Dynamic Kinematic Assessment with 3D Motion Analysis After Arthroscopic Bankart Repair: A Mid- to Long-Term Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Prospective Cohort
2.3. Long-Term Cohort
2.4. Control Group
2.5. Motion Analysis Protocol
2.6. Measurement Protocol
2.6.1. Calibration and Baseline
2.6.2. Maximum Range of Motion (ROM)
2.6.3. Activities of Daily Living (ADLs)
- Apron tying (apron)—reaching posteriorly with the hand to place the palm centrally on the back.
- Neck grip (neck)—reaching to the nape of the neck at the hairline.
- Opposite axilla wash (wash)—simulating washing the contralateral axilla with a cloth.
- Book reaching (book)—reaching at eye level to grasp a book on an adjustable shelf.
2.6.4. Activity-Related Range of Motion (AROM)
2.6.5. Statistical Analysis
3. Results
3.1. Short-Term Postoperative Outcomes
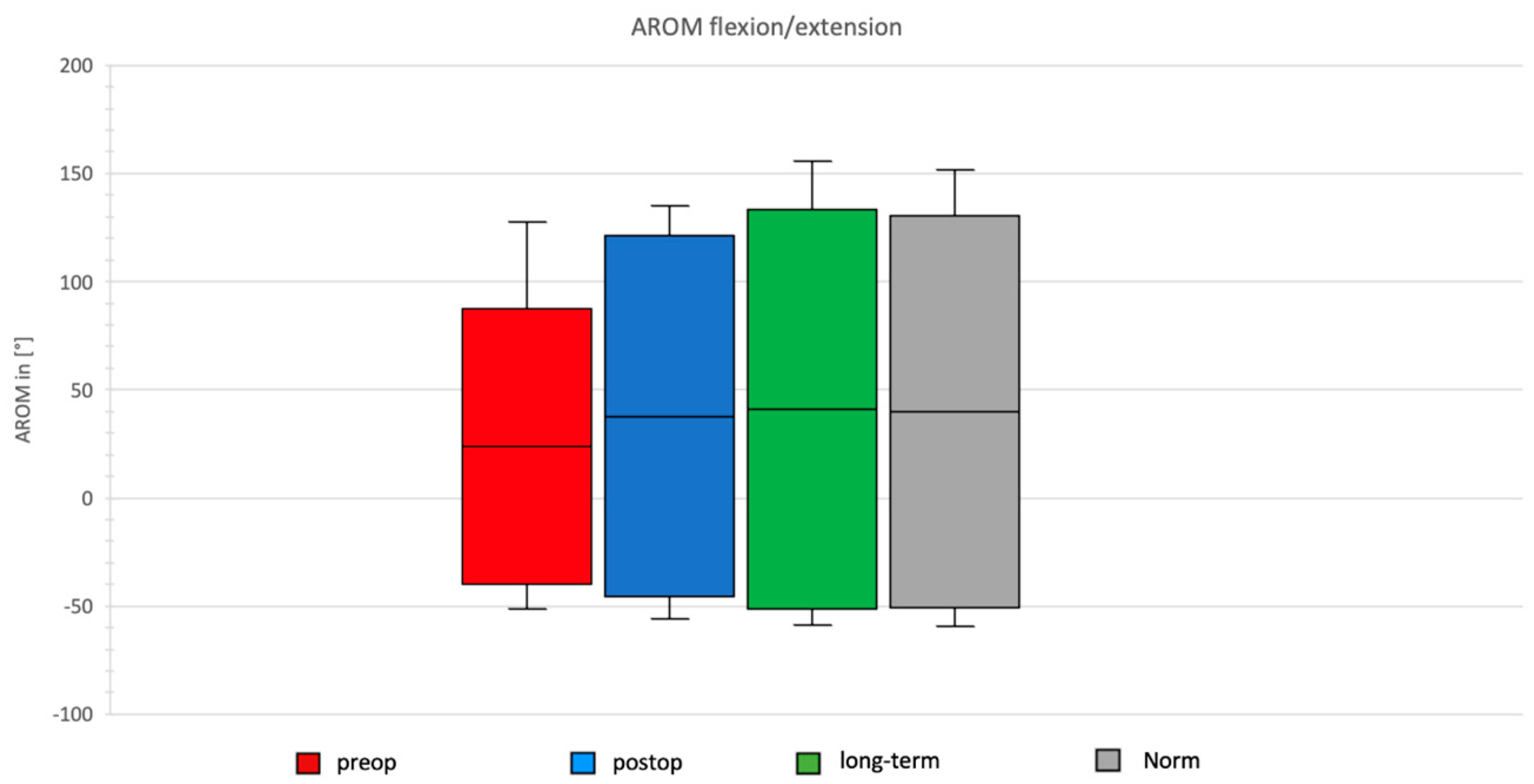
- Forward flexion increased by 58° (from 90° to 148°).
