Influence of Endurance Training, High-Intensity Interval Training, and Acute Exercise on Left Ventricular Mechanics: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Clinical Findings
3.3. Baseline Characteristics and Hemodynamic Responses Across Exercise Modalities
3.4. Structural and Functional Echocardiographic Parameters Across Exercise Modalities
3.5. Myocardial Strain and Deformation Parameters Across Exercise Modalities
3.6. Left Ventricular Rotational Mechanics, Torsion, and Twisting Responses Across Exercise Modalities
3.7. Biomarker Assessment
3.8. Between-Study Heterogeneity
3.9. NIH Quality Assessment of Included Studies
3.10. Publication Bias Assessment
4. Discussion
4.1. Summary of Main Findings
4.2. Comparison with Literature Data
4.3. Clinical Implications
4.4. Limitations of the Included Studies
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baggish, A.L.; Wood, M.J. Athlete’s Heart and Cardiovascular Care of the Athlete: Scientific and Clinical Update. Circulation 2011, 123, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Zholshybek, N.; Khamitova, Z.; Toktarbay, B.; Jumadilova, D.; Khissamutdinov, N.; Dautov, T.; Rakhmanov, Y.; Bekbossynova, M.; Gaipov, A.; Salustri, A. Cardiac Imaging in Athlete’s Heart: Current Status and Future Prospects. Cardiovasc. Ultrasound 2023, 21, 21. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Spataro, A.; Proschan, M.A.; Spirito, P. The Upper Limit of Physiologic Cardiac Hypertrophy in Highly Trained Elite Athletes. N. Engl. J. Med. 1991, 324, 295–301. [Google Scholar] [CrossRef]
- Spirito, P.; Pelliccia, A.; Proschan, M.A.; Granata, M.; Spataro, A.; Bellone, P.; Caselli, G.; Biffi, A.; Vecchio, C.; Maron, B.J. Morphology of the “Athlete’s Heart” Assessed by Echocardiography in 947 Elite Athletes Representing 27 Sports. Am. J. Cardiol. 1994, 74, 802–806. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial Strain Imaging: How Useful Is It in Clinical Decision Making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Voigt, J.U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic Implications of Global LV Dysfunction: A Systematic Review and Meta-Analysis of Global Longitudinal Strain and Ejection Fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11 Pt 1, 260–274. [Google Scholar] [CrossRef]
- Murray, J.; Bennett, H.; Bezak, E.; Perry, R.; Boyle, T. The Effect of Exercise on Left Ventricular Global Longitudinal Strain. Eur. J. Appl. Physiol. 2022, 122, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Notomi, Y.; Lysyansky, P.; Setser, R.M.; Shiota, T.; Popović, Z.B.; Martin-Miklovic, M.G.; Weaver, J.A.; Oryszak, S.J.; Greenberg, N.L.; White, R.D.; et al. Measurement of Ventricular Torsion by Two-Dimensional Ultrasound Speckle Tracking Imaging. J. Am. Coll. Cardiol. 2005, 45, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.B.; Baggish, A.L. Exercise-Induced Cardiac Remodeling. Prog. Cardiovasc. Dis. 2012, 54, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L.; Wang, F.; Weiner, R.B.; Elinoff, J.M.; Tournoux, F.; Boland, A.; Picard, M.H.; Hutter, A.M., Jr.; Wood, M.J. Training-Specific Changes in Cardiac Structure and Function: A Prospective and Longitudinal Assessment of Competitive Athletes. J. Appl. Physiol. 2008, 104, 1121–1128. [Google Scholar] [CrossRef]
- La Gerche, A.; Burns, A.T.; Mooney, D.J.; Inder, W.J.; Taylor, A.J.; Bogaert, J.; Macisaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise-Induced Right Ventricular Dysfunction and Structural Remodelling in Endurance Athletes. Eur. Heart J. 2012, 33, 998–1006. [Google Scholar] [CrossRef]
- George, K.; Whyte, G.P.; Green, D.J.; Oxborough, D.; Shave, R.E.; Gaze, D.; Somauroo, J. The Endurance Athlete’s Heart: Acute Stress and Chronic Adaptation. Br. J. Sports Med. 2012, 46 (Suppl. S1), i29–i36. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Perhonen, M.; Howden, E.; Peshock, R.M.; Zhang, R.; Adams-Huet, B.; Haykowsky, M.J.; Levine, B.D. Cardiac Remodeling in Response to 1 Year of Intensive Endurance Training. Circulation 2014, 130, 2152–2161. [Google Scholar] [CrossRef]
- Ko, J.M.; So, W.Y.; Park, S.E. Narrative Review of High-Intensity Interval Training: Positive Impacts on Cardiovascular Health and Disease Prevention. J. Cardiovasc. Dev. Dis. 2025, 12, 158. [Google Scholar] [CrossRef]
