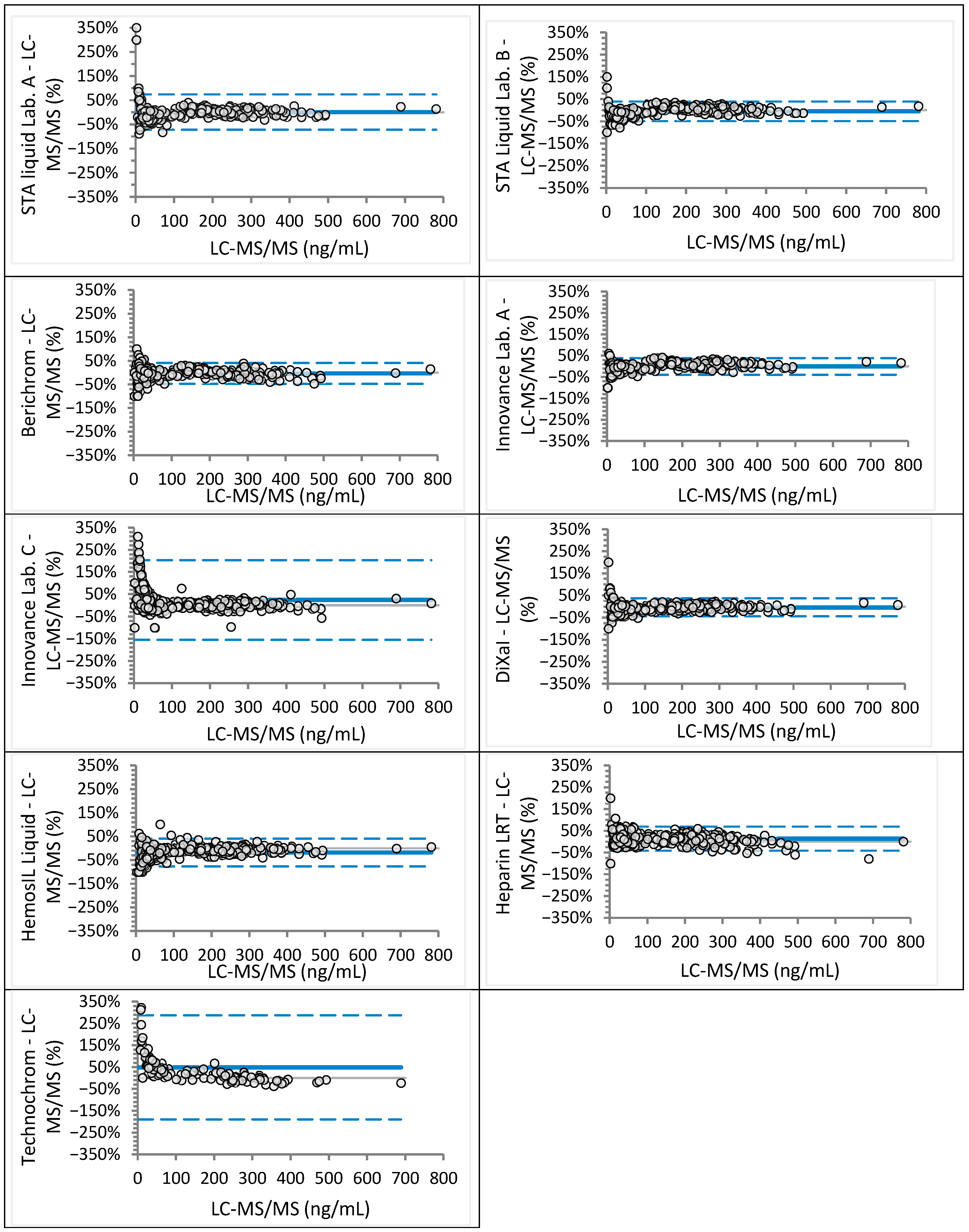
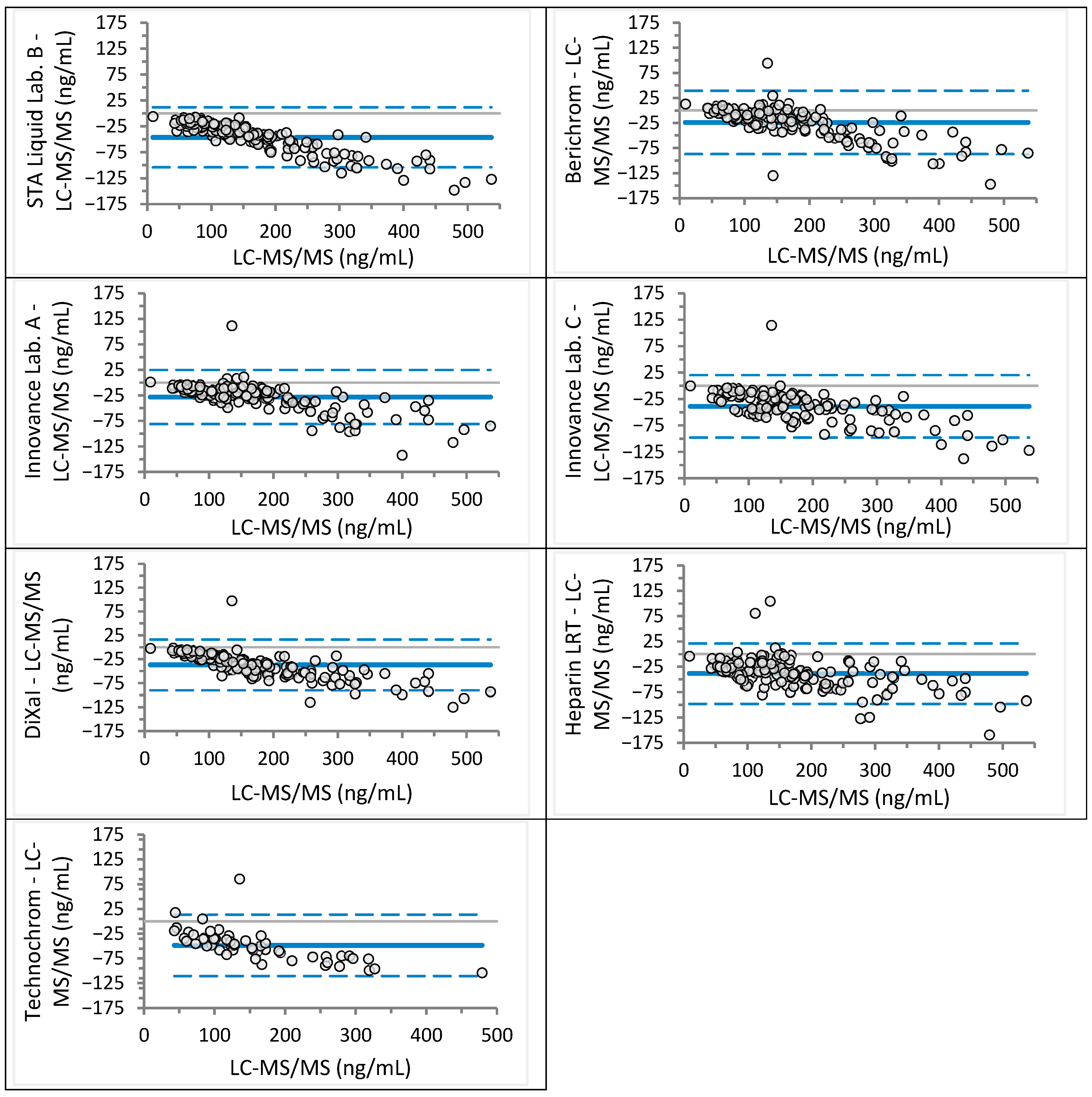
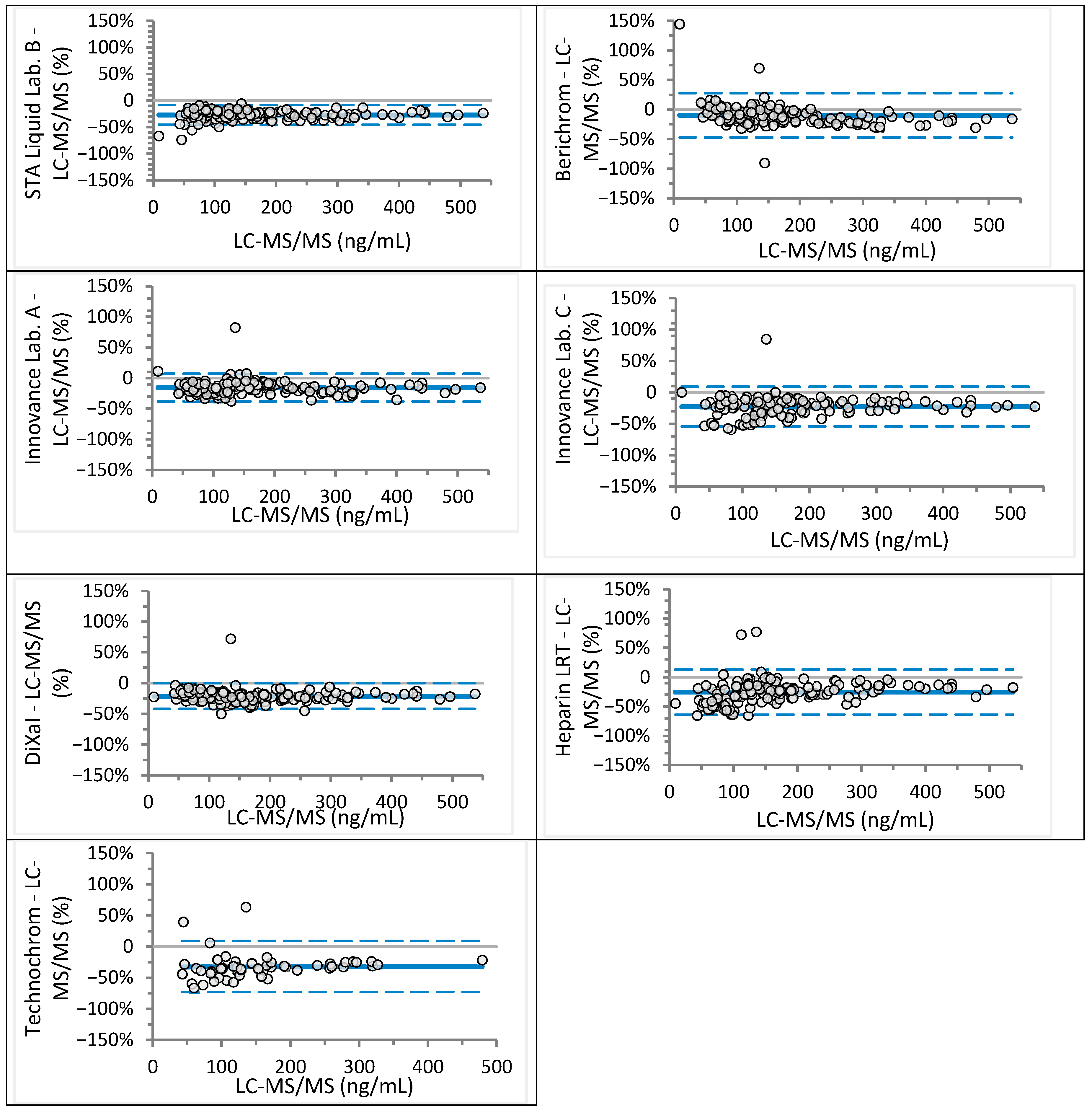
| Laboratory | Analyzer (Manufacturer) | Anti-Xa Assay (Manufacturer) | Rivaroxaban Calibrator and Control Set (Manufacturer) | Apixaban Calibrator and Control Set (Manufacturer) |
|---|---|---|---|---|
| Lab. A | CS-2500 (Sysmex, Kobe, Japan) | STA-Liquid Anti-Xa (Diagnostica Stago, Asnières sur Seine, France) | STA-Rivaroxaban Calibrator, STA-Rivaroxaban Control (Diagnostica Stago) | / |
| Berichrom Heparin (Siemens Healthineers, Marburg, Germany) | STA-Apixaban Calibrator, STA-Apixaban Control (Diagnostica Stago) | |||
| Innovance Heparin (Siemens Healthineers) | ||||
| Biophen DiXaI (Hyphen Biomed, Neuville-sur-Oise, Francija) | Biophen Rivaroxaban Plasma Calibrator, Biophen Rivaroxaban Calibrator Low, Biophen Rivaroxaban Control Plasma, Biophen Rivaroxaban Control Low (Hyphen Biomed) | Biophen Apixaban Plasma Calibrator, Biophen Apixaban Calibrator Low, Biophen Apixaban Control Plasma, Biophen Apixaban Control Low (Hyphen Biomed) | ||
| HemosIL Liquid Anti-Xa (Werfen, Bedford, MA, United States) | HemosIL Rivaroxaban Calibrators, HemosIL Rivaroxaban Controls (Werfen) | / | ||
