From Evidence to Practice: The Growing Role of Angiography-Derived Physiology
Abstract
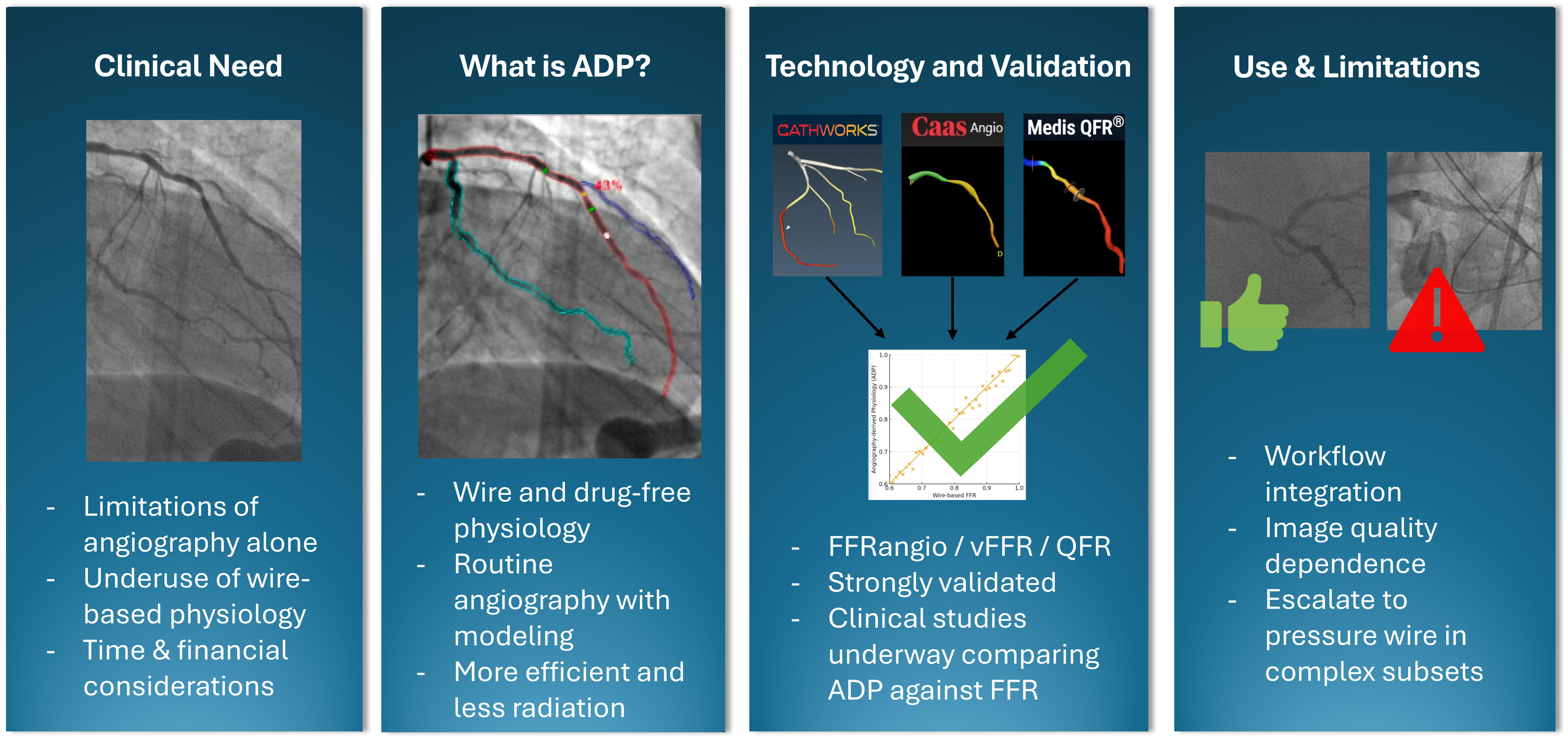
1. Introduction
1.1. What Is Angiography-Derived Physiology
1.2. Angiography-Derived Physiology: Technologies and How They Differ
1.3. Angiography-Derived Physiology: Validation
1.4. Randomized Control Trials of Angiography Derived Coronary Physiology
1.5. Special Lesions
1.6. Acute Coronary Syndrome
1.7. Left Main Coronary Artery Disease
1.8. CABG and Graft Patency
1.9. Bifurcation Lesions
1.10. Diffuse Serial Disease
1.11. Chronic Total Occlusion
1.12. Aortic Stenosis
1.13. Microvascular Dysfunction
1.14. Post-PCI Coronary Physiology
1.15. Intravascular Imaging
2. Limitations
2.1. Image Quality
2.2. Hemodynamic Assumptions
2.3. Limited Validation in Special Subsets
2.4. Lack of Microvascular or Perfusion Information
3. How to Integrate ADP into Daily Practice
3.1. Case Selection
3.2. Image Acquisition
3.3. Interpretation and Adjudication
3.4. Training and Operator Dependence
3.5. Workflow Integration
3.6. Cost and Reimbursement
3.7. Regulatory and Guidelines
3.8. Post-Procedural Quality Review
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| ADP | Angiography-Derived Physiology |
| AS | Aortic Stenosis |
| CABG | Coronary Artery Bypass Grafts |
| CAD | Coronary Artery Disease |
| CFR | Coronary Flow |
| CTO | Chronic Total Occlusion |
| FFR | Fractional Flow Reserve |
| FDA | U.S. Food And Drug Administration |
| IVI | Intravascular Imaging |
| IVUS | Intravascular Ultrasound |
| LM | Left Main |
| MACE | Major Adverse Cardiac Events |
| MI | Myocardial Infarction |
| MLA | Minimal Lumen Area |
| NHPR | Non-Hyperemic Pressure Ratio |
| NPV | Negative Predictive Value |
| OCT | Optical Coherence Tomography |
| PCI | Percutaneous Coronary Intervention |
| PPV | Positive Predictive Value |
| QFR | Quantitative Flow Ratio |
| STEMI | ST Elevation Myocardial Infarction |
| TAVI | Transcatheter Aortic Valve Implantation |
| vFFR | Vessel FFR |
References
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119, Erratum in Circulation 2023, 148, e148. https://doi.org/10.1161/CIR.0000000000001183; Erratum in Circulation 2023, 5, e186. https://doi.org/10.1161/CIR.0000000000001195. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.C.; Remkes, W.S.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188. [Google Scholar] [CrossRef]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van ’t Veer, M.; Klauss, V.; Manoharan, G.; Engström, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef]
- Fearon, W.F.; Nishi, T.; De Bruyne, B.; Boothroyd, D.B.; Barbato, E.; Tonino, P.A.; Jüni, P.; Pijls, N.H.; Hlatky, M.A. Clinical Outcomes and Cost-Effectiveness of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention in Patients with Stable Coronary Artery Disease: Three-Year Follow-Up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation 2018, 137, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Amponsah, D.K.; Mahendiran, T.; Mizukami, T.; Wilgenhof, A.; Fearon, W.F. Advancements and future perspectives in coronary angiography-derived fractional flow reserve. Prog. Cardiovasc. Dis. 2025, 88, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Strepkos, D.; Sara, J.D.S.; Carvalho, P.E.P.; Alexandrou, M.; Mutlu, D.; Ser, O.S.; Seto, A.H.; Fearon, W.F.; Rangan, B.V.; Mastrodemos, O.C.; et al. Angiography-Derived Fractional Flow Reserve: Newer Data and Future Directions. Am. J. Cardiol. 2025, 238, 1–8. [Google Scholar] [CrossRef]
- Parikh, R.V.; Liu, G.; Plomondon, M.E.; Sehested, T.S.G.; Hlatky, M.A.; Waldo, S.W.; Fearon, W.F. Utilization and Outcomes of Measuring Fractional Flow Reserve in Patients with Stable Ischemic Heart Disease. J. Am. Coll. Cardiol. 2020, 75, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Pfau, S.E. Coronary Physiology in the Cardiac Catheterization Laboratory. J. Clin. Med. 2019, 8, 255. [Google Scholar] [CrossRef]