- Del Punta, L.; De Biase, N.; Armenia, S.; Di Fiore, V.; Maremmani, D.; Gargani, L.; Mazzola, M.; De Carlo, M.; Mengozzi, A.; Lomonaco, T.; et al. Combining Cardiopulmonary Exercise Testing with Echocardiography: A Multiparametric Approach to the Cardiovascular and Cardiopulmonary Systems. Eur. Heart J. Imaging Methods Pract. 2023, 1, qyad021. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Nottin, S.; Doucende, G.; Schuster, I.; Tanguy, S.; Dauzat, M.; Obert, P. Alteration in Left Ventricular Strains and Torsional Mechanics after Ultralong Duration Exercise in Athletes. Circ. Cardiovasc. Imaging 2009, 2, 323–330. [Google Scholar] [CrossRef]
- George, K.; Shave, R.; Oxborough, D.; Cable, T.; Dawson, E.; Artis, N.; Gaze, D.; Hew-Butler, T.; Sharwood, K.; Noakes, T. Left Ventricular Wall Segment Motion after Ultra-Endurance Exercise in Humans Assessed by Myocardial Speckle Tracking. Eur. J. Echocardiogr. 2009, 10, 238–243. [Google Scholar] [CrossRef]
- Chan-Dewar, F.; Oxborough, D.; Shave, R.; Gregson, W.; Whyte, G.; George, K. Left Ventricular Myocardial Strain and Strain Rates in Sub-Endocardial and Sub-Epicardial Layers before and after a Marathon. Eur. J. Appl. Physiol. 2010, 109, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, D.; Shave, R.; Warburton, D.; Williams, K.; Oxborough, A.; Charlesworth, S.; Foulds, H.; Hoffman, M.D.; Birch, K.; George, K. Dilatation and Dysfunction of the Right Ventricle Immediately after Ultraendurance Exercise: Exploratory Insights from Conventional Two-Dimensional and Speckle Tracking Echocardiography. Circ. Cardiovasc. Imaging 2011, 4, 253–263. [Google Scholar] [CrossRef]
- Unnithan, V.B.; Rowland, T.; George, K.; Lindley, M.R.; Roche, D.M. Regional and Global Left Ventricular Function Following a Simulated 5 km Race in Sports-Trained Adolescents. Pediatr. Cardiol. 2015, 36, 322–328. [Google Scholar] [CrossRef][Green Version]
- Stewart, G.M.; Yamada, A.; Haseler, L.J.; Kavanagh, J.J.; Koerbin, G.; Chan, J.; Sabapathy, S. Altered Ventricular Mechanics after 60 min of High-Intensity Endurance Exercise: Insights from Exercise Speckle-Tracking Echocardiography. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H875–H883. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.M.; Chan, J.; Yamada, A.; Kavanagh, J.J.; Haseler, L.J.; Shiino, K.; Sabapathy, S. Impact of High-Intensity Endurance Exercise on Regional Left and Right Ventricular Myocardial Mechanics. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.P.; Mahure, C.; Mungulmare, K.; Grewal, H.K.; Bansal, M. Myocardial Fatigue in Recreational Marathon Runners: A Speckle-Tracking Echocardiography Study. Indian Heart J. 2018, 70 (Suppl. S3), S229–S234. [Google Scholar] [CrossRef]
- Oxborough, D.L.; Spence, A.; George, K.P.; Van Oorschot, F.; Thijssen, D.H.T.; Green, D.J. Impact of 24 Weeks of Supervised Endurance versus Resistance Exercise Training on Left Ventricular Mechanics in Healthy Untrained Humans. J. Appl. Physiol. 2019, 126, 1095–1102. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Christou, G.A.; Sotiriou, P.G.; Anifanti, M.A.; Koutlianos, N.A.; Tsironi, M.P.; Christou, K.A.; Vassilikos, V.P.; Deligiannis, A.P.; Kouidi, E.J. Impact of a 246 km Ultra-Marathon Running Race on Heart: Insights from Advanced Deformation Analysis. Eur. J. Sport Sci. 2022, 22, 1287–1295. [Google Scholar] [CrossRef]
- Birat, A.; Ratel, S.; Dodu, A.; Grossoeuvre, C.; Dupont, A.C.; Rance, M.; Morel, C.; Nottin, S. A Long-Duration Race Induces a Decrease of Left Ventricular Strains, Twisting Mechanics and Myocardial Work in Trained Adolescents. Eur. J. Sport Sci. 2023, 23, 1394–1404. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Pelliccia, A.; Alvino, F.; Solari, M.; Loffreno, A.; Cameli, M.; Focardi, M.; Bonifazi, M.; Mondillo, S. Effects of Training on LV Strain in Competitive Athletes. Heart 2015, 101, 1834–1839. [Google Scholar] [CrossRef]
- Egelund, J.; Jørgensen, P.G.; Mandrup, C.M.; Fritz-Hansen, T.; Stallknecht, B.; Bangsbo, J.; Nyberg, M.; Hellsten, Y. Cardiac Adaptations to High-Intensity Aerobic Training in Premenopausal and Recent Postmenopausal Women: The Copenhagen Women Study. J. Am. Heart Assoc. 2017, 6, e005469. [Google Scholar] [CrossRef]