| Lab. B | CS-2100i (Sysmex) | STA-Liquid Anti-Xa (Diagnostica Stago) | STA-Rivaroxaban Calibrator, STA-Rivaroxaban Control (Diagnostica Stago) | STA-Apixaban Calibrator, STA-Apixaban Control (Diagnostica Stago) |
| Lab. C | CS-5100 (Sysmex) | Innovance Heparin (Siemens Healthineers) | Technoview Rivaroxaban CAL High Set, Technoview Rivaroxaban CAL Set, Technoview Rivaroxaban High, Medium and Low Control (Technoclone) | Technoview Apixaban CAL Set, Technoview Apixaban Low and High Control (Technoclone, Vienna, Austria) |
| Biophen Heparin LRT (Hyphen Biomed) | ||||
| Ceveron s100 (Technoclone) | Technochrom anti-Xa (Technoclone) |
| Anti-Xa Assay (ng/mL) | Regression Equation | Slope 95% CI | Intercept 95% CI | 95% CI at 30 ng/mL | 95% CI at 50 ng/mL |
|---|---|---|---|---|---|
| STA Liquid Lab. A | y = 1.02x − 2.77 | 1.00 to 1.05 | −4.45 to −1.00 | 27–30 | 47–50 |
| STA Liquid Lab. B | y = 1.06x − 5.68 | 1.03 to 1.08 | −6.91 to −4.00 | 25–27 | 46–48 |
| Berichrom | y = 0.99x − 0.84 | 0.96 to 1.01 | −2.25 to 0.63 | 28–30 | 47–50 |
| Innovance Lab. A | y = 1.09x − 4.31 | 1.07 to 1.12 | −5.38 to −3.18 | 28–29 | 49–51 |