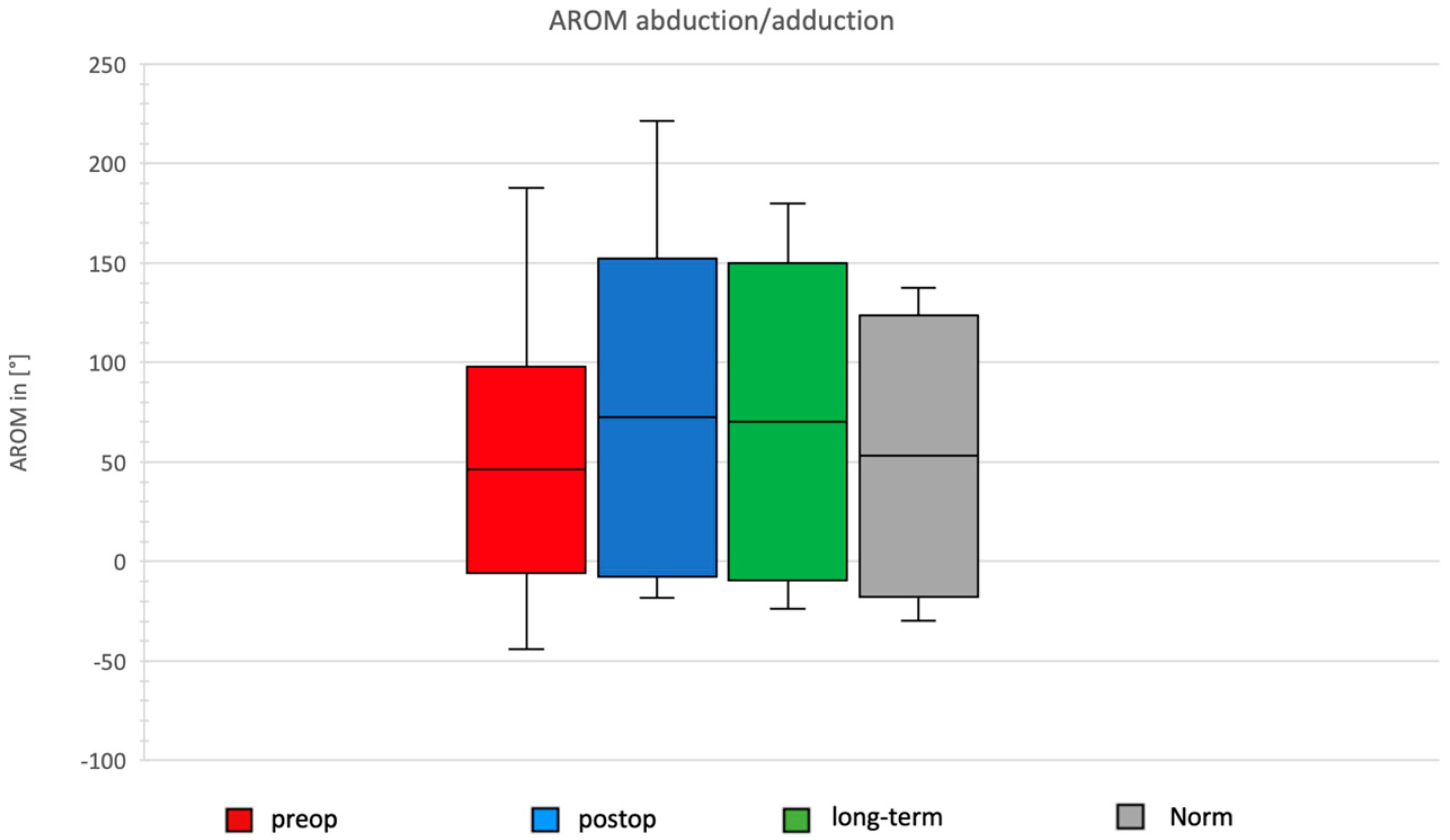
- Abduction improved by 70° (from 76° to 146°).
