Complement Inhibitors in Generalized Myasthenia Gravis: Comparison of Administration Schedules, Efficacy, and Safety
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Inclusion Criteria
- Initiation of treatment with Eculizumab, Ravulizumab, or Zilucoplan, as part of clinical practice.
- Age ≥ 18 years at the time of informed consent and treatment initiation.
- MG diagnosis in accordance with national guidelines and based on clinical, serological (positive anti-AChR antibody test), and neurophysiological (positive repetitive nerve stimulation test and/or single-fiber electromyography) criteria.
- Myasthenia Gravis Foundation of America (MGFA) classification IIa (only for Zilucoplan, according to prescription requirement in Italy), IIb, IIIa, IIIb, IVa, and IVb.
- Post-intervention status unchanged or worsened after treatment with corticosteroids and at least one other non-steroid immunosuppressant (NSIST), administered at adequate dosages and for an adequate duration, but with persistent symptoms or side effects that impaired functionality, as assessed by both the patient and the treating physician.
2.2. Data Collection
2.3. Treatment Schedules and Vaccinations
2.4. Clinical Scales and Outcome Measures
2.4.1. Myasthenia Gravis Activity of Daily Living (MG-ADL)
2.4.2. Quantitative Myasthenia Gravis Score (QMG)
2.4.3. Myasthenia Gravis Composite Scale (MGC)