| Innovance Lab. C | y = 1.00x + 7.00 | 0.97 to 1.03 | 2.32 to 10.44 | 33–40 | 53–59 |
| DiXaI | y = 1.00x − 1.95 | 0.97 to 1.01 | −3.22 to −1.07 | 27–29 | 47–48 |
| HemosIL Liquid | y = 0.98x − 7.38 | 0.95 to 1.00 | −9.18 to −5.53 | 20–24 | 40–43 |
| Heparin LRT | y = 1.11x + 1.30 | 1.07 to 1.14 | −0.05 to 3.39 | 34–36 | 56–59 |
| Technochrom | y = 0.91x + 24.18 | 0.85 to 0.96 | 19.68 to 27.74 | 48–55 | 66–73 |
| Anti-Xa Assay (ng/mL) | Regression Equation | Slope 95% CI | Intercept 95% CI | 95% CI at 30 ng/mL | 95% CI at 50 ng/mL |
|---|---|---|---|---|---|
| STA Liquid Lab. B | y = 0.79x − 0.58 | 0.77 to 0.81 | −3.02 to 1.81 | 21–25 | 37–41 |
| Berichrom | y = 0.18x + 13.86 | 0.79 to 0.84 | 11.55 to 17.06 | 36–41 | 53–57 |
| Innovance Lab. A | y = 0.89x − 1.90 | 0.87 to 0.91 | −4.40 to 0.43 | 23–27 | 40–44 |
| Innovance Lab. C | y = 0.87x − 6.26 | 0.85 to 0.90 | −10.19 to −3.01 | 17–23 | 34–40 |
| DiXaI | y = 0.81x − 0.05 | 0.79 to 0.83 | −2.35 to 2.20 | 22–26 | 39–42 |
| Heparin LRT | y = 0.91x − 15.97 | 0.88 to 0.94 | −21.38 to −11.92 | 7–15 | 25–32 |
| Technochrom | y = 0.81x − 17.79 | 0.76 to 0.85 | −26.69 to −11.43 | 0–11 | 16–27 |