- Other movements (extension, external and internal rotation, elbow motion, and pronation/supination) showed non-significant trends toward improvement.
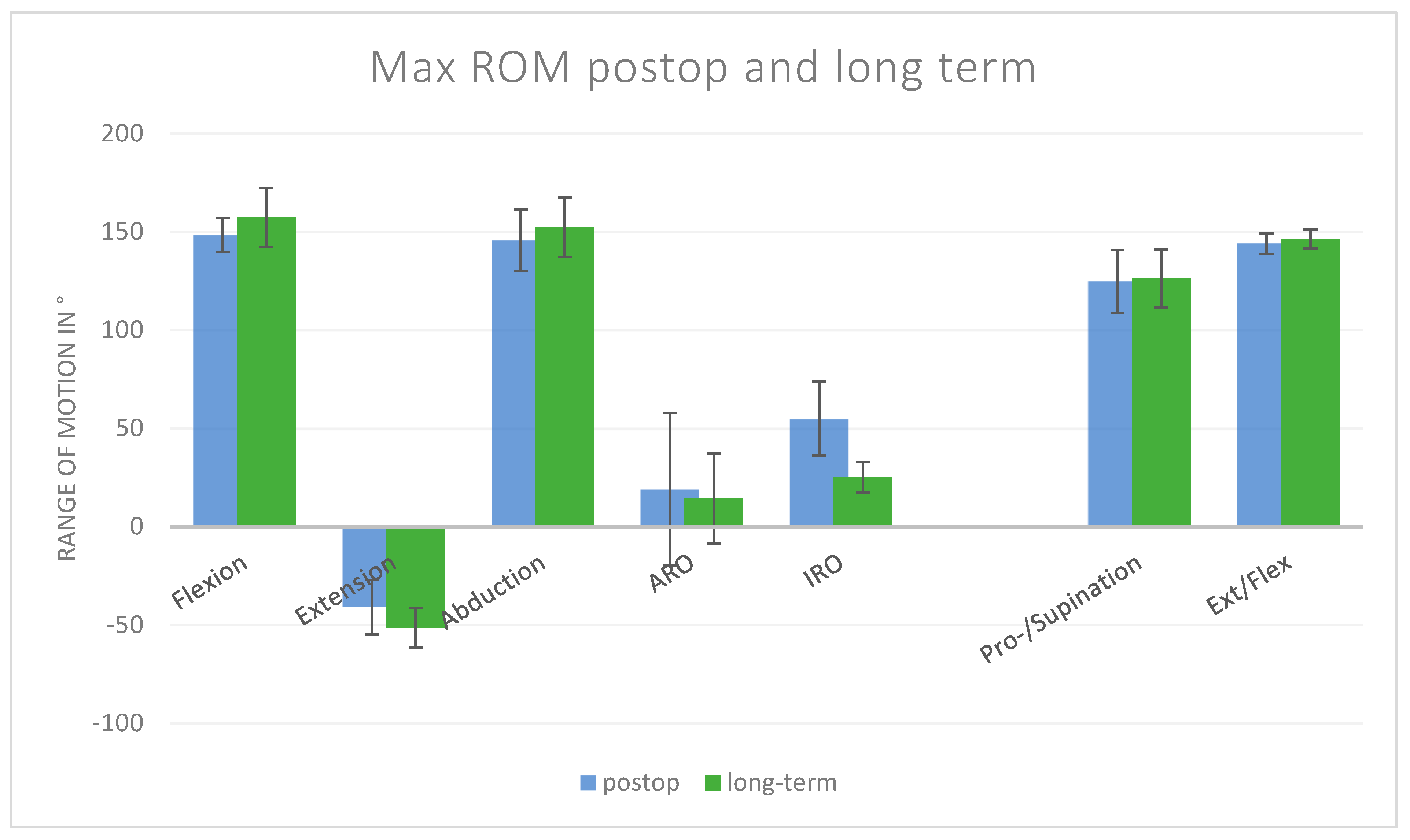
3.2. Long-Term Outcomes
- Forward flexion improved by an additional 9° compared to postoperative values (148° vs. 157°).