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Enrolled Patients
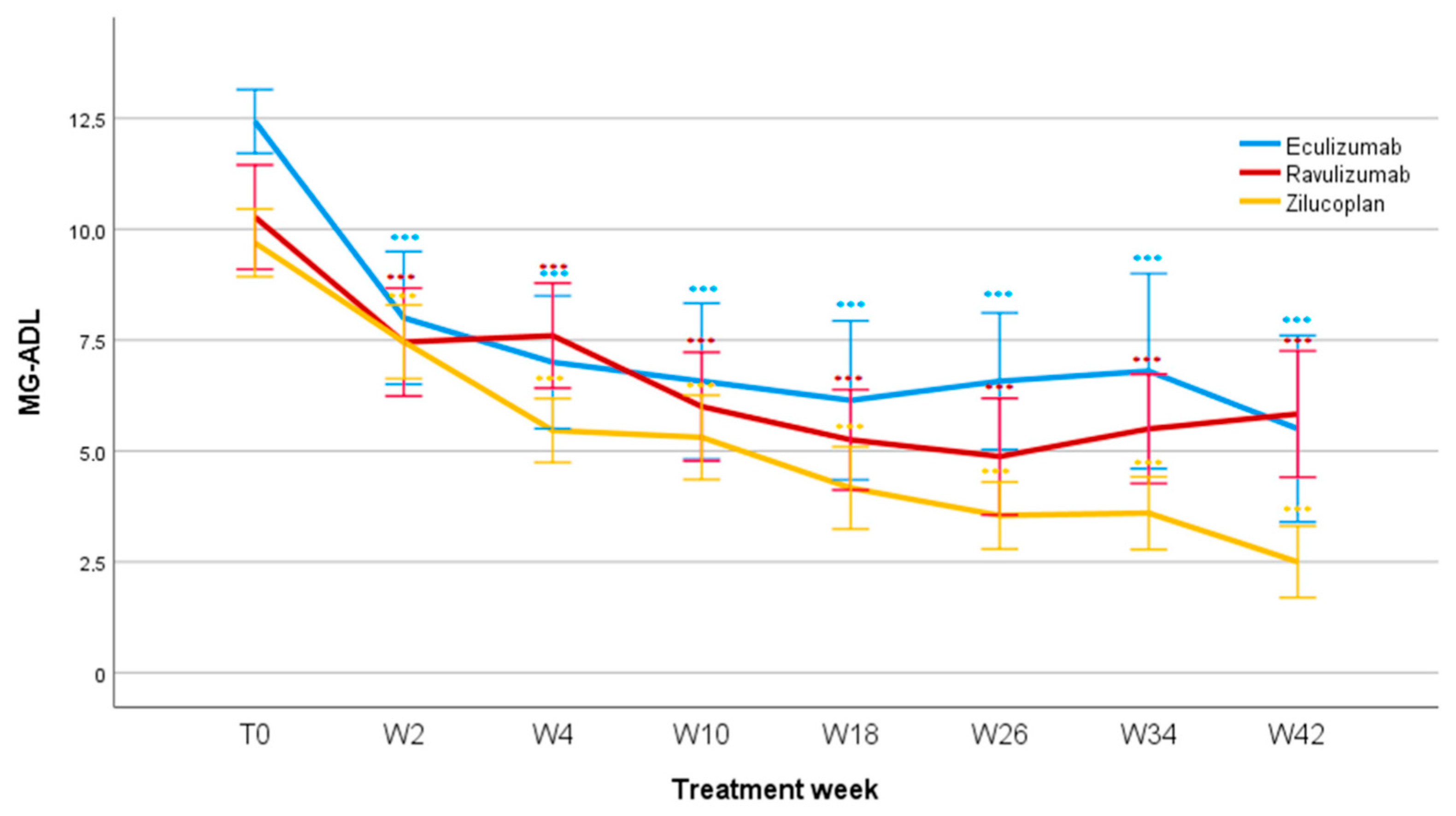
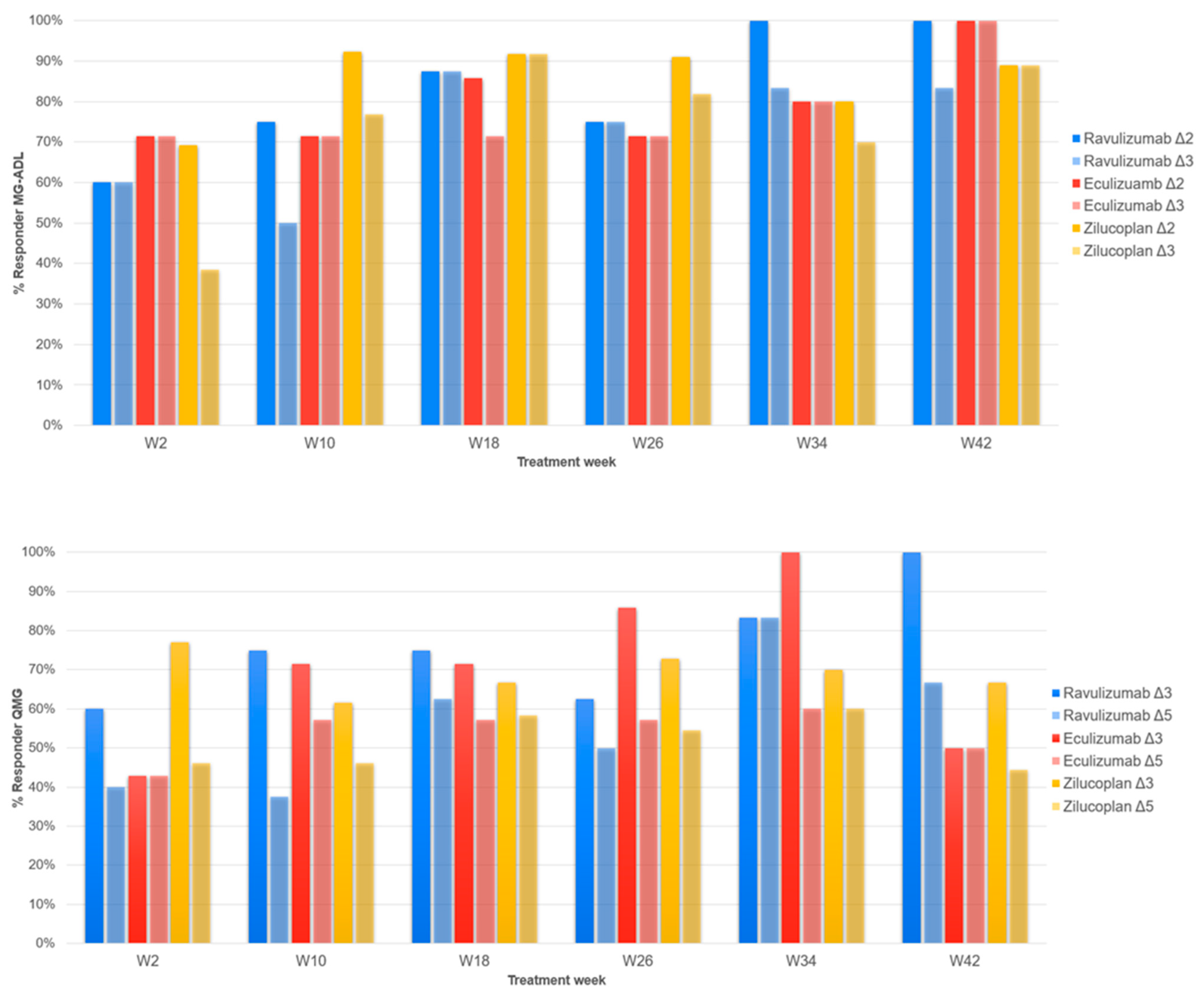
3.2. MG-ADL
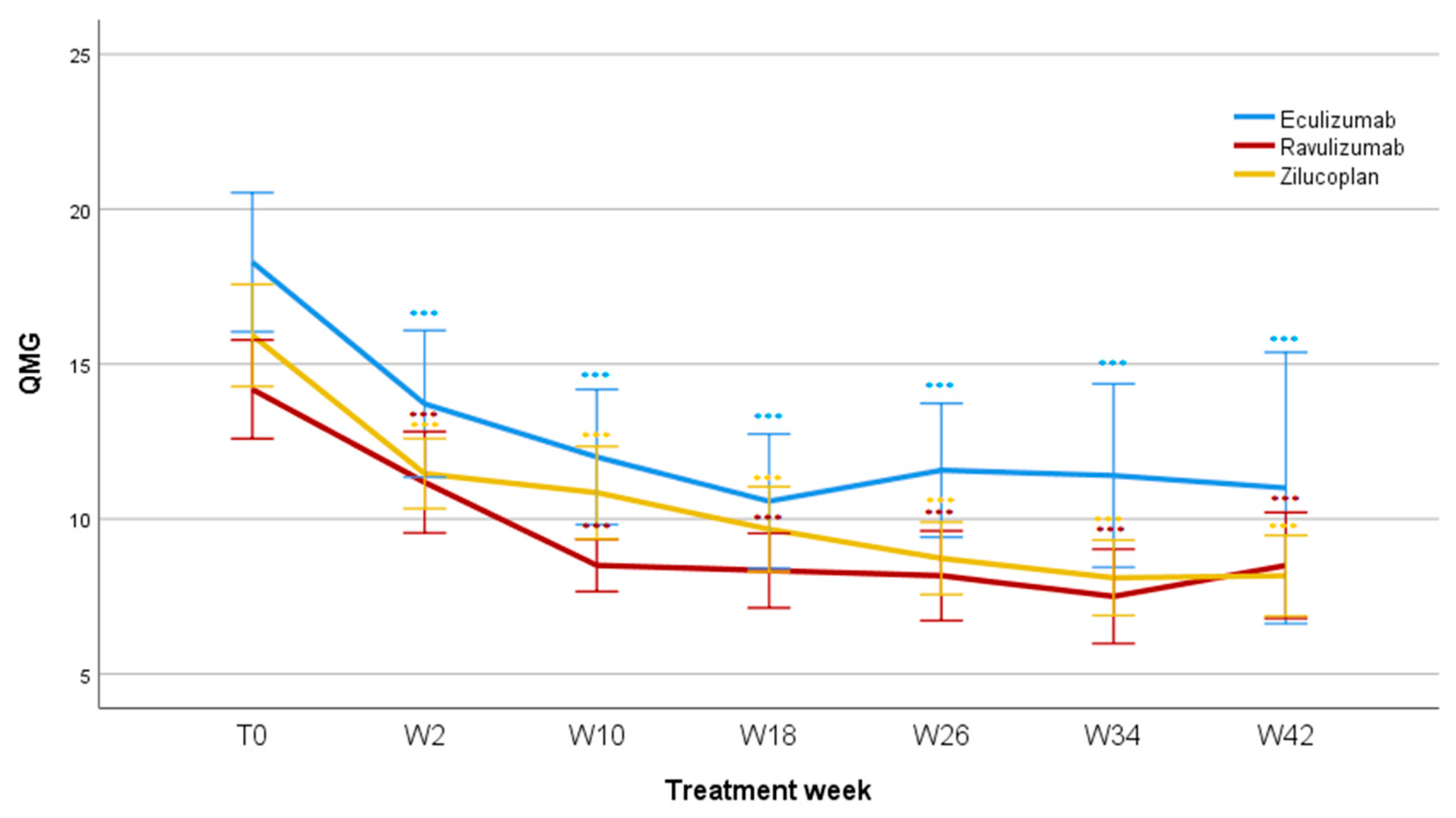
3.3. QMG
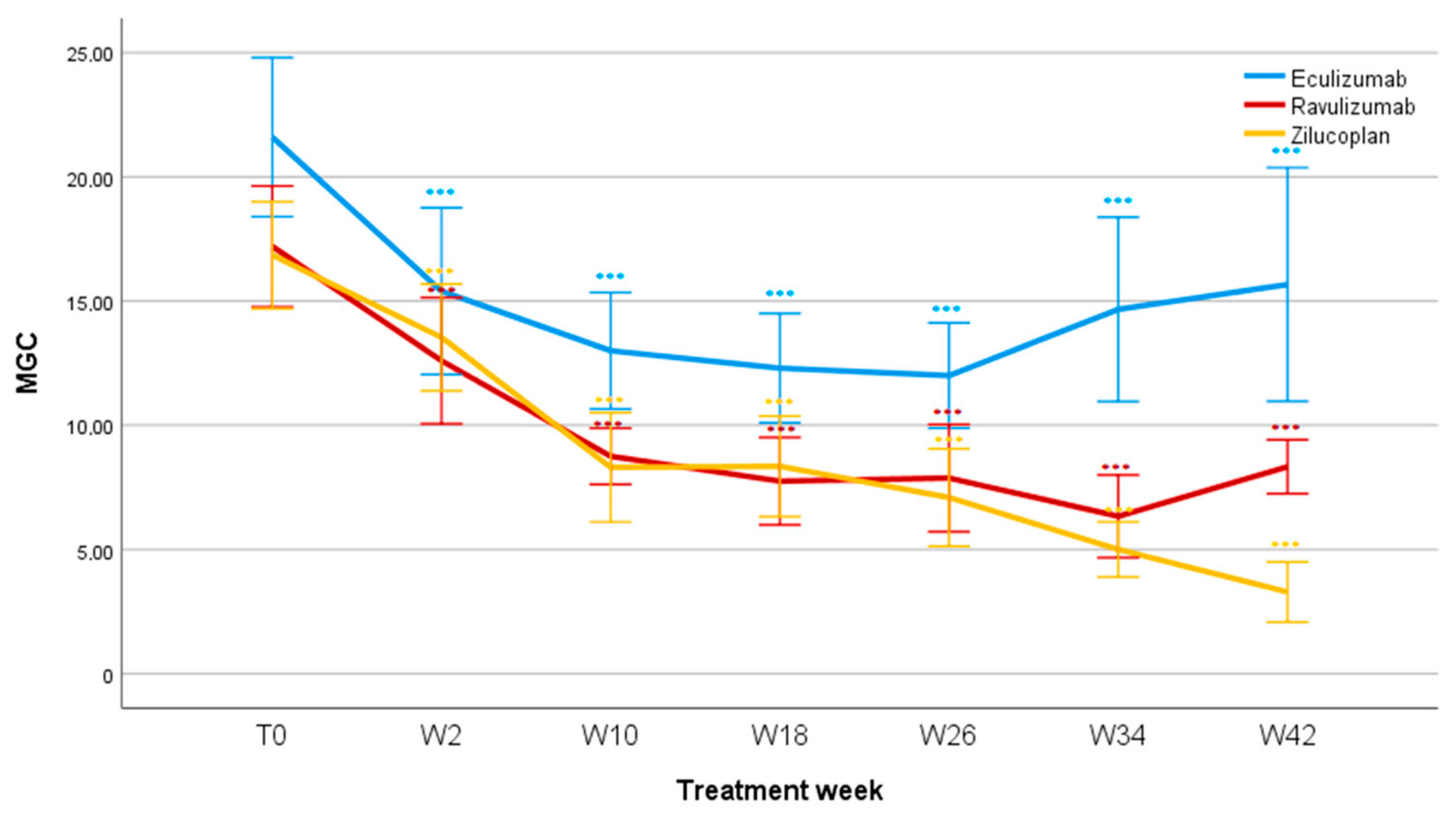
3.4. MGC
3.5. Minimal Symptom Expression
- MSE in Eculizumab: W2: 0; W18: 1 (14%); W26: 1 (14%); W34: 1 (14%); W42: 1 (14%).
- MSE in Ravulizumab: W2: 1 (9%); W18: 1 (9%); W26: 3 (27%); W34: 2 (18%); W42: 1 (9%).