- Pijls, N.H.; De Bruyne, B.; Peels, K.; Van der Voort, P.H.; Bonnier, H.J.; Bartunek, J.J.; Koolen, J.J. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med. 1996, 334, 1703–1708. [Google Scholar] [CrossRef]
- Pijls, N.H.; De Bruyne, B.; Bech, G.J.; Liistro, F.; Heyndrickx, G.R.; Bonnier, H.J.; Koolen, J.J. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery: Validation in humans. Circulation 2000, 102, 2371–2377. [Google Scholar] [CrossRef]
- Sen, S.; Escaned, J.; Malik, I.S.; Mikhail, G.W.; Foale, R.A.; Mila, R.; Tarkin, J.; Petraco, R.; Broyd, C.; Jabbour, R.; et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: Results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol. 2012, 59, 1392–1402. [Google Scholar] [CrossRef]
- Shpilfoygel, S.D.; Close, R.A.; Valentino, D.J.; Duckwiler, G.R. X-ray videodensitometric methods for blood flow and velocity measurement: A critical review of literature. Med. Phys. 2000, 27, 2008–2023. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. K232147—CAAS Workstation vFFR. FDA 510(k) Premarket Notification. 2023. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf23/K232147.pdf (accessed on 10 August 2025).
- US Food and Drug Administration. K182149—FFRangio (CathWorks). FDA 510(k) Premarket Notification. 2018. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf18/K182149.pdf (accessed on 10 August 2025).
- US Food and Drug Administration. K182611—QAngio XA 3D (Includes QFR). FDA 510(k) Premarket Notification. 2018. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf18/K182611.pdf (accessed on 10 August 2025).
- Masdjedi, K.; van Zandvoort, L.J.C.; Balbi, M.M.; Gijsen, F.J.H.; Ligthart, J.M.R.; Rutten, M.C.M.; Lemmert, M.E.; Wilschut, J.M.; Diletti, R.; de Jaegere, P.; et al. Validation of a three-dimensional quantitative coronary angiography-based software to calculate fractional flow reserve: The FAST study. EuroInterventon 2020, 16, 591–599. [Google Scholar] [CrossRef]
- Pie Medical Imaging. CAAS Workstation—vFFR & Pullback Tool; IVUS Co-Registration. Available online: https://www.piemedicalimaging.com/download_file/890/233 (accessed on 31 August 2025).
- Pie Medical Imaging, B.V. CAAS vFFR. Available online: https://www.piemedicalimaging.com/product/caas-workstation/vffr (accessed on 7 November 2025).
- Fearon, W.F.; Achenbach, S.; Engström, T.; Assali, A.; Shlofmitz, R.; Jeremias, A.; Fournier, S.; Kirtane, A.J.; Kornowski, R.; Greenberg, G.; et al. FAST-FFR Study Investigators. Accuracy of fractional flow reserve derived from coronary angiography. Circulation 2019, 139, 477–484. [Google Scholar] [CrossRef]
- CathWorks. FFRangio System Overview & Functional Toolset (Lesion Impact, Pullback, Vessel Sizing). Available online: https://cath.works/cathworks-ffrangio/ (accessed on 31 August 2025).
- Tu, S.; Westra, J.; Yang, J.; von Birgelen, C.; Ferrara, A.; Pellicano, M.; Nef, H.; Tebaldi, M.; Murasato, Y.; Lansky, A.; et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: The international multicenter FAVOR pilot study. JACC Cardiovasc. Interv. 2016, 9, 2024–2035. [Google Scholar] [CrossRef]
- Medis Medical Imaging. Medis QFR 3.0—AI-Assisted Workflow & Residual QFR Tool. Available online: https://medisimaging.com/news/medis-qfr/medis-qfr-3-0-launches-in-the-u-s/ (accessed on 31 August 2025).
- Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; OxAMI Study Investigators; Ribichini, F.; Ferreira, V.M.; et al. Angiography-derived index of microcirculatory resistance (IMRangio) as a novel pressure-wire-free tool to assess coronary microvascular dysfunction in acute coronary syndromes and stable coronary artery disease. Int. J. Cardiovasc. Imaging 2021, 37, 1801–1813. [Google Scholar] [CrossRef]
- Medis Medical Imaging System, B.V. Medis QFR. Available online: https://medisimaging.com/software-solutions/medis-qfr/ (accessed on 7 November 2025).