- O’Driscoll, J.M.; Wright, S.M.; Taylor, K.A.; Coleman, D.A.; Sharma, R.; Wiles, J.D. Cardiac Autonomic and Left Ventricular Mechanics Following High Intensity Interval Training: A Randomized Crossover Controlled Study. J. Appl. Physiol. 2018, 125, 1030–1040. [Google Scholar] [CrossRef]
- Grace, F.; Herbert, P.; Elliott, A.D.; Richards, J.; Beaumont, A.; Sculthorpe, N.F. High-Intensity Interval Training (HIIT) Improves Resting Blood Pressure, Metabolic Capacity and Heart Rate Reserve without Compromising Cardiac Function in Sedentary Aging Men. Exp. Gerontol. 2018, 109, 75–81. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsai, H.H.; Fu, T.C.; Hsu, C.C.; Wang, J.S. High-Intensity Interval Training Improves Left Ventricular Contractile Function. Med. Sci. Sports Exerc. 2019, 51, 1420–1428. [Google Scholar] [CrossRef]
- Edwards, J.J.; Wiles, J.D.; Vadaszy, N.; Taylor, K.A.; O’Driscoll, J.M. Left Ventricular Mechanical, Cardiac Autonomic and Metabolic Responses to a Single Session of High Intensity Interval Training. Eur. J. Appl. Physiol. 2022, 122, 383–394. [Google Scholar] [CrossRef]
- Kösemen, D.S.; Çetin, S.; Demirci, D.; Babaoğlu, K. Evaluation of the Left Ventricular Myocardium Using Layer-Specific Strain Analysis in Adolescent Athletes Performing High-Intensity Interval Training. Pediatr. Cardiol. 2024, 45, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Stefani, L.; Pedrizzetti, G.; De Luca, A.; Mercuri, R.; Innocenti, G.; Galanti, G. Real-Time Evaluation of Longitudinal Peak Systolic Strain (Speckle Tracking Measurement) in Left and Right Ventricles of Athletes. Cardiovasc. Ultrasound 2009, 7, 17. [Google Scholar] [CrossRef]
- Liang, C.; Ma, Y.; Gao, C.; Zhang, J.; Yang, M.; Chen, G.; Fu, S.; Zhu, T. Two-Dimensional Strain Echocardiography Technology for Evaluation of Myocardial Strain in Swimming Athletes after High-Intensity Exercise. Echocardiography 2017, 34, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Mikołajczyk, R.; Waśkiewicz, Z.; Gąsior, Z.; Mizia-Stec, K.; Kawecki, D.; Rosemann, T.; Nikolaidis, P.T.; Knechtle, B. Left Ventricular Systolic Function Assessed by Speckle Tracking Echocardiography in Athletes with and without Left Ventricle Hypertrophy. J. Clin. Med. 2019, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Kandels, J.; Metze, M.; Hagendorff, A.; Marshall, R.P.; Hepp, P.; Laufs, U.; Stöbe, S. The Impact of Upright Posture on Left Ventricular Deformation in Athletes. Int. J. Cardiovasc. Imaging 2023, 39, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Kandels, J.; Stöbe, S.; Kogel, A.; Hepp, P.; Riepenhof, H.; Droste, J.N.; Stoeggl, T.; Marshall, R.P.; Rudolph, U.; Laufs, U.; et al. Effect of Maximum Exercise on Left Ventricular Deformation and Its Correlation with Cardiopulmonary Exercise Capacity in Competitive Athletes. Echo Res. Pract. 2023, 10, 17. [Google Scholar] [CrossRef]
- Oxborough, D.; Birch, K.; Shave, R.; George, K. “Exercise-Induced Cardiac Fatigue”—A Review of the Echocardiographic Literature. Echocardiography 2010, 27, 1130–1140. [Google Scholar] [CrossRef]
- Lord, R.N.; Utomi, V.; Oxborough, D.L.; Curry, B.A.; Brown, M.; George, K.P. Left Ventricular Function and Mechanics Following Prolonged Endurance Exercise: An Update and Meta-Analysis with Insights from Novel Techniques. Eur. J. Appl. Physiol. 2018, 118, 1291–1299. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-Intensity Interval Training in Patients with Lifestyle-Induced Cardiometabolic Disease: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Kemi, O.J.; Wisløff, U. High-Intensity Aerobic Exercise Training Improves the Heart in Health and Disease. J. Cardiopulm. Rehabil. Prev. 2010, 30, 2–11. [Google Scholar] [CrossRef] [PubMed]
- McGregor, G.; Powell, R.; Begg, B.; Birkett, S.T.; Nichols, S.; Ennis, S.; McGuire, S.; Prosser, J.; Fiassam, O.; Hee, S.W.; et al. High-Intensity Interval Training in Cardiac Rehabilitation: A Multi-Centre Randomized Controlled Trial. Eur. J. Prev. Cardiol. 2023, 30, 745–755. [Google Scholar] [CrossRef]