- MSE in Zilucoplan: W2: 0; W18: 2 (15%); W26: 2 (15%); W34: 2 (15%); W42: 4 (31%).
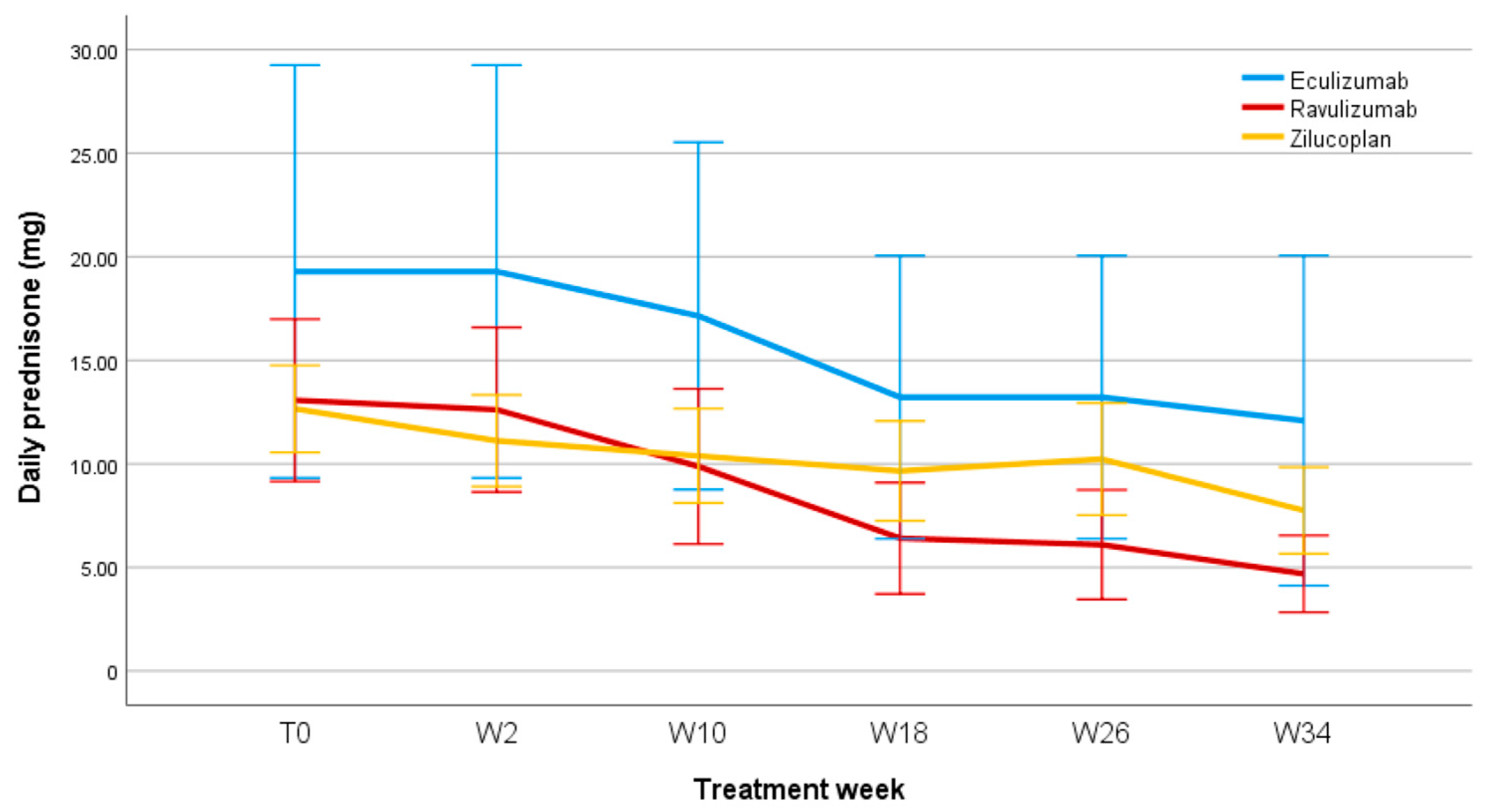
3.6. Steroid-Sparing Effect
3.7. Thymectomy
3.8. Safety and Adverse Events
4. Discussion
4.1. Comparison with Previous Clinical Trial
4.2. Steroid-Sparing Effect
4.3. Safety and Tolerability
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Primers 2019, 5, 30. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Verschuuren, J.J. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015, 14, 1023–1036. [Google Scholar] [CrossRef]
- Punga, A.R.; Maddison, P.; Heckmann, J.M.; Guptill, J.T.; Evoli, A. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022, 21, 176–188. [Google Scholar] [CrossRef]
- Nel, M.; Heckmann, J.M. Epidemiology and genetics of myasthenia gravis. In Current Clinical Neurology; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Pham, M.C.; Masi, G.; Patzina, R.; Obaid, A.H.; Oxendine, S.R.; Oh, S.; Payne, A.S.; Nowak, R.J.; O’Connor, K.C. Individual myasthenia gravis autoantibody clones can efficiently mediate multiple mechanisms of pathology. Acta Neuropathol. 2023, 146, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Role of complement, anti-complement therapeutics, and other targeted immunotherapies in myasthenia gravis. Expert Rev. Clin. Immunol. 2022, 18, 691–701. [Google Scholar] [CrossRef]
- Howard, J.F.; Utsugisawa, K.; Benatar, M.; Murai, H.; Barohn, R.J.; Illa, I.; Jacob, S.; Vissing, J.; Burns, T.M.; Kissel, J.T.; et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017, 16, 976–986. [Google Scholar] [CrossRef]
- Meisel, A.; Annane, D.; Vu, T.; Mantegazza, R.; Katsuno, M.; Aguzzi, R.; Frick, G.; Gault, L.; Howard, J.F.; the CHAMPION MG Study Group. Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: Results from the phase 3 CHAMPION MG open-label extension. J. Neurol. 2023, 270, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.F.; Bresch, S.; Genge, A.; Hewamadduma, C.; Hinton, J.; Hussain, Y.; Juntas-Morales, R.; Kaminski, H.J.; Maniaol, A.; Mantegazza, R.; et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023, 22, 395–406. [Google Scholar] [CrossRef]
- Wolfe, G.I.; Herbelin, L.; Nations, S.P.; Foster, B.; Bryan, W.W.; Barohn, R.J. Myasthenia gravis activities of daily living profile. Neurology 1999, 52, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Barohn, R.J.; McIntire, D.; Herbelin, L.; Wolfe, G.I.; Nations, S.; Bryan, W.W. Reliability testing of the quantitative myasthenia gravis score. Ann. N. Y. Acad. Sci. 1998, 841, 769–772. [Google Scholar] [CrossRef]