- Kornowski, R.; Lavi, I.; Pellicano, M.; Xaplanteris, P.; Vaknin-Assa, H.; Assali, A.; Valtzer, O.; Lotringer, Y.; De Bruyne, B. Fractional Flow Reserve Derived From Routine Coronary Angiograms. J. Am. Coll. Cardiol. 2016, 68, 2235–2237. [Google Scholar] [CrossRef]
- Witberg, G.; De Bruyne, B.; Fearon, W.F.; Achenbach, S.; Engstrom, T.; Matsuo, H.; Kornowski, R. Diagnostic Performance of Angiogram-Derived Fractional Flow Reserve: A Pooled Analysis of 5 Prospective Cohort Studies. J. Am. Coll. Cardiol. 2020, 13, 488–497. [Google Scholar] [CrossRef]
- Xu, B.; Tu, S.; Qiao, S.; Qu, X.; Chen, Y.; Yang, J.; Guo, L.; Sun, Z.; Li, Z.; Tian, F.; et al. FAVOR II China Study Group. Diagnostic accuracy of angiography-based quantitative flow ratio (QFR) for assessment of coronary stenosis severity in China: FAVOR II China study. J. Am. Coll. Cardiol. 2017, 70, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Westra, J.; Andersen, B.K.; Campo, G.; Matsuo, H.; Koltowski, L.; Eftekhari, A.; Liu, T.; Di Serafino, L.; Di Girolamo, D.; Escaned, J.; et al. Diagnostic Performance of In-Procedure Angiography-Derived Quantitative Flow Reserve Compared to Pressure-Derived Fractional Flow Reserve: The FAVOR II Europe-Japan Study. J. Am. Heart Assoc. 2018, 7, e009603. [Google Scholar] [CrossRef]
- Westra, J.; Tu, S.; Winther, S.; Nissen, L.; Vestergaard, M.-B.; Andersen, B.K.; Holck, E.N.; Fox Maule, C.; Johansen, J.K.; Andreasen, L.N.; et al. Evaluation of Coronary Artery Stenosis by Quantitative Flow Ratio During Invasive Coronary Angiography: The WIFI II Study (Wire-Free Functional Imaging II). Circ. Cardiovasc. Imaging 2018, 11, e007107. [Google Scholar] [CrossRef]
- Westra, J.; Tu, S.; Tu, S.; Campo, G.; Qiao, S.; Matsuo, H.; Qu, X.; Koltowski, L.; Chang, Y.; Liu, T.; et al. Diagnostic performance of quantitative flow ratio in prospectively enrolled patients: An individual patient-data meta-analysis. Catheter. Cardiovasc. Interv. 2019, 94, 693–701. [Google Scholar] [CrossRef]
- Neleman, T.; Masdjedi, K.; Van Zandvoort, L.J.C.; Tomaniak, M.; Ligthart, J.M.R.; Witberg, K.T.; Vermaire, A.A.; Boersma, E.; Van Mieghem, N.M.; Daemen, J. Extended validation of novel 3D quantitative coronary angiography-based software to calculate vFFR: The FAST EXTEND study. JACC Cardiovasc. Imaging 2021, 14, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Masdjedi, K.; Tanaka, N.; Van Belle, E.; Porouchani, S.; Linke, A.; Woitek, F.J.; Bartorelli, A.L.; Ali, Z.A.; den Dekker, W.K.; Wilschut, J.; et al. Vessel fractional flow reserve (vFFR) for the assessment of stenosis severity: The FAST II study. EuroIntervention 2022, 17, 1498–1505. [Google Scholar] [CrossRef]
- Xu, B.; Tu, S.; Song, L.; Jin, Z.; Yu, B.; Fu, G.; Zhou, Y.; Wang, J.; Chen, Y.; Pu, J.; et al. FAVOR III China Study Group. Angiographic quantitative flow ratio–guided coronary intervention (FAVOR III China): A multicentre, randomised, sham-controlled trial. Lancet 2021, 398, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xu, B.; Tu, S.; Guan, C.; Jin, Z.; Yu, B.; Fu, G.; Zhou, Y.; Wang, J.; Chen, Y.; et al. 2-Year Outcomes of Angiographic Quantitative Flow Ratio-Guided Coronary Interventions. JACC 2022, 80, 2089–2101. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.K.; Holm, N.R.; Mogensen, L.J.H.; Maillard, L.; Råmunddal, T.; Erriquez, A.; Christiansen, E.H.; Escaned, J. FAVOR III Europe Study Group. Quantitative flow ratio versus fractional flow reserve for coronary revascularisation guidance (FAVOR III Europe): A multicentre, randomised, non-inferiority trial. Lancet 2024, 404, 1835–1846, Erratum in Lancet 2025, 404, 2542. https://doi.org/10.1016/S0140-6736(24)02753-3. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Tsai, T.Y.; Sharif, F.; Wykrzykowska, J.; Serruys, P.W. QFR vs FFR for intermediate coronary stenosis. Lancet 2025, 406, 334. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.K.; Holm, N.R.; Mogensen, L.J.H.; Maillard, L.; Råmunddal, T.; Erriquez, A.; Christiansen, E.H.; Escaned, J. Coronary revascularisation deferral based on quantitative flow ratio or fractional flow reserve: A post hoc analysis of the FAVOR III Europe trial. EuroIntervention 2025, 21, e161–e170. [Google Scholar] [CrossRef]
- Matsuo, H.; PROVISION Investigators. PROVISION Trial—Highlights of the First Randomized Clinical Trial Comparing Angiography-Based Physiology (FFRangio) Versus Wire-Based FFR Guidance. In Proceedings of the Transcatheter Cardiovascular Therapeutics (TCT) 2024, Washington, DC, USA, 27–30 October 2024; Available online: https://www.tctmd.com/slide/provision-trial-highlights-first-rct-comparing-angio-based-physiology-versus-ffr (accessed on 31 August 2025).
- Redfors, B.; Madhavan, M.V.; Kirtane, A.J.; Fearon, W.F.; Yeh, R.W.; Cohen, D.J.; Al-Lamee, R.; Jeremias, A.; Witberg, G.; Sharma, R.P.; et al. ALL-RISE Investigators. FFRangio-guided versus pressure wire-guided PCI: Design and rationale of the multicentre, randomised ALL-RISE trial. EuroIntervention 2025, 21, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Scoccia, A.; Byrne, R.A.; Banning, A.P.; Landmesser, U.; Van Belle, E.; Amat-Santos, I.J.; Sabaté, M.; Tijssen, J.G.P.; Spitzer, E.; Daemen, J. Fractional flow reserve or 3D-quantitative-coronary-angiography-based vessel-FFR guided revascularization: Rationale and study design of the prospective randomized FAST III trial. Am. Heart J. 2023, 260, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Comparison of Vessel-FFR Versus FFR in Intermediate Coronary Stenoses (LIPSIASTRATEGY); ClinicalTrials.gov Identifier: NCT03497637; ClinicalTrials.gov Website. 2025. Available online: https://clinicaltrials.gov/study/NCT03497637 (accessed on 31 August 2025).