| Assay | Rivaroxaban Concentration | Apixaban Concentration |
|---|---|---|
| dRVVT Lab. A | 0.928 (0.912–0.942) | 0.844 (0.811–0.872) |
| dRVVT Lab. B | 0.924 (0.906–0.938) | 0.834 (0.798–0.863) |
| dRVVT Lab. C | 0.898 (0.875–0.917) | 0.742 (0.690–0.786) |
| APTT | 0.783 (0.737–0.821) | 0.327 (0.229–0.418) |
| dRVVT (s)—Rivaroxaban | Regression Equation | Slope 95% CI | Intercept 95% CI |
|---|---|---|---|
| Lab. A and Lab. B | y = 0.91x +0.12 | 0.90 to 0.93 | −0.43 to 0.63 |
| Lab. A and Lab. C | y = 0.97x − 0.93 | 0.96 to 0.98 | 1.56 to −0.44 |
| Lab. C and Lab. B | y = 0.94x + 1.06 | 0.92 to 0.95 | 0.39 to 1.68 |
| dRVVT (s)—Apixaban | |||
| Lab. A and Lab. B | y = 0.87x + 2.91 | 0.85 to 0.89 | 2.05 to 3.85 |
| Lab. A and Lab. C | y = 1.06x − 3.20 | 1.03 to 1.10 | 4.97 to −1.65 |
| Lab. C and Lab. B | y = 0.82x + 5.66 | 0.79 to 0.84 | 4.50 to 6.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božič Mijovski, M.; Mavri, A.; Antovic, J.P.; Malmström, R.E.; Coen Herak, D. Multicenter Evaluation of Different Anti-Xa Assays and Diluted Russell’s Viper Venom Time in Ex Vivo Plasma Samples from Patients Treated with Rivaroxaban or Apixaban. J. Clin. Med. 2025, 14, 8274. https://doi.org/10.3390/jcm14238274