- Burns, T.M.; Conaway, M.R.; Cutter, G.R.; Sanders, D.B. Construction of an efficient evaluative instrument for myasthenia gravis: The MG composite. Muscle Nerve 2008, 38, 1553–1562. [Google Scholar] [CrossRef]
- Di Stefano, V.; Iacono, S.; Militello, M.; Leone, O.; Rispoli, M.G.; Ferri, L.; Ajdinaj, P.; Lanza, P.; Lupica, A.; Crescimanno, G.; et al. Comorbidity in myasthenia gravis: Multicentric, hospital-based, and controlled study of 178 Italian patients. Neurol. Sci. 2024, 45, 3481–3494. [Google Scholar] [CrossRef]
- Katzberg, H.D.; Barnett, C.; Merkies, I.S.J.; Bril, V. Minimal clinically important difference in myasthenia gravis: Outcomes from a randomized trial. Muscle Nerve 2014, 49, 661–665. [Google Scholar] [CrossRef]
- Saccà, F.; Pane, C.; Espinosa, P.E.; Sormani, M.P.; Signori, A. Efficacy of innovative therapies in myasthenia gravis: A systematic review, meta-analysis and network meta-analysis. Eur. J. Neurol. 2023, 30, 3854–3867. [Google Scholar] [CrossRef]
- Howard, J.F.; Nowak, R.J.; Wolfe, G.I.; Freimer, M.L.; Vu, T.H.; Hinton, J.L.; Benatar, M.; Duda, P.W.; MacDougall, J.E.; Farzaneh-Far, R.; et al. Clinical Effects of the Self-administered Subcutaneous Complement Inhibitor Zilucoplan in Patients with Moderate to Severe Generalized Myasthenia Gravis: Results of a Phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial. JAMA Neurol. 2020, 77, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Mantegazza, R.; Annane, D.; Katsuno, M.; Meisel, A.; Nicolle, M.W.; Bril, V.; Aguzzi, R.; Frick, G.; Howard, J.F., Jr.; et al. Long-Term Efficacy and Safety of Ravulizumab in Adults With Anti-Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis: Final Results From the Phase 3 CHAMPION MG Open-Label Extension. Eur. J. Neurol. 2025, 32, e70158. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, D.; Erra, C.; Tuccillo, F.; De Martino, B.M.; Fasolino, A.; Habetswallner, F. Eculizumab in refractory myasthenia gravis: A real-world single-center experience. Neurol. Sci. 2025, 46, 951–959. [Google Scholar] [CrossRef]
- Rossini, E.; Di Stefano, V.; Iorio, R.; Habetswallner, F.; Maestri, M.; Vinciguerra, C.; Pennisi, E.M.; Di Martino, G.; Rini, N.; Falso, S.; et al. Ravulizumab for generalized Myasthenia Gravis: A multicenter real-life experience. J. Neurol. 2025, 272, 1–13. [Google Scholar] [CrossRef]
- Pane, C.; Di Stefano, V.; Cuomo, N.; Sarnataro, A.; Vinciguerra, C.; Bevilacqua, L.; Brighina, F.; Rini, N.; Puorro, G.; Marsili, A.; et al. A real-life experience with eculizumab and efgartigimod in generalized myasthenia gravis patients. J. Neurol. 2024, 271, 6209–6219. [Google Scholar] [CrossRef]