- Puymirat, E.; Cayla, G.; Simon, T.; Steg, P.G.; Montalescot, G.; Durand-Zaleski, I.; le Bras, A.; Gallet, R.; Khalife, K.; Morelle, J.-F.; et al. Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction. N. Engl. J. Med. 2021, 385, 297–308. [Google Scholar] [CrossRef]
- Milzi, A.; Dettori, R.; Marx, N.; Reith, S.; Burgmaier, M. Quantitative flow ratio (QFR) identifies functional relevance of non-culprit lesions in coronary angiographies of patients with acute myocardial infarction. Clin. Res. Cardiol. 2021, 110, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Sejr-Hansen, M.; Westra, J.; Thim, T.; Biering-Sørensen, T.; Bøtker, A.E.; Sand, N.P.; Terkelsen, C.J.; Davies, J.R.; Christiansen, E.H.; Holm, N.R.; et al. Quantitative flow ratio for immediate assessment of nonculprit lesions in patients with ST-segment elevation myocardial infarction-An iSTEMI substudy. Catheter. Cardiovasc. Interv. 2019, 94, 686–692. [Google Scholar] [CrossRef]
- Wu, K.C.; Zerhouni, E.A.; Judd, R.M.; Lugo-Olivieri, C.H.; Barouch, L.A.; Schulman, S.P.; Blumenthal, R.S.; Lima, J.A.C. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998, 97, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Groenland, F.T.W.; Scoccia, A.; Ziedses des Plantes, A.C.; Neleman, T.; Van Mieghem, N.M.; Daemen, J. Acute vs staged vessel fractional flow reserve in STEMI patients with multivessel disease: The FAST-STAGED study. IJC Heart Vasc. 2023, 45, 101192. [Google Scholar] [CrossRef] [PubMed]
- Van der Eijk, J.A.; Groenland, F.T.W.; Scoccia, A.; Ziedses des Plantes, A.C.; Huang, J.; Nuis, R.-J.; Wilschut, J.M.; den Dekker, W.K.; Diletti, R.; Kardys, I.; et al. TCT-226 Validation of Coronary Angiography–Based FFR Compared with Pressure Wire–Based FFR to Guide Revascularization of Intermediate Coronary Lesions in Nonculprit Vessels in Patients Presenting with ST-Segment Elevation Myocardial Infarction (FAST STEMI II). J. Am. Coll. Cardiol. 2024, 84 (Suppl. 18), B27. [Google Scholar] [CrossRef]
- Skalidis, I.; Meier, D.; De Bruyne, B.; Collet, C.; Sonck, J.; Mahendiran, T.; Rotzinger, D.; Qanadli, S.D.; Eeckhout, E.; Muller, O.; et al. Diagnostic performance of angiography-derived fractional flow reserve in patients with NSTEMI. Catheter. Cardiovasc. Interv. 2023, 101, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Palop, R.; Carrillo, P.; Leithold, G.; Lozano, I.; Nieto, A.; Frutos, A.; Garcia, J.; Freites, A.; Lacunza, J.; Duran, J.M.; et al. Accuracy of the angiography-based quantitative flow ratio in intermediate left main coronary artery lesions and comparison with visual estimation. Int. J. Cardiol. 2023, 383, 8–14. [Google Scholar] [CrossRef]
- Yuasa, S.; Lauri, F.M.; Mejia-Renteria, H.; Liontou, C.; Lee, H.-J.; Tanigaki, T.; Nakayama, M.; Warisawa, T.; Uchiyama, T.; Matsuo, H.; et al. Angiography-derived functional assessment of left main coronary stenoses. Catheter. Cardiovasc. Interv. 2023, 101, 1045–1052. [Google Scholar] [CrossRef]
- Tomaniak, M.; Masdjedi, K.; van Zandvoort, L.J.; Neleman, T.; Tovar Forero, M.N.; Vermaire, A.; Kochman, J.; Kardys, I.; den Dekker, W.; Wilschut, J.; et al. Correlation between 3D-QCA based FFR and quantitative lumen assessment by IVUS for left main coronary artery stenoses. Catheter. Cardiovasc. Interv. 2021, 97, E495–E501. [Google Scholar] [CrossRef]