Božič Mijovski M, Mavri A, Antovic JP, Malmström RE, Coen Herak D. Multicenter Evaluation of Different Anti-Xa Assays and Diluted Russell’s Viper Venom Time in Ex Vivo Plasma Samples from Patients Treated with Rivaroxaban or Apixaban. Journal of Clinical Medicine. 2025; 14(23):8274. https://doi.org/10.3390/jcm14238274
Chicago/Turabian StyleBožič Mijovski, Mojca, Alenka Mavri, Jovan P. Antovic, Rickard E. Malmström, and Désirée Coen Herak. 2025. "Multicenter Evaluation of Different Anti-Xa Assays and Diluted Russell’s Viper Venom Time in Ex Vivo Plasma Samples from Patients Treated with Rivaroxaban or Apixaban" Journal of Clinical Medicine 14, no. 23: 8274. https://doi.org/10.3390/jcm14238274
APA StyleBožič Mijovski, M., Mavri, A., Antovic, J. P., Malmström, R. E., & Coen Herak, D. (2025). Multicenter Evaluation of Different Anti-Xa Assays and Diluted Russell’s Viper Venom Time in Ex Vivo Plasma Samples from Patients Treated with Rivaroxaban or Apixaban. Journal of Clinical Medicine, 14(23), 8274. https://doi.org/10.3390/jcm14238274