- Siddiqi, Z.A.; Nowak, R.J.; Mozaffar, T.; O’Brien, F.; Yountz, M.; Patti, F.; the REGAIN Study Group. Eculizumab in refractory generalized myasthenia gravis previously treated with rituximab: Subgroup analysis of REGAIN and its extension study. Muscle Nerve 2021, 64, 662–669. [Google Scholar] [CrossRef]
- Vinciguerra, C.; Bevilacqua, L.; Toriello, A.; Iovino, A.; Piscosquito, G.; Calicchio, G.; Barone, P. Starting eculizumab as rescue therapy in refractory myasthenic crisis. Neurol. Sci. 2023, 44, 3707–3709. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.F.; Bresch, S.; Farmakidis, C.; Freimer, M.; Genge, A.; Hewamadduma, C.; Hinton, J.; Hussain, Y.; Juntas-Morales, R.; Kaminski, H.J.; et al. Long-term safety and efficacy of zilucoplan in patients with generalized myasthenia gravis: Interim analysis of the RAISE-XT open-label extension study. Herapeutic Adv. Neurol. Disord. 2024, 17, 17562864241243186. [Google Scholar] [CrossRef] [PubMed]
| Eculizumab | Ravulizumab | Zilucoplan | Total | |
|---|---|---|---|---|
| Patients (n, %) | 7 (23%) | 11 (35%) | 13 (42%) | 31 (100%) |
| Female (n, %) | 5 (71%) | 5 (45%) | 11 (85%) | 21 (68%) |
| Male (n, %) | 2 (29%) | 6 (55%) | 2 (15%) | 10 (32%) |
| Age (years, mean) | 66.3 | 59.8 | 54.4 | 59 |
| Age at Diagnosis (years, mean) | 63.9 | 52.4 | 44.9 | 51.8 |
| Disease Duration (years, mean) | 2.4 | 7.4 | 9.46 | 7.2 |
| EOMG (n, %) | 1 (14%) | 4 (36%) | 8 (62%) | 13 (42%) |
| LOMG (n, %) | 6 (86%) | 7 (64%) | 5 (38%) | 18 (58%) |
| Thymectomy (n, %) | 2 (29%) | 4 (36%) | 6 (46%) | 12 (39%) |
| Thymoma (n, %) | 2 (29%) | 3 (27%) | 2 (15%) | 7 (23%) |
| Other Pathologies (n, %) | 6 (86%) | 7 (64%) | 13 (100%) | 26 (84%) |
| IVIG/PLEX Before T0 (n, %) | 7 (100%) | 10 (91%) | 11 (85%) | 28 (90%) |
| Myasthenic Crisis Before T0 (n, %) | 2 (29%) | 4 (36%) | 2 (15%) | 8 (26%) |
| Pyridostigmine (n, %) | 3 (42.8%) | 9 (81.8%) | 13 (100%) | 25 (81%) |
| Steroid-Dose (MG/DAY) | 27 | 13.06 | 12.65 | 17.57 |
| NSIST (n, %) | 4 (57%) | 7 (64%) | 7 (54%) | 18 (58%) |
| AZA (n, %) | 3 (42.8%) | 5 (45.4%) | 5 (38.5%) | 13 (42%) |
| AZA-Dose (mg/day, mean) | 116,66 | 105 | 110 | |
| Other NSIST (n, %) | 0 (0%) | 2 (18.2%) | 2 (15.4%) | 4 (13%) |
| Antibodies | ||||
| Seropositive (n, %) | 7 (100%) | 11 (100%) | 13 (100%) | 31 (100%) |
| AChR (n, %) | 7 (100%) | 11 (100%) | 13 (100%) | 31 (100%) |
| MuSK (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| MGFA Class | ||||