- Dowling, C.; Nelson, A.J.; Lim, R.Y.; Zhang, J.M.; Cheng, K.; Smith, J.A.; Seneviratne, S.; Malaiapan, Y.; Zaman, S.; Wong, D.T. Quantitative flow ratio to predict long-term coronary artery bypass graft patency in patients with left main coronary artery disease. Int. J. Cardiovasc. Imaging 2022, 38, 2811–2818. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Z.; Hou, Z.; Wang, Y.; Song, L.; Xu, B.; Guan, C.; Ning, Y.; Feng, W.; Zhang, Y. Impact of Preoperative Quantitative Flow Ratio of the Left Anterior Descending Artery on Internal Mammary Artery Graft Patency and Midterm Patient Outcomes After Coronary Artery Bypass Grafting. J. Am. Heart Assoc. 2023, 12, e029134. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Zimmermann, F.M.; De Bruyne, B.; Piroth, Z.; van Straten, A.H.M.; Szekely, L.; Davidavicius, G.; Kalinauskas, G.; Mansour, S.; Kharbanda, R.; et al. Fractional Flow Reserve-Guided PCI as Compared with Coronary Bypass Surgery. N. Engl. J. Med. 2022, 386, 128–137. [Google Scholar] [CrossRef]
- Wang, T.; Liu, D.; Guan, C.; Son, L.; Yang, W. TCT-217 Angiographic Quantitative Flow Ratio-Guided Coronary Intervention in Bifurcation Lesion: A Subgroup Study of FAVOR III China. J. Am. Coll. Cardiol. 2024, 84 (Suppl. 18), B23. [Google Scholar] [CrossRef]
- Scarsini, R.; Fezzi, S.; Pesarini, G.; Del Sole, P.A.; Venturi, G.; Mammone, C.; Marcoli, M.; Gambaro, A.; Tavella, D.; Pighi, M.; et al. Impact of physiologically diffuse versus focal pattern of coronary disease on quantitative flow reserve diagnostic accuracy. Catheter. Cardiovasc. Interv. 2022, 99, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, J.; Ding, X.; Gong, X.; Zhou, L.; Chen, H. QFR measurements post CTO percutaneous coronary intervention: Can the long term outcome be predicted? IJC Heart Vasc. 2025, 59, 101689. [Google Scholar] [CrossRef]
- Dowling, C.; Michail, M.; Zhang, J.M.; Comella, A.; Thakur, U.; Gooley, R.; McCormick, L.; Brown, A.J.; Wong, D.T.L. Diagnostic performance of quantitative flow ratio, non-hyperaemic pressure indices and fractional flow reserve for the assessment of coronary lesions in severe aortic stenosis. Cardiovasc. Diagn. Ther. 2022, 12, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Sejr-Hansen, M.; Christiansen, E.H.; Ahmad, Y.; Vendrik, J.; Westra, J.; Holm, N.R.; Thim, T.; Seligman, H.; Hall, K.; Sen, S.; et al. Performance of quantitative flow ratio in patients with aortic stenosis undergoing transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2022, 99, 68–73. [Google Scholar] [CrossRef]
- De Maria, G.L.; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.; et al. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, S.; Tebaldi, M.; Brugaletta, S.; Cerrato, E.; Erriquez, A.; Passarini, G.; Ielasi, A.; Spitaleri, G.; Di Girolamo, D.; Mezzapelle, G.; et al. Prognostic Value of QFR Measured Immediately After Successful Stent Implantation: The International Multicenter Prospective HAWKEYE Study. JACC Cardiovasc Interv. 2019, 12, 2079–2088. [Google Scholar] [CrossRef]
- Terentes-Printzios, D.; Gkini, K.-P.; Oikonomou, D.; Gardikioti, V.; Aznaouridis, K.; Dima, I.; Tsioufis, K.; Vlachopoulos, C. Prognostic Value of Post-PCI Angiography-Derived Fractional Flow Reserve: A Systematic Review and Meta-Analysis of Cohort Studies. J. Pers. Med. 2023, 13, 1251. [Google Scholar] [CrossRef]