- Wang, C.; Xing, J.; Zhao, B.; Wang, Y.; Zhang, L.; Wang, Y.; Zheng, M.; Liu, G. The Effects of High-Intensity Interval Training on Exercise Capacity and Prognosis in Heart Failure and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Cardiovasc. Ther. 2022, 2022, 4273809. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Cheng, Y.; Zhang, M.; Zhao, Y.; Zhang, T.; Dong, J.; Xing, J.; Zhen, Y.; Wang, C. High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Aerobic Capacity and Functional Capacity in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2024, 11, 1302109. [Google Scholar] [CrossRef]
- Kb, S.; Vaishali, K.; Kadavigere, R.; Sukumar, S.; Kn, S.; Pullinger, S.A.; Bommasamudram, T. Effects of High-Intensity Interval Training versus Moderate-Intensity Continuous Training on Vascular Function among Individuals with Overweight and Obesity—A Systematic Review. Int. J. Obes. 2024, 48, 1517–1533. [Google Scholar] [CrossRef]
- Bhella, P.S.; Hastings, J.L.; Fujimoto, N.; Shibata, S.; Carrick-Ranson, G.; Palmer, M.D.; Boyd, K.N.; Adams-Huet, B.; Levine, B.D. Impact of Lifelong Exercise “Dose” on Left Ventricular Compliance and Distensibility. J. Am. Coll. Cardiol. 2014, 64, 1257–1266. [Google Scholar] [CrossRef]
- La Gerche, A.; Heidbüchel, H. Can Intensive Exercise Harm the Heart? You Can Get Too Much of a Good Thing. Circulation 2014, 130, 992–1002. [Google Scholar] [CrossRef]
- Andersen, L.J.; Hansen, P.R.; Søgaard, P.; Madsen, J.K.; Bech, J.; Krustrup, P. Improvement of Systolic and Diastolic Heart Function after Physical Training in Sedentary Women. Scand. J. Med. Sci. Sports 2010, 20 (Suppl. S1), 50–57. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior Cardiovascular Effect of Aerobic Interval Training versus Moderate Continuous Training in Heart Failure Patients: A Randomized Study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.; Picco, J.M.; Díaz-González, L.; Valenzuela, P.L.; Gonzalez-Dávila, E.; Santos-Lozano, A.; Matile, P.; Wolff, D.; Boraita, A.; Lucia, A. Exercise-Induced Cardiac Fatigue in Recreational Ultramarathon Runners at Moderate Altitude: Insights from Myocardial Deformation Analysis. Front. Cardiovasc. Med. 2022, 8, 744393. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Wang, Y.; Liu, H.; Kong, Z.; Qi, F. Effects of High-Intensity Interval vs. Moderate-Intensity Continuous Training on Cardiac Rehabilitation in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 845225. [Google Scholar] [CrossRef] [PubMed]
- Mascia, G.; Olivotto, I.; Brugada, J.; Arbelo, E.; Di Donna, P.; Della Bona, R.; Canepa, M.; Porto, I. Sport Practice in Hypertrophic Cardiomyopathy: Running to Stand Still? Int. J. Cardiol. 2021, 345, 77–82. [Google Scholar] [CrossRef]
- Paldino, A.; Rossi, M.; Dal Ferro, M.; Tavčar, I.; Behr, E.; Sharma, S.; Papadakis, M.; Sinagra, G.; Finocchiaro, G. Sport and Exercise in Genotype Positive (+) Phenotype Negative (−) Individuals: Current Dilemmas and Future Perspectives. Eur. J. Prev. Cardiol. 2023, 30, 871–883. [Google Scholar] [CrossRef]
- Negishi, T.; Negishi, K.; Thavendiranathan, P.; Cho, G.Y.; Popescu, B.A.; Vinereanu, D.; Kurosawa, K.; Penicka, M.; Marwick, T.H.; SUCCOUR Investigators; et al. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc. Imaging 2017, 10, 518–522. [Google Scholar] [CrossRef]
- Rösner, A.; Barbosa, D.; Aarsæther, E.; Kjønås, D.; Schirmer, H.; D’hooge, J. The Influence of Frame Rate on Two-Dimensional Speckle-Tracking Strain Measurements: A Study on Silico-Simulated Models and Images Recorded in Patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Fagiani, V.; Nicolosi, G.L.; Lombardo, M. The Influence of Pectus Excavatum on Biventricular Mechanics: A Systematic Review and Meta-Analysis. Minerva Cardiol. Angiol. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]


| Study Name, Publication Year and Country | Size (%Males) | Mean Age (Yrs) | Repeated Measures Assessment | Software | Endurance Exercise | Influence on Biventricular Mechanics |
|---|---|---|---|---|---|---|
| Nottin S. (2009) France [20] | 23 (100) | 40 | within 3 days before and within 45 min of the race completion | GE | 14 h triathlon race | Decreased LV longitudinal, circumferential and radial strains; slightly reduced/delayed twist; depressed/delayed untwisting |
| George K. (2009) U.K. [21] | 19 (100) | 41 | 24 h prior to the race and within 60 min of race finish | GE (>40 and <90 frames per second) | 89-km Comrades Marathon | Post–race significant reduction in LV–GLS, LV–GCS and LV–GRS |