| I (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IIA (n, %) | 0 (0%) | 0 (0%) | 2 (15%) | 2 (6%) |
| IIB (n, %) | 0 (0%) | 3 (27%) | 5 (38%) | 6 (19%) |
| IIIA (n, %) | 2 (29%) | 1 (9%) | 2 (15%) | 5 (16%) |
| IIIB (n, %) | 4 (57%) | 5 (45%) | 4 (31%) | 13 (42%) |
| IVA (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IVB (n, %) | 1 (14%) | 1 (9%) | 0 (0%) | 2 (6%) |
| V (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| QMG | 18.28 | 14.2 | 15.92 | 16.13 |
| MG-ADL | 12.42 | 10.4 | 9.69 | 10.84 |
| MGC | 21.6 | 17.2 | 16.8 | 18.53 |
| Eculizumab | Ravulizumab | Zilucoplan | |
|---|---|---|---|
| Adverse Events | 3 (43%) | 5 (45%) | 8 (62%) |
| Nausea | 0 (0%) | 3 (27%) | 2 (15%) |
| Fever | 0 (0%) | 2 (18%) | 0 (0%) |
| Dizziness | 0 (0%) | 1 (9%) | 0 (0%) |
| General Malaise | 0 (0%) | 1 (9%) | 0 (0%) |
| Upper Resp Tract Infection | 0 (0%) | 1 (9%) | 0 (0%) |
| Pneumonia | 1 (14%) | 1 (9%) | 0 (0%) |
| Bronchitis | 0 (0%) | 1 (9%) | 0 (0%) |
| Headache | 1 (14%) | 0 (0%) | 4 (31%) |
| Diarrhea | 1 (14%) | 0 (0%) | 0 (0%) |
| Skin Rash | 0 (0%) | 1 (9%) | 0 (0%) |
| Infusion Site Reaction | 0 (0%) | 0 (0%) | 8 (62%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Martino, G.; Rini, N.; Bonaventura, A.; Trovato, M.; Maccora, S.; Lima, S.M.; La Seta, C.; Brighina, F.; Di Stefano, V. Complement Inhibitors in Generalized Myasthenia Gravis: Comparison of Administration Schedules, Efficacy, and Safety. J. Clin. Med. 2025, 14, 8205. https://doi.org/10.3390/jcm14228205
Di Martino G, Rini N, Bonaventura A, Trovato M, Maccora S, Lima SM, La Seta C, Brighina F, Di Stefano V. Complement Inhibitors in Generalized Myasthenia Gravis: Comparison of Administration Schedules, Efficacy, and Safety. Journal of Clinical Medicine. 2025; 14(22):8205. https://doi.org/10.3390/jcm14228205
Chicago/Turabian StyleDi Martino, Giuseppe, Nicasio Rini, Alessia Bonaventura, Mauro Trovato, Simona Maccora, Salvatore Maria Lima, Concetta La Seta, Filippo Brighina, and Vincenzo Di Stefano. 2025. "Complement Inhibitors in Generalized Myasthenia Gravis: Comparison of Administration Schedules, Efficacy, and Safety" Journal of Clinical Medicine 14, no. 22: 8205. https://doi.org/10.3390/jcm14228205
APA StyleDi Martino, G., Rini, N., Bonaventura, A., Trovato, M., Maccora, S., Lima, S. M., La Seta, C., Brighina, F., & Di Stefano, V. (2025). Complement Inhibitors in Generalized Myasthenia Gravis: Comparison of Administration Schedules, Efficacy, and Safety. Journal of Clinical Medicine, 14(22), 8205. https://doi.org/10.3390/jcm14228205