- Masdjedi, K.; van Zandvoort, L.J.C.; Balbi, M.M.; Nuis, R.-J.; Wilschut, J.; Diletti, R.; de Jaegere, P.P.T.; Zijlstra, F.; Van Mieghem, N.M.; Daemen, J. Validation of novel 3-dimensional quantitative coronary angiography based software to calculate fractional flow reserve post stenting. Catheter. Cardiovasc. Interv. 2021, 98, 671–677. [Google Scholar] [CrossRef]
- Neleman, T.; Scoccia, A.; Masdjedi, K.; Tomaniak, M.; Ligthart, J.M.R.; Witberg, K.T.; Vermaire, A.; Wolff, Q.; Visser, L.; Cummins, P.; et al. The prognostic value of angiography-based vessel fractional flow reserve after percutaneous coronary intervention: The FAST Outcome study. Int. J. Cardiol. 2022, 359, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [CrossRef]
- Neleman, T.; van Zandvoort, L.J.C.; Tovar Forero, M.N.; Masdjedi, K.; Ligthart, J.M.R.; Witberg, K.T.; Groenland, F.T.W.; Cummins, P.; Lenzen, M.J.; Boersma, E.; et al. FFR-Guided PCI Optimization Directed by High-Definition IVUS Versus Standard of Care: The FFR REACT Trial. JACC Cardiovasc. Interv. 2022, 15, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Kedhi, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Fabris, E.; IJsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The COMBINE OCT-FFR trial. Eur. Heart J. 2021, 42, 4671–4679. [Google Scholar] [CrossRef]
- Buonpane, A.; De Caterina, A.R.; Trimarchi, G.; Di Muro, F.M.; Galante, D.; Zella, S.; Pizzino, F.; Ciardetti, M.; Paradossi, U.; Concistrè, G.; et al. Unveiling the Causes of Acute and Non-Acute Myocardial Ischemic Syndromes: The Role of Optical Coherence Tomography. Medicina 2025, 61, 1218. [Google Scholar] [CrossRef]
- Zuo, W.; Sun, R.; Zhang, X.; Qu, Y.; Ji, Z.; Su, Y.; Zhang, R.; Ma, G. The Association Between Quantitative Flow Ratio and Intravascular Imaging-defined Vulnerable Plaque Characteristics in Patients with Stable Angina and Non-ST-segment Elevation Acute Coronary Syndrome. Front. Cardiovasc. Med. 2021, 8, 690262. [Google Scholar] [CrossRef]
- Milzi, A.; Dettori, R.; Burgmaier, K.; Marx, N.; Reith, S.; Burgmaier, M. Quantitative Flow Ratio Is Related to Intraluminal Coronary Stenosis Parameters as Assessed with Optical Coherence Tomography. J. Clin. Med. 2021, 10, 1856. [Google Scholar] [CrossRef]
- Geng, L.; Yuan, Y.; Du, P.; Gao, L.; Wang, Y.; Li, J.; Guo, W.; Huang, Y.; Zhang, Q. The association between intravascular ultrasound-derived echo-attenuation and quantitative flow ratio in intermediate coronary lesions. Cardiovasc. Diagn. Ther. 2021, 11, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, Y.; Wu, P.; Yang, Y.; Lu, X.; Meng, H. Association between optical coherence tomography and functionally severe stenosis assessed by quantitative flow ratio in coronary intermediate lesions. PLoS ONE 2025, 20, e0324872. [Google Scholar] [CrossRef]
- Ziedses des Plantes, A.C.; Scoccia, A.; Groenland, F.T.W.; Tovar Forero, M.N.; Tomaniak, M.; Kochman, J.; Wojakowski, W.; Roleder-Dylewska, M.; Ameloot, K.; Adriaenssens, T.; et al. Association of vessel fractional flow reserve (vFFR) with luminal obstruction and plaque characteristics as detected by optical coherence tomography (OCT) in patients with NSTE-ACS: The FAST OCT study. Eur. Heart J. Cardiovasc. Imaging 2024, 26, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ziedses des Plantes, A.C.; Scoccia, A.; Groenland, F.T.W.; Tovar Forero, M.N.; Tomaniak, M.; Kochman, J.; Wojakowski, W.; Roleder-Dylewska, M.; Ameloot, K.; Adriaenssens, T.; et al. Association between post-PCI vessel fractional flow reserve (vFFR) and optical coherence tomography (OCT) findings: Results from the FAST OCT study. IJC Heart Vasc. 2025, 59, 101706. [Google Scholar] [CrossRef]