| Chan-Dewar F. (2010) U.K. [22] | 14 (100) | 32 | 24 h prior to the race and within 60 min of race completion | GE (between 40 and 80 frames per second) | 42.2-km London marathon | Mild reduction in LV–GLS, significant reduction in ACS; not altered rotation and unchanged LV torsion |
| Oxborough D.L. (2011) U.K. [23] | 16 (75) | 42 | 24 h before starting the race, and within 1 h of race completion | GE (<90 frames/second) | 161-km ultramarathon | Reduced LV strain in all planes (longitudinal, circumferential and radial); unchanged LV torsion; LASr impairment; RV dilatation with reduced RV strain. |
| Unnithan V.B. (2015) U.K. [24] | 20 (100) | 15.2 | Prior to and 45–min post–race | GE | 5 km cross–country race | Minor transient decrease in LV–GLS 45–min post–race. |
| Stewart G.M. (2015) Australia [25] | 15 (100) | 28 | 1.5 h before and after CRIT60 | GE (NS) | 60-min endurance cycling intervention (CRIT60) | Decreased LV–GLS and RV–GLS; unchanged LV torsion; increased cTnT. |
| Stewart G.M. (2017) Australia [26] | 23 (100) | 28 | Before and after 90-min CRIT | GE (frame rate of 50–80 frames/sec) | 90-min endurance cycling intervention | Transient reductions in LV–GLS, LV–GCS and RV–GLS |
| Sengupta S.P. (2018) India [27] | 50 (88) | 40.8 | Before training and within 10 days of completion of marathon | GE (frame rate of 50–80 frames/sec) | 42.2-km marathon | Decreased LV–GLS and LV–GCS; unchanged LV–GRS; increased NT–proBNP |
| Oxborough D.L. (2019) U.K. [28] | 23 (100) | 27.4 | Baseline and after 24–wk training | GE (NS) | 24-wk endurance training program | Unchanged LV–GLS; mild reduction in LV–GCS and LV–GRS; increased basal rotation |
| Pagourelias E.D. (2022) Greece [29] | 27 (70.4) | 45 | 24 h before starting the race, and within 10 min after finishing | GE (NS) | 246 km ultra–marathon running race | Decreased LV–GLS and RV–GLS; unchanged LASr and RASr |
| Birat A. (2023) France [30] | 20 (100) | 15.8 | Immediately before and 24-h after the race | GE (NS) | 68.5-km competitive adventure race | Unchanged LV–GLS; reduced LV global myocardial work, LV twisting and untwisting |
| Study Name, Publication Year and Country | Size (%Males) | Mean Age (Yrs) | Repeated Measures Assessment | Software | HIIT Program | Influence on LV Mechanics |
|---|---|---|---|---|---|---|
| D’Ascenzi F. (2015) Italy [31] | 91 (60%) | 23.0 | Before the competitive period and 18 ± 2 weeks later | GE (60–90 frames/s) | An 18-week, intensive training programme | Improved LV–GLS (↑BLS, MLS). Unchanged LV basal rotation, apical rotation, torsion, twisting and untwisting rate. |
| Egelund J. (2017) Denmark [32] | 37 (0%) | 53.5 | Before and after the intervention period | GE | 12-week period of high-intensity aerobic cycle training | Improved LV–GLS. |
| O’Driscoll J.M. (2018) U.K. [33] | 40 (100%) | 21.0 | Baseline and postintervention measures | GE (frame rates > 60 Hz) | 2-wk HIIT intervention | Improved LV–GLS. Improvement in LV apical rotation, ACS, ARS and LV torsion. |
| Grace F. (2018) Australia [34] | 22 (100%) | 62.7 | Pre/post HIIT design | GE | 6 weeks of HIIT | Improved LV–GLS. |
| Huang YC. (2019) Taiwan [35] | 18 (100%) | 21.4 | Two days before pretraining and two days after post-training | Siemens | High-intensity interval training for 6 weeks | Improved LV–GLS, LV–GCS and LV–GRS. Increased LV peak basal rotation, peak apical rotation and LV torsion. |
| Edwards J.J. (2022) U.K. [36] | 50 (50%) | 22.9 | Baseline and following HIIT in the recovery period | GE (frame rates > 60 Hz) | HIIT (three 30 s periods of maximal intensity cycling) | Improved LV–GLS, BRS and ACS. Increased apical rotation and LV torsion. |
| Kösemen D.S. (2024) Turkey [37] | 19 (100%) | 16.8 | Pre/post HIIT design | GE | 8-week HIIT program | Improved LV–GLS and LV–GCS. |
| Study Name, Publication Year and Country | Size (%Males) | Mean Age (Yrs) | Repeated Measures Assessment | Software | Acute Test | Influence on LV Mechanics |
|---|---|---|---|---|---|---|
| Stefani L. (2009) Italy [38] | 32 (100) | 25.0 | At rest and at the peak of effort | My Lab 30 echocardiograph/X–Strain software | Isometric stress test performed with a graduated handle dynamometer held in the dominant arm for 3 min | Improved LV–GLS and RV–GLS; significant increase in LV and RV mid-apical longitudinal strain |
| Liang C. (2017) China [39] | 15 (60) | 16.3 | 30 min before and immediately after the training program | GE (frame rate > 70 frames per second) | 200 m high-intensity (increasing-load) exercise in swimming athletes (one-time intensive exercise) | Reduced LV–GLS, due to exercise–induced greater impairment in LS of basal and middle segments |
| Żebrowska A. (2019) Poland [40] | 14 (100) | 27.2 | Before and immediately after stress test | GE | Incremental stress test on a cycling ergometer | Mild reduction in LV–GLS, BCS and basal rotation |
| Kandels J. (June 2023) Germany [41] | 50 (100) | 25.7 | Athletes examined in left lateral position and with upright posture | GE | Upright posture | Upright posture produced a marked reduction in global LV deformation with predominant regional depression in basal inferior/posterolateral segments. |
| Kandels J. (October 2023) Germany [42] | 19 (100) | 22.1 | At rest and 5 min after CPET | GE | Incremental CPET in football players | Mild reduction in LV–GLS; unchanged global myocardial work |
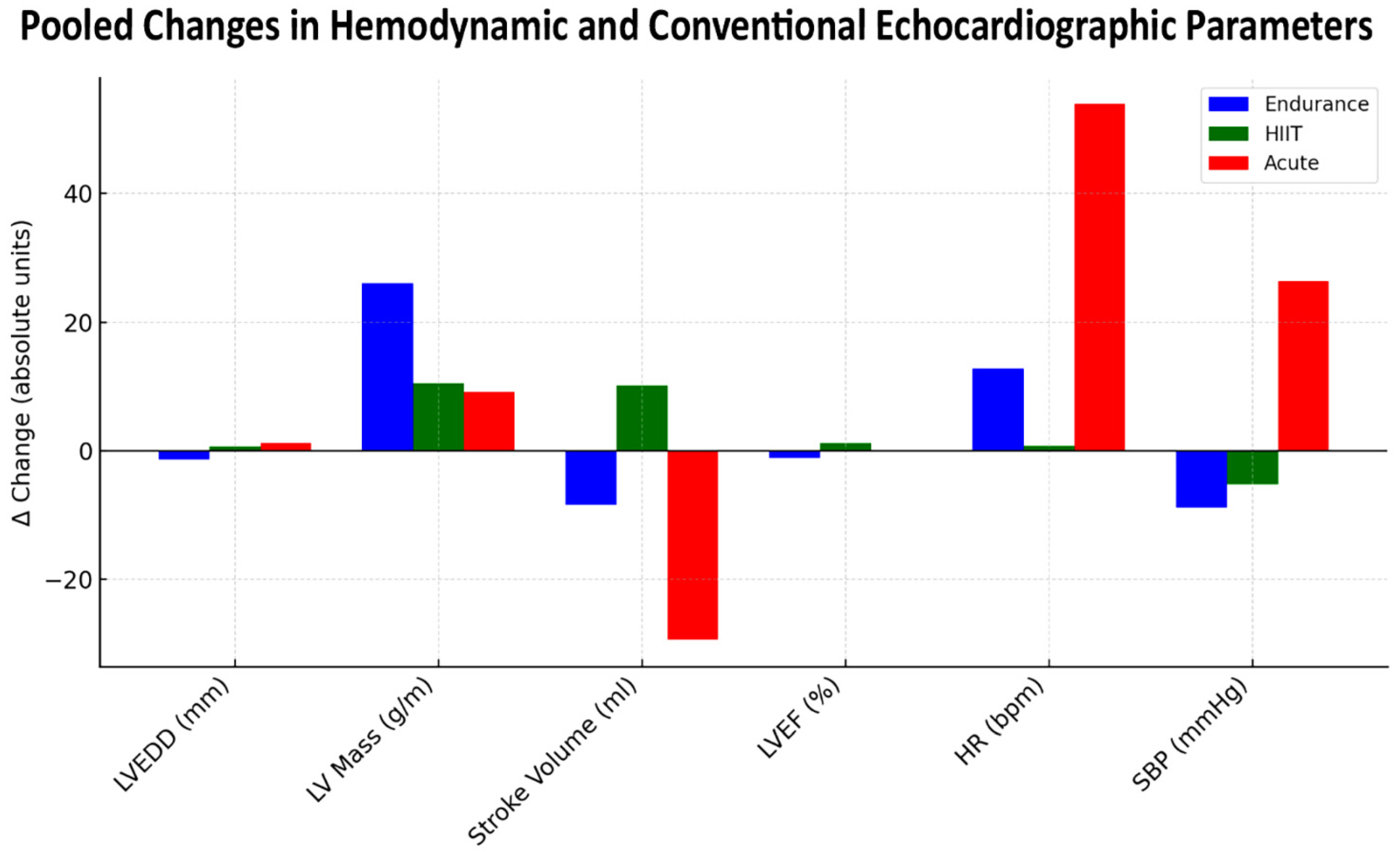
| Parameter | Endurance (n = 250) | HIIT (n = 277) | Acute Tests (n = 130) | p-Value |
|---|---|---|---|---|
| %males | 93.9 (70.4, 100.0) | 72.9 (0.0, 100.0) | 92.0 (60.0, 100.0) | 0.119 |
| Mean age (yrs) | 32.3 (15.2, 45.0) | 31.6 (16.8, 62.7) | 23.3 (16.3, 27.2) | 0.296 |
| Resting BMI (Kg/m2) | 23.1 (20.2, 26.3) | 24.4 (21.5, 29.4) | 23.4 (22.4, 24.1) | 0.850 |
| Post–stress BMI (Kg/m2) | 22.5 (19.9, 24.3) | 23.9 (21.5, 29.1) | NR | 0.602 |
| Delta BMI (Kg/m2) | −0.5 (−1.3, 0.1) | −0.1 (−0.3, 0.3) | NR | 0.093 |
| Resting HR (bpm) | 61.1 (52.0, 74.1) | 66.4 (62.2, 70.4) | 67.5 (63.0, 79.1) | 0.038 |
| Post-stress HR (bpm) | 73.9 (58.0, 86.6) | 67.1 (57.7, 100.0) | 121.3 (61.0, 185.0) | 0.001 |
| Delta HR (bpm) | 12.8 (−9.6, 24.0) | 0.7 (−5.5, 31.0) | 53.9 (−18.1, 122.0) | 0.005 |
| Rest SBP (mmHg) | 122.9 (118.0, 134.0) | 120.9 (113.0, 135.0) | 125.2 (118.6, 133.8) | 0.508 |
| Post-stress SBP (mmHg) | 114.0 (107.0, 122.0) | 115.7 (110.0, 130.0) | 153.3 (125.3, 185.7) | <0.001 |
| Delta SBP (mmHg) | −8.9 (−12.0, −3.0) | −5.2 (−15.0, 0.0) | 26.4 (−3.0, 67.1) | <0.001 |
| Rest DBP (mmHg) | 73.8 (63.0, 82.0) | 73.7 (67.6, 87.0) | 73.8 (60.0, 82.2) | 0.999 |
| Post-stress DBP (mmHg) | 70.3 (60.0, 79.0) | 68.0 (59.0, 82.0) | 70.3 (60.0, 78.0) | 0.806 |
| Delta DBP (mmHg) | −3.5 (–9.0, 0.0) | −5.6 (−10.0, −2.8) | –8.1 (−18.6, −1.4) | 0.267 |
| Parameter | Endurance (n = 250) | HIIT (n = 277) | Acute Tests (n = 130) | p-Value |
|---|---|---|---|---|
| Resting IVS (mm) | 9.5 (9.0, 11.4) | 9.1 (7.4, 10.9) | 9.9 (9.6, 10.2) | 0.331 |
| Post-stress IVS (mm) | 10.7 (10.0, 11.4) | 9.7 (7.4, 11.7) | 10.2 (9.7, 10.8) | 0.303 |
| Delta IVS (mm) | 0.8 (0.0, 1.4) | 0.5 (0.0, 1.0) | 0.4 (−0.2, 1.0) | 0.388 |
| Resting LVEDD (mm) | 51.5 (47.0, 55.5) | 47.1 (42.4, 51.0) | 51.6 (48.2, 57.5) | 0.021 |
| Post-stress LVEDD (mm) | 48.8 (46.0, 52.1) | 47.7 (43.4, 52.0) | 54.1 (53.4, 54.8) | 0.008 |
| Delta LVEDD (mm) | −1.4 (−3.4, −0.7) | 0.6 (−1.7, 2.1) | 1.2 (−4.1, 6.6) | 0.174 |
| Resting LV mass (g) | 142.3 (120.2, 157.8) | 160.0 (109.0, 223.0) | 212.7 (206.4, 219.0) | 0.011 |
| Post-stress LV mass (g) | 168.3 (160.3, 179.6) | 170.5 (94.3, 241.0) | 221.8 (221.0, 222.6) | 0.079 |
| Delta LV mass (g) | 26.0 (16.0, 40.1) | 10.5 (−14.7, 22.5) | 9.1 (2.0, 16.2) | 0.087 |
| Resting LVEDV (mL) | 126.8 (61.4, 169.0) | 101.5 (93.3, 115.8) | 140.9 (117.0, 164.8) | 0.197 |
| Post-stress LVEDV (mL) | 117.4 (72.8, 162.0) | 99.7 (85.3, 116.2) | 148.2 (138.6, 157.9) | 0.040 |
| Delta LVEDV (mL) | −5.1 (−20.0, 11.4) | −1.8 (−8, 2.2) | 7.3 (−26.2, 40.9) | 0.59 |
| Resting LVESV (mL) | 48.0 (21.9, 66.0) | 40.3 (40.2, 40.3) | 52.9 (48.3, 57.5) | 0.338 |
| Post-stress LVESV (mL) | 46.9 (20.3, 61.0) | 37.6 (32.3, 41.8) | 56.4 (50.0, 62.7) | 0.118 |
| Delta LVESV (mL) | −1.1 (−5.0, 3.0) | −2.7 (−8.0, 1.5) | 3.5 (−7.5, 14.4) | 0.390 |
| Resting LVEF (%) | 64.8 (56.0, 75.1) | 60.0 (55.6, 65.1) | 61.7 (59.7, 65.8) | 0.096 |
| Post-stress LVEF (%) | 63.6 (54.0, 76.8) | 61.1 (53.5, 67.7) | 62.7 (61.1, 64.3) | 0.666 |