- Dan, K.; Witberg, G.; Itabashi, F.; Maeda, T.; Kikuta, Y.; Okabe, K.; Tanigaki, T.; Nanasato, M.; Hikichi, Y.; Yokoi, H.; et al. Comparison of angiogram-based physiological assessment system sizing tool and intravascular ultrasound imaging measurements. Cardiovasc. Revascularization Med. 2025, 78, 80–85. [Google Scholar] [CrossRef]
- Chang, C.C.; Lee, Y.H.; Chuang, M.J.; Hsueh, C.H.; Lu, Y.W.; Tsai, Y.L.; Chou, R.H.; Wu, C.H.; Lu, T.M.; Huang, P.H.; et al. Agreement Between Invasive Wire-Based and Angiography-Based Vessel Fractional Flow Reserve Assessment on Intermediate Coronary Stenoses. Front. Cardiovasc. Med. 2021, 8, 707454. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Serruys, P.W.; Kotoku, N.; Zhou, J.; Kageyama, S.; Masuda, S.; Revaiah, P.C.; Wang, B.; He, X.; Tsai, T.Y.; et al. Anonymous Comparison of Various Angiography-Derived Fractional Flow Reserve Software with Pressure-Derived Physiological Assessment. JACC Cardiovasc. Interv. 2023, 16, 1778–1790. [Google Scholar] [CrossRef]

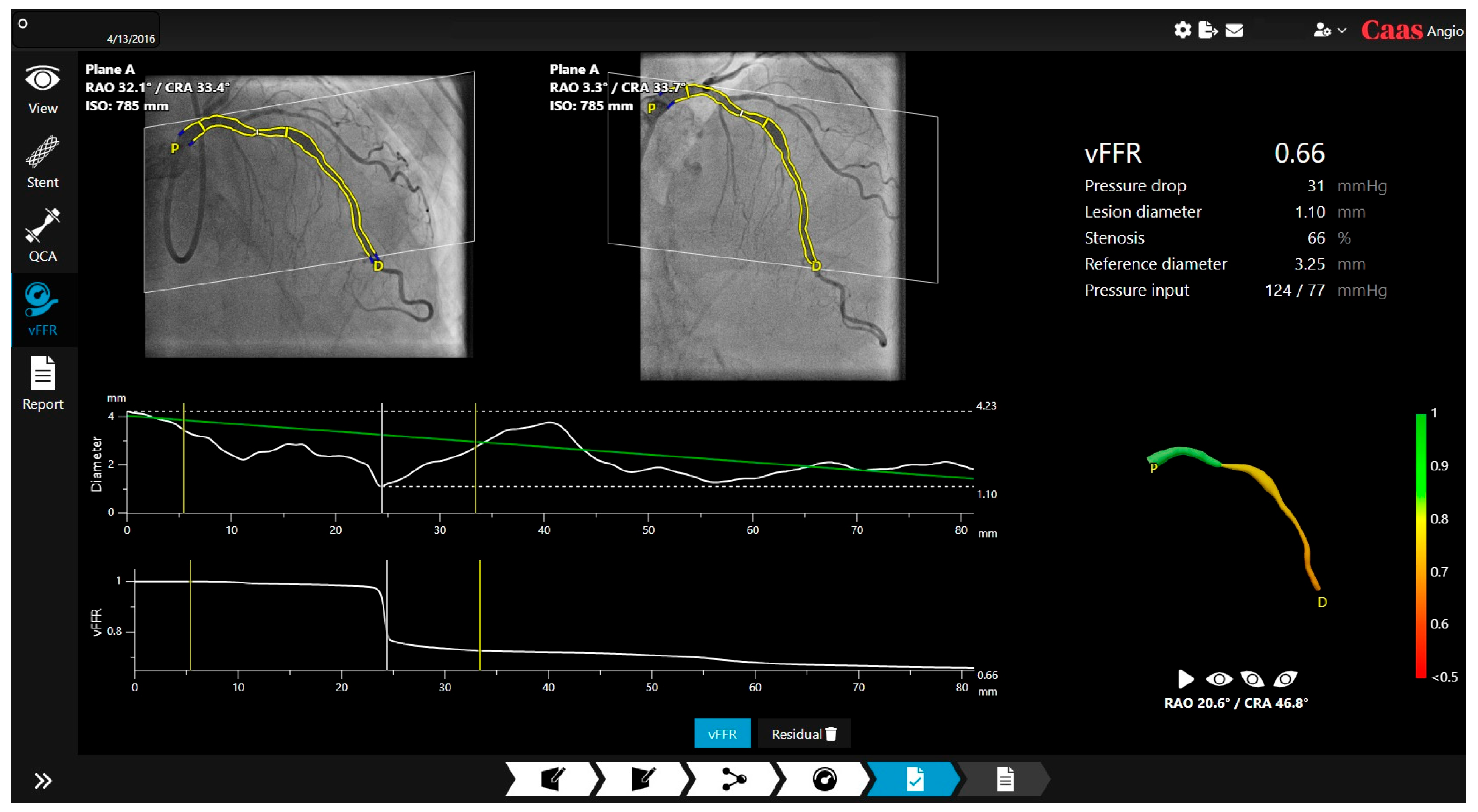
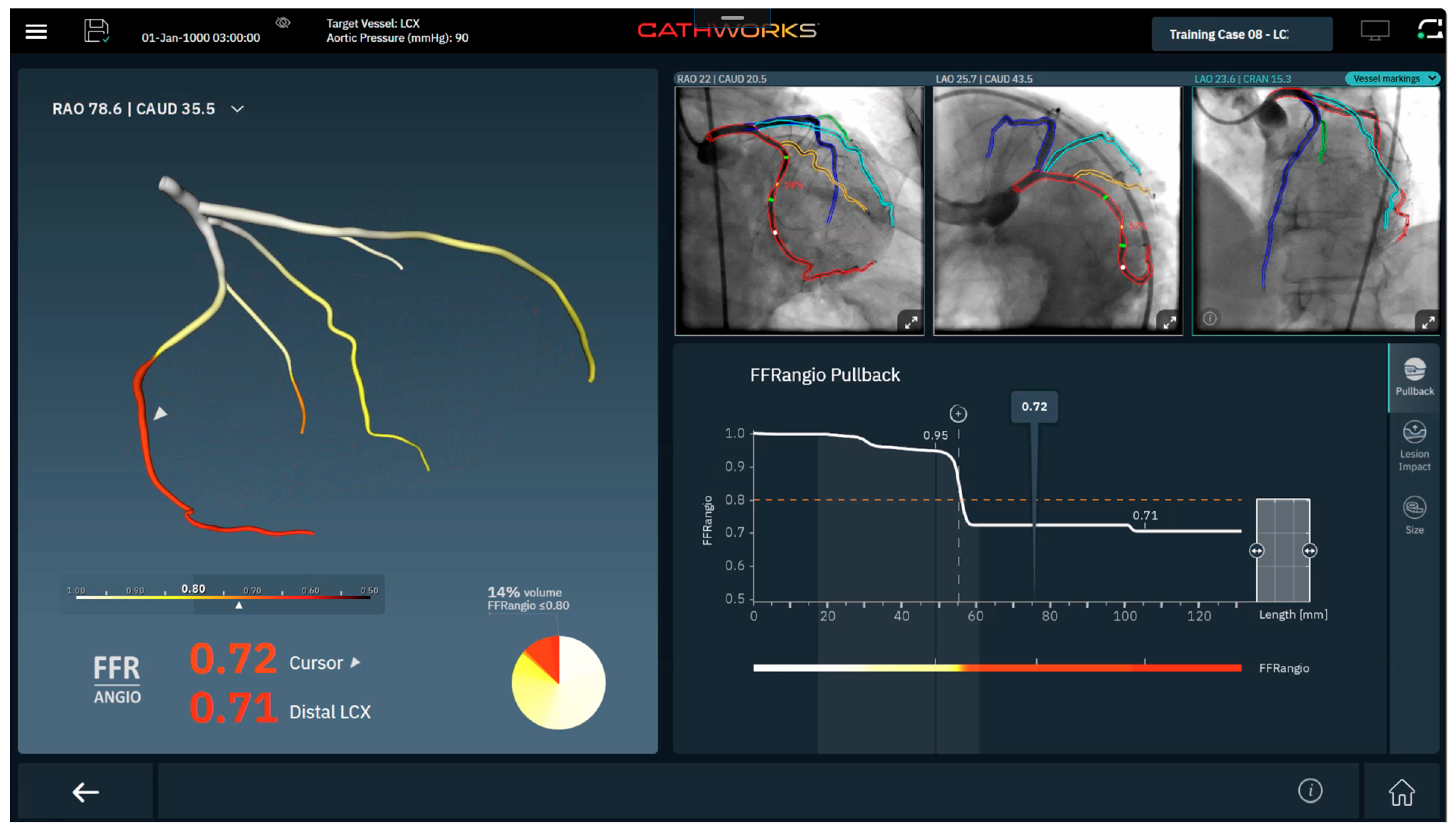
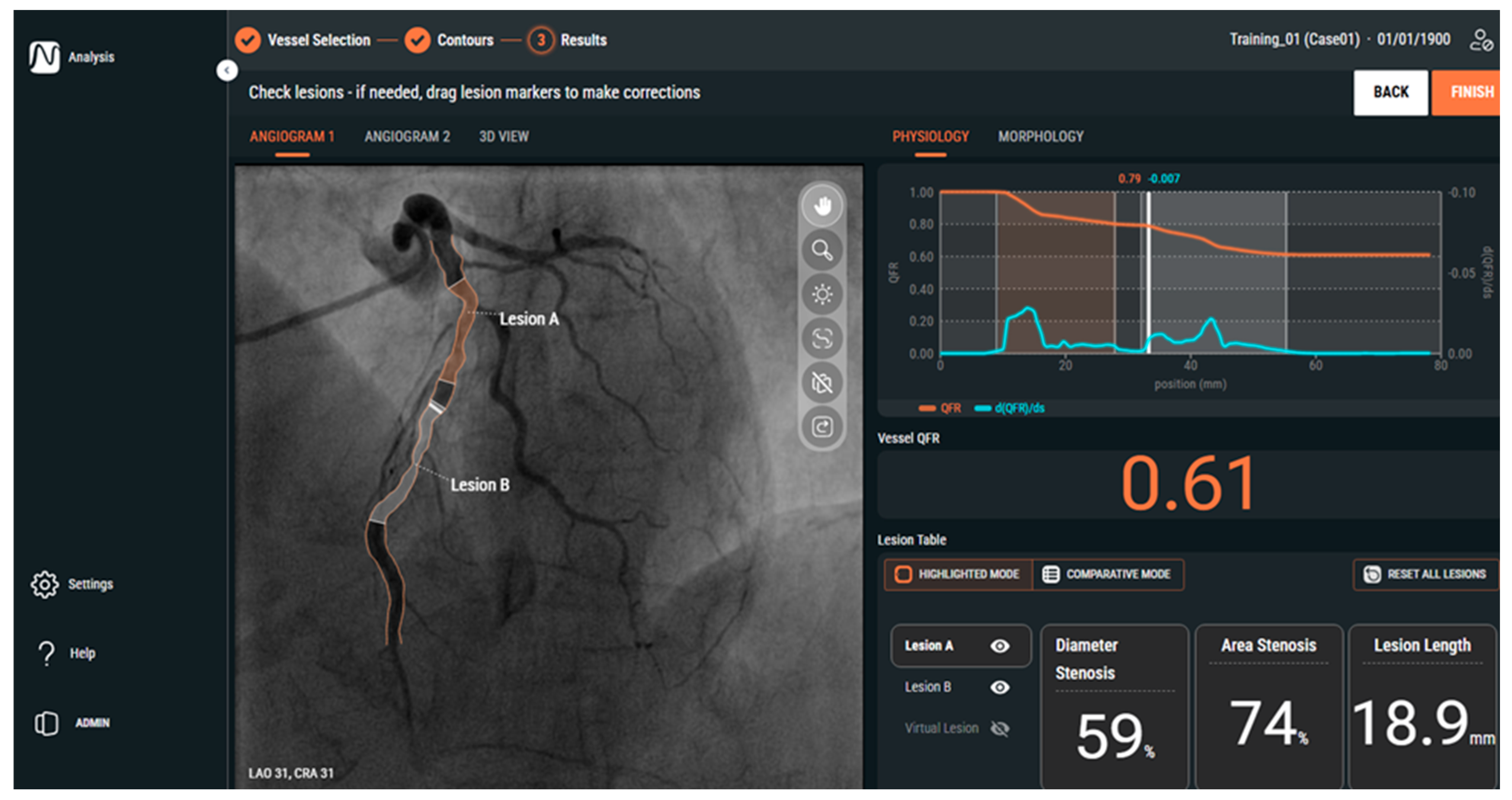


| Feature | QFR (Medis) | FFRangio (CathWorks) | vFFR (Pie Medical) |
|---|---|---|---|
| Angiographic Requirements | 2 orthogonal views, ≥25° apart | ≥2 orthogonal views, 30°apart | 2 orthogonal views, ≥25° apart |
| FDA-cleared cutoff | ≤0.80 | ≤0.80 | ≤0.80 |
| Outputs | Lesion level QFR, stent sizing, post-PCI prediction (investigational) | Multilevel tree analysis, pullback, lesion impact tool, vessel sizing | Lesion-level vFFR, virtual pullback, residual vFFR (investigational) |
| Unique features | Microvascular assessment using IMRangio (investigational) | Whole-vessel tree map; lesion impact tool | Residual physiology prediction |
| Best studied in | ACS, CABG graft patency, diffuse CAD | Multivessel CAD, cost effectiveness | Left main validation (vs. IVUS), diffuse CAD |
| Platform | Study | N (lesions) | Accuracy (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| QFR | FAVOR II China | 308 | 93 | 95 | 92 |
| QFR | FAVOR II Europe-Japan | 329 | 87 | 87 | 87 |
| vFFR | FAST-EXTEND | 294 | 88 | 75 | 94 |
| vFFR | FAST II | 334 | 90 | 81 | 95 |
| FFRangio | FAST-FFR | 301 | 92 | 94 | 91 |
| Platform | Trial | Comparator | N | Primary Outcomes | Key Results |
|---|---|---|---|---|---|
| QFR | FAVOR III China | Angiography-guided PCI | 3825 | 1-year MACE | QFR reduced MACE (HR 0.55), fewer PCIs |
| QFR | FAVOR III Europe | Wire-based FFR | 2000 | 1-year MACE | QFR not non-inferior, more stents, increased MI |
| FFRangio | Provision | Wire-based FFR | 401 | Revascularization rate | Met non-inferiority, decreased cost and radiation |
| FFRangio | ALLRISE | Wire-based FFR | ~1924 | 1-year MACE | Pending |
| vFFR | FAST III | Wire-based FFR | ~1700 | 1-year MACE | Pending |
| vFFR | LIPISIASTRATEGY | Wire-based FFR | ~2000 | 1-year MACE | Pending |
| Lesion Subsets | QFR | vFFR | FFRangio |
|---|---|---|---|