| Delta LVEF (%) | −1.1 (−9.0, 7.3) | 1.2 (−2.1, 5.0) | −0.1 (−1.5, 1.4) | 0.499 |
| Resting SV (mL) | 88.7 (68.7, 100.0) | 65.8 (55.1, 75.6) | 90.3 (86.0, 94.7) | <0.001 |
| Post-stress SV (mL) | 80.4 (66.9, 95.0) | 75.9 (55.8, 103.8) | 65.3 (65.3, 65.3) | 0.367 |
| Delta SV (mL) | −8.4 (−17.0, −1.8) | 10.1 (0.7, 38.1) | −29.4 (−29.4, −29.4) | <0.001 |
| Resting E/A | 1.9 (1.4, 2.8) | 1.6 (1.1, 1.9) | 1.8 (1.8, 1.9) | 0.307 |
| Post-stress E/A | 1.5 (1.1, 2.3) | 1.6 (1.2, 2.0) | 1.2 (1.2, 1.2) | 0.369 |
| Delta E/A | −0.4 (−0.8, 0.2) | 0.0 (−0.7, 0.2) | −0.6 (−0.6, −0.6) | 0.005 |
| Resting E/e’ | 6.8 (5.1, 9.0) | 5.6 (4.4, 7.0) | 4.9 (4.9, 4.9) | 0.135 |
| Post-stress E/e’ | 6.6 (4.6, 8.3) | 5.5 (4.1, 7.5) | 5.4 (5.4, 5.4) | 0.267 |
| Delta E/e’ | −0.2 (−0.7, 0.5) | −0.1 (−0.4, 0.5) | 0.5 (0.5, 0.5) | 0.114 |
| Resting LAV (mL) | 46.9 (22.8, 62.0) | 49.9 (48.5, 51.2) | 7.7 (7.7, 7.7) | 0.017 |
| Post-stress LAV (mL) | 42.0 (19.0, 57.0) | 60.4 (55.2, 65.6) | 18.2 (18.2, 18.2) | 0.024 |
| Delta LAV (mL) | −4.9 (−6.0, −3.8) | 10.6 (6.7, 14.4) | 10.5 (10.5, 10.5) | <0.001 |
| Resting RVIT (mm) | 41.5 (41.0, 42.0) | NR | NR | / |
| Post-stress RVIT (mm) | 44.0 (43.0, 45.0) | NR | NR | / |
| Delta RVIT (mm) | 2.5 (2.0, 3.0) | NR | NR | / |
| Resting TAPSE (mm) | 27.0 (25.0, 29.0) | 24.1 (23.1, 25.0) | 19.0 (19.0, 19.0) | 0.004 |
| Post-stress TAPSE (mm) | 24.0 (22.0, 26.0) | 23.8 (22.7, 25.0) | 20.0 (20.0, 20.0) | 0.056 |
| Delta TAPSE (mm) | −3.0 (−3.0, −3.0) | −0.2 (−0.4, 0.0) | 1.0 (1.0, 1.0) | <0.001 |
| Resting RV-FAC (%) | 47.3 (43.0, 50.0) | NR | NR | / |
| Post-stress RV-FAC (%) | 42.3 (38.0, 46.0) | NR | NR | / |
| Delta RV-FAC (%) | −5.0 (−7.0, −3.0) | NR | NR | / |
| Resting sPAP (mmHg) | 28.0 (28.0, 28.0) | NR | 23.7 (23.7, 23.7) | <0.001 |
| Post-stress sPAP (mmHg) | 25.0 (25.0, 25.0) | NR | 22.5 (22.5, 22.5) | <0.001 |
| Delta sPAP (mmHg) | −3.0 (−3.0, −3.0) | NR | −1.2 (−1.2, −1.2) | <0.001 |
| Parameter | Endurance (n = 250) | HIIT (n = 277) | Acute Tests (n = 130) | p-Value |
|---|---|---|---|---|
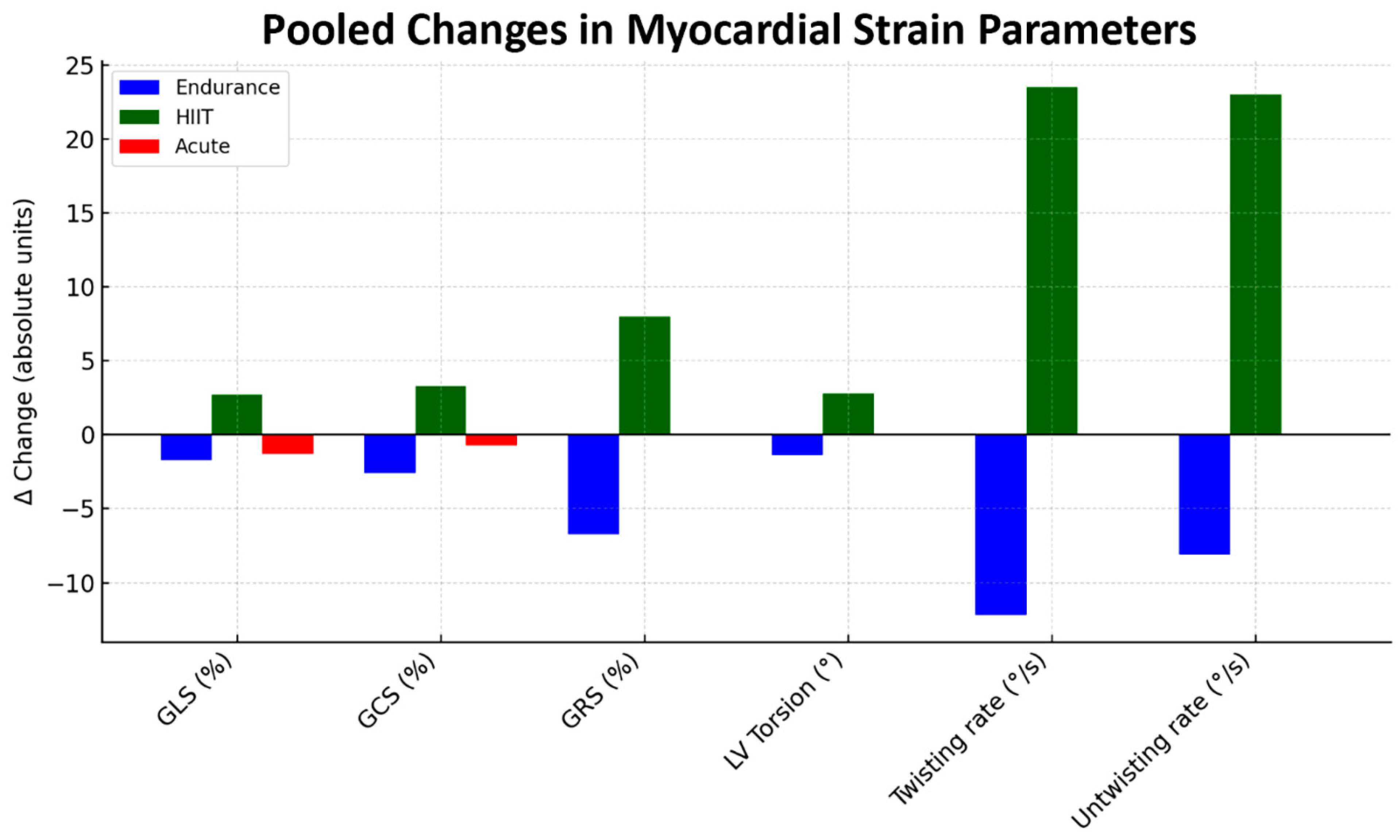
| Resting LV–GLS (%) | 19.2 (16.1, 21.8) | 17.6 (12.1, 20.2) | 18.4 (17.1, 19.4) | 0.239 |
| Post-stress LV–GLS (%) | 17.5 (14.0, 20.5) | 20.3 (13.3, 29.2) | 17.1 (13.5, 21.3) | 0.103 |
| Delta LV–GLS (%) | −1.7 (−4.0, 0.1) | 2.7 (0.6, 10.9) | −1.3 (−5.0, 4.2) | 0.002 |
| Resting LV–BLS (%) | 18.5 (16.6, 20.1) | 18.7 (17.3, 20.1) | 17.1 (16.0, 18.2) | 0.329 |
| Post-stress LV–BLS (%) | 16.6 (15.5, 18.7) | 19.4 (18.2, 20.6) | 15.1 (11.6, 18.5) | 0.110 |
| Delta LV–BLS (%) | −1.9 (−3.3, −1.0) | 0.7 (0.5, 0.9) | −2.0 (−6.6, 2.5) | 0.363 |
| Resting LV–MLS (%) | 20.1 (19.9, 20.4) | 20.2 (20.2, 20.2) | 18.6 (18.1, 19.2) | 0.002 |
| Post-stress LV–MLS (%) | 18.5 (17.6, 19.3) | 20.8 (20.6, 20.9) | 19.2 (14.3, 24.1) | 0.630 |
| Delta LV–MLS (%) | −1.7 (−2.3, −1.1) | 0.6 (0.4, 0.7) | 0.6 (−4.9, 6.0) | 0.627 |
| Resting LV–ALS (%) | 25.0 (24.6, 25.8) | 22.2 (20.3, 24.0) | 19.0 (18.1, 19.8) | <0.001 |
| Post-stress LV–ALS (%) | 24.6 (23.4, 26.6) | 22.7 (21.0, 24.3) | 22.4 (20.7, 24.1) | 0.203 |
| Delta LV–ALS (%) | −0.5 (−2.1, 1.9) | 0.5 (0.3, 0.7) | 3.4 (0.9, 6.0) | 0.059 |
| Resting LV–GCS (%) | 20.6 (17.2, 24.0) | 15.2 (14.2, 16.2) | 15.9 (15.9, 15.9) | 0.007 |
| Post-stress LV–GCS (%) | 18.0 (14.7, 22.2) | 18.4 (17.8, 19.1) | 15.2 (15.2, 15.2) | 0.326 |
| Delta LV–GCS (%) | −2.6 (−4.5, −1.0) | 3.3 (1.6, 4.9) | −0.7 (−0.7, −0.7) | <0.001 |
| Resting LV–BCS (%) | 21.2 (16.7, 25.0) | 18.3 (14.4, 23.2) | 15.9 (15.9, 15.9) | 0.218 |
| Post-stress LV–BCS (%) | 19.4 (13.5, 25.0) | 20.4 (17.0, 24.7) | 15.2 (15.2, 15.2) | 0.320 |
| Delta LV–BCS (%) | −1.8 (−3.2, 0.0) | 2.1 (1.5, 2.6) | −0.7 (−0.7, −0.7) | 0.005 |
| Resting LV–MACS (%) | 24.0 (18.3, 26.9) | 18.3 (14.9, 21.8) | NR | 0.110 |
| Post-stress LV–MACS (%) | 21.5 (15.9, 25.0) | 22.3 (18.2, 26.4) | NR | 0.814 |
| Delta LV–MACS (%) | −2.4 (−3.2, −1.7) | 3.9 (3.3, 4.6) | NR | <0.001 |
| Resting LV–GRS (%) | 46.3 (31.9, 53.4) | 26.0 (26.0, 26.0) | NR | 0.010 |
| Post-stress LV–GRS (%) | 39.6 (30.8, 47.0) | 34.0 (34.0, 34.0) | NR | 0.292 |
| Delta LV–GRS (%) | −6.7 (−13.1, −1.0) | 8.0 (8.0, 8.0) | NR | 0.004 |
| Resting LV–BRS (%) | 42.0 (36.3, 47.8) | 39.3 (39.3, 39.3) | NR | 0.567 |