| ACS (non-culprit lesions) | Strong diagnostic performance vs. FFR (AUC ~0.89) with suggested triage cutoffs < 0.75 (treat) and >0.92 (defer); good agreement between acute and staged measurements | FAST-STAGED: High accuracy of acute vs. staged vFFR with (diagnostic accuracy ~94%). FAST-STEMI II: Modest diagnostic performance vs. FFR (accuracy ~71.8%) with discordance mainly in microvascular dysfunction. | NSTEMI Population: High diagnostic accuracy vs. FFR for intermediate non-culprit lesions (diagnostic accuracy ~97%) |
| Left Main disease | Excellent agreement with FFR in intermediate lesions (diagnostic accuracy ~91%) | vFFR correlates strongly with IVUS-derived MLA; vFFR ≤ 0.80 correlates well with MLA < 6.0 mm2 | Limited: LM and ostial lesions were largely excluded from early validation |
| CABG/Graft patency | Pre-operative QFR > 0.80 is associated with increased graft occlusion; pre-op LAD QFR > 0.80 independently associated with internal mammary graft failure and adverse outcomes increased graft failure risk | Limited: No dedicated graft outcome studies. | Limited: No dedicated graft outcomes studies. |
| Bifurcation lesions | Post-PCI QFR in main and side branches identifies functionally incomplete revascularization and predicts higher long-term adverse | Bifurcation subsets within FAST-EXTENDED and FAST II show good diagnostic agreement with FFR | Limited data |
| Diffuse/serial disease | QFR pullback mimics pressure-wire pullback | vFFR incorporates press-drop analysis to evaluate individual lesions in serial disease | Limited: Whole tree-mapping highlights segments in diffuse/serial disease |
| Chronic Total Occlusion | Lower post-PCI QFR values after CTO intervention associated with worse clinical outcomes | Limited | Limited |
| Severe AS | Good agreement with FFR (diagnostic accuracy ~84%); Post-TAVI QFR maintains good agreement (diagnostic accuracy ~83%) | Limited | Limited |
| Microvascular dysfunction | QFR-derived IMRangio provides an angiography-based estimate of microcirculatory resistance showing early validation vs. wire-based IMR | Limited | Limited |
| Post-PCI assessment | HAWKEYE: Post-PCI QFR ≤ 0.89 is associated with a ~3-fold higher vessel-oriented composite events | Validated against FFR to detect suboptimal post-PCI physiology; lower vFFR associated with higher target failure at long-term follow-up | Limited |
| Intravascular Imaging | Lower QFR associated with IVUS/OCT high-risk plaque features, smaller lumen dimensions, and greater stenosis severity | Reduced vFFR correlates with adverse OCT-derived lumen metrics and high-risk characteristics | Limited: Early data shows reasonable agreement between FFRangio derived vessel sizing and IVUS measurements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amponsah, D.K.; Fearon, W.F. From Evidence to Practice: The Growing Role of Angiography-Derived Physiology. J. Clin. Med. 2025, 14, 8219. https://doi.org/10.3390/jcm14228219
Amponsah DK, Fearon WF. From Evidence to Practice: The Growing Role of Angiography-Derived Physiology. Journal of Clinical Medicine. 2025; 14(22):8219. https://doi.org/10.3390/jcm14228219
Chicago/Turabian StyleAmponsah, Daniel K., and William F. Fearon. 2025. "From Evidence to Practice: The Growing Role of Angiography-Derived Physiology" Journal of Clinical Medicine 14, no. 22: 8219. https://doi.org/10.3390/jcm14228219
APA StyleAmponsah, D. K., & Fearon, W. F. (2025). From Evidence to Practice: The Growing Role of Angiography-Derived Physiology. Journal of Clinical Medicine, 14(22), 8219. https://doi.org/10.3390/jcm14228219