| Post-stress LV–BRS (%) | 41.0 (30.6, 51.3) | 23.2 (23.2, 23.2) | NR | 0.105 |
| Delta LV–BRS (%) | −1.1 (−5.7, 3.5) | −16.1 (−16.1, 16.1) | NR | 0.022 |
| Resting LV–MARS (%) | 42.0 (39.4, 44.6) | 35.5 (35.5, 35.5) | NR | 0.044 |
| Post-stress LV–MARS (%) | 33.9 (31.1, 36.7) | 47.5 (47.5, 47.5) | NR | 0.007 |
| Delta LV–MARS (%) | −8.1 (−13.5, −2.7) | 12.0 (12.0, 12.0) | NR | 0.015 |
| Resting RV–GLS (%) | 26.7 (22.9, 28.4) | NR | 24.4 (24.4, 24.4) | 0.235 |
| Post-stress RV–GLS (%) | 23.1 (21.2, 24.0) | NR | 25.4 (25.4, 25.4) | 0.034 |
| Delta RV–GLS (%) | −3.6 (−4.9, −1.7) | NR | 1.0 (1.0, 1.0) | 0.006 |
| Resting LASr (%) | 42.0 (38.9, 45.2) | NR | NR | / |
| Post-stress LASr (%) | 35.5 (34.0, 36.9) | NR | NR | / |
| Delta LASr (%) | −6.6 (−8.3, −4.9) | NR | NR | / |
| Resting RASr (%) | 44.6 (44.6, 44.6) | NR | NR | / |
| Post-stress RASr (%) | 44.3 (44.3, 44.3) | NR | NR | / |
| Delta RASr (%) | −0.3 (−0.3, −0.3) | NR | NR | / |
| Parameter | Endurance (n = 250) | HIIT (n = 277) | Acute Tests (n = 130) | p-Value |
|---|---|---|---|---|
| Resting LV basal rotation (°) | 4.1 (2.2, 6.5) | 5.0 (4.1, 5.6) | 6.8 (6.8, 6.8) | 0.045 |
| Post-stress LV basal rotation (°) | 4.6 (3.7, 5.7) | 6.0 (5.2, 7.5) | 6.1 (6.1, 6.1) | 0.022 |
| Delta LV basal rotation (°) | 0.5 (−1.3, 2.3) | 1.0 (0.3, 1.9) | −0.7 (−0.7, −0.7) | 0.130 |
| Resting LV apical rotation (°) | 7.5 (3.7, 10.4) | 5.7 (2.1, 7.9) | NR | 0.250 |
| Post-stress LV apical rotation (°) | 6.2 (2.0, 9.4) | 7.5 (4.0, 12.4) | NR | 0.478 |
| Delta LV apical rotation (°) | −1.3 (−2.6, 0.2) | 1.8 (−1.1, 4.5) | NR | 0.007 |
| Resting LV torsion (°) | 11.2 (6.7, 15.9) | 9.2 (6.2, 10.6) | NR | 0.277 |
| Post-stress LV torsion (°) | 9.7 (5.7, 15.1) | 11.9 (9.0, 16.2) | NR | 0.294 |
| Delta LV torsion (°) | −1.4 (−3.9, 1.4) | 2.8 (−0.3, 5.7) | NR | 0.003 |
| Resting LV twisting (basal rotation rate) °/s | 59.9 (51.0, 68.4) | 54.7 (48.3, 58.3) | NR | 0.230 |
| Post-stress LV twisting (basal rotation rate) °/s | 47.7 (2.9, 67.7) | 78.2 (59.4, 114.0) | NR | 0.088 |
| Delta LV twisting (basal rotation rate) °/s | −12.2 (−65.5, 13.4) | 23.5 (1.8, 55.7) | NR | 0.112 |
| Resting LV untwisting (basal rotation rate) °/s | 54.2 (27.7, 82.6) | 53.9 (26.7, 94.4) | NR | 0.980 |
| Post-stress LV untwisting (basal rotation rate) °/s | 46.1 (38.0, 54.3) | 76.9 (46.3, 98.3) | NR | 0.016 |
| Delta LV untwisting (basal rotation rate) °/s | −8.1 (−28.3, 13.8) | 23.0 (−2.4, 50.3) | NR | 0.034 |
| Resting LV twisting (apical rotation rate) °/s | 63.4 (55.0, 69.6) | 51.8 (45.8, 61.3) | NR | 0.013 |
| Post-stress LV twisting (apical rotation rate) °/s | 61.9 (52.0, 76.4) | 85.0 (61.0, 132.7) | NR | 0.137 |
| Delta LV twisting (apical rotation rate) °/s | −1.5 (−10.6, 11.1) | 33.2 (12.9, 71.4) | NR | 0.017 |
| Resting LV untwisting (apical rotation rate) °/s | 53.0 (29.0, 68.1) | 60.2 (26.7, 94.4) | NR | 0.612 |
| Post-stress LV untwisting (apical rotation rate) °/s | 47.1 (18.4, 71.6) | 85.3 (59.8, 116.0) | NR | 0.025 |
| Delta LV untwisting (apical rotation rate) °/s | −5.8 (−10.6, 3.5) | 25.1 (3.9, 41.5) | NR | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Baravelli, M. Influence of Endurance Training, High-Intensity Interval Training, and Acute Exercise on Left Ventricular Mechanics: A Systematic Review. J. Clin. Med. 2025, 14, 8210. https://doi.org/10.3390/jcm14228210
Sonaglioni A, Nicolosi GL, Lombardo M, Baravelli M. Influence of Endurance Training, High-Intensity Interval Training, and Acute Exercise on Left Ventricular Mechanics: A Systematic Review. Journal of Clinical Medicine. 2025; 14(22):8210. https://doi.org/10.3390/jcm14228210
Chicago/Turabian StyleSonaglioni, Andrea, Gian Luigi Nicolosi, Michele Lombardo, and Massimo Baravelli. 2025. "Influence of Endurance Training, High-Intensity Interval Training, and Acute Exercise on Left Ventricular Mechanics: A Systematic Review" Journal of Clinical Medicine 14, no. 22: 8210. https://doi.org/10.3390/jcm14228210
APA StyleSonaglioni, A., Nicolosi, G. L., Lombardo, M., & Baravelli, M. (2025). Influence of Endurance Training, High-Intensity Interval Training, and Acute Exercise on Left Ventricular Mechanics: A Systematic Review. Journal of Clinical Medicine, 14(22), 8210. https://doi.org/10.3390/jcm14228210