- Abduction increased by 7° (146° vs. 152°).
- Extension tended to increase by 10° (non-significant).
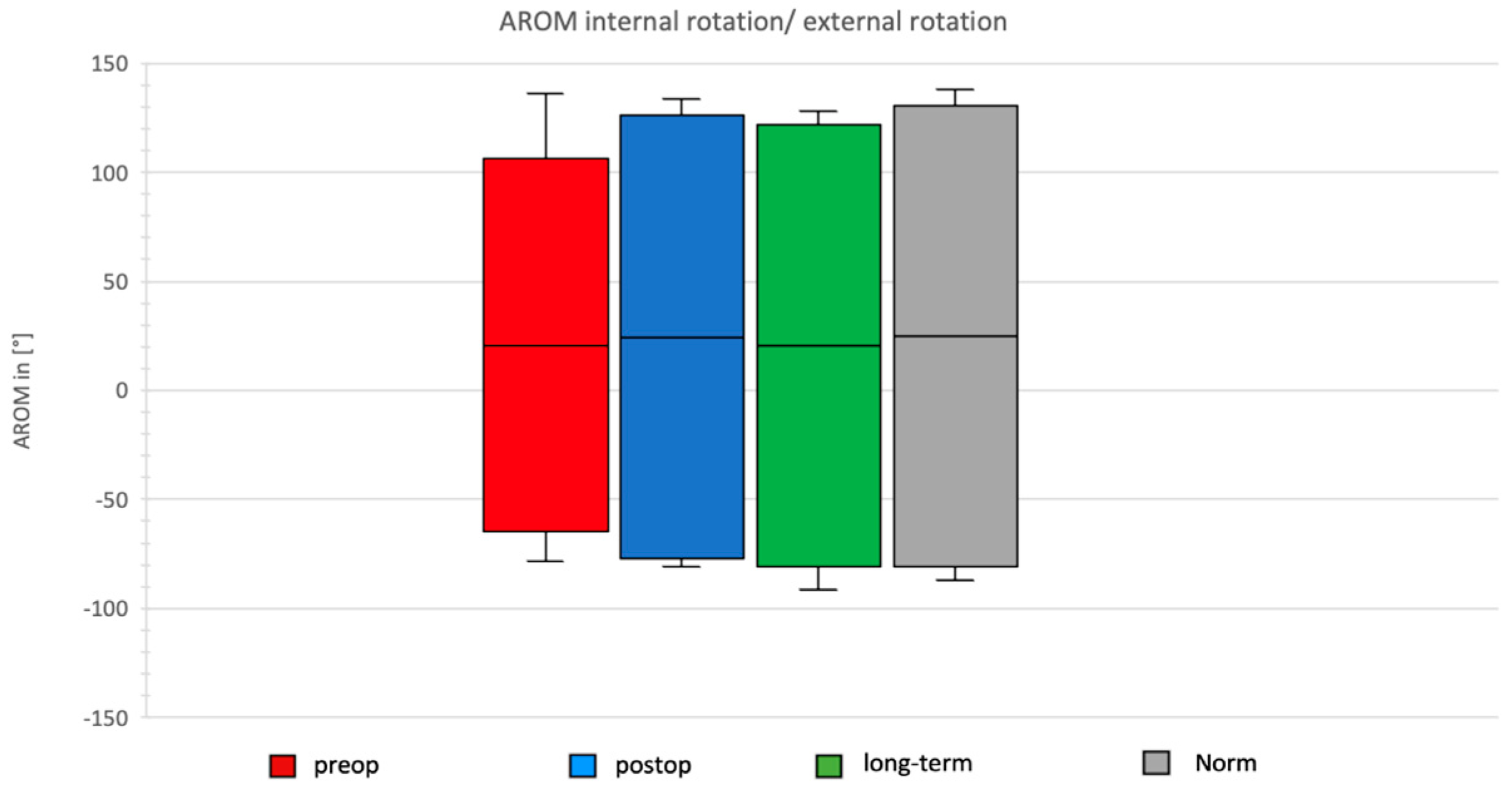
- External rotation decreased by 5° (19° vs. 14°).
- Internal rotation decreased by 30° (55° vs. 25°; p < 0.05).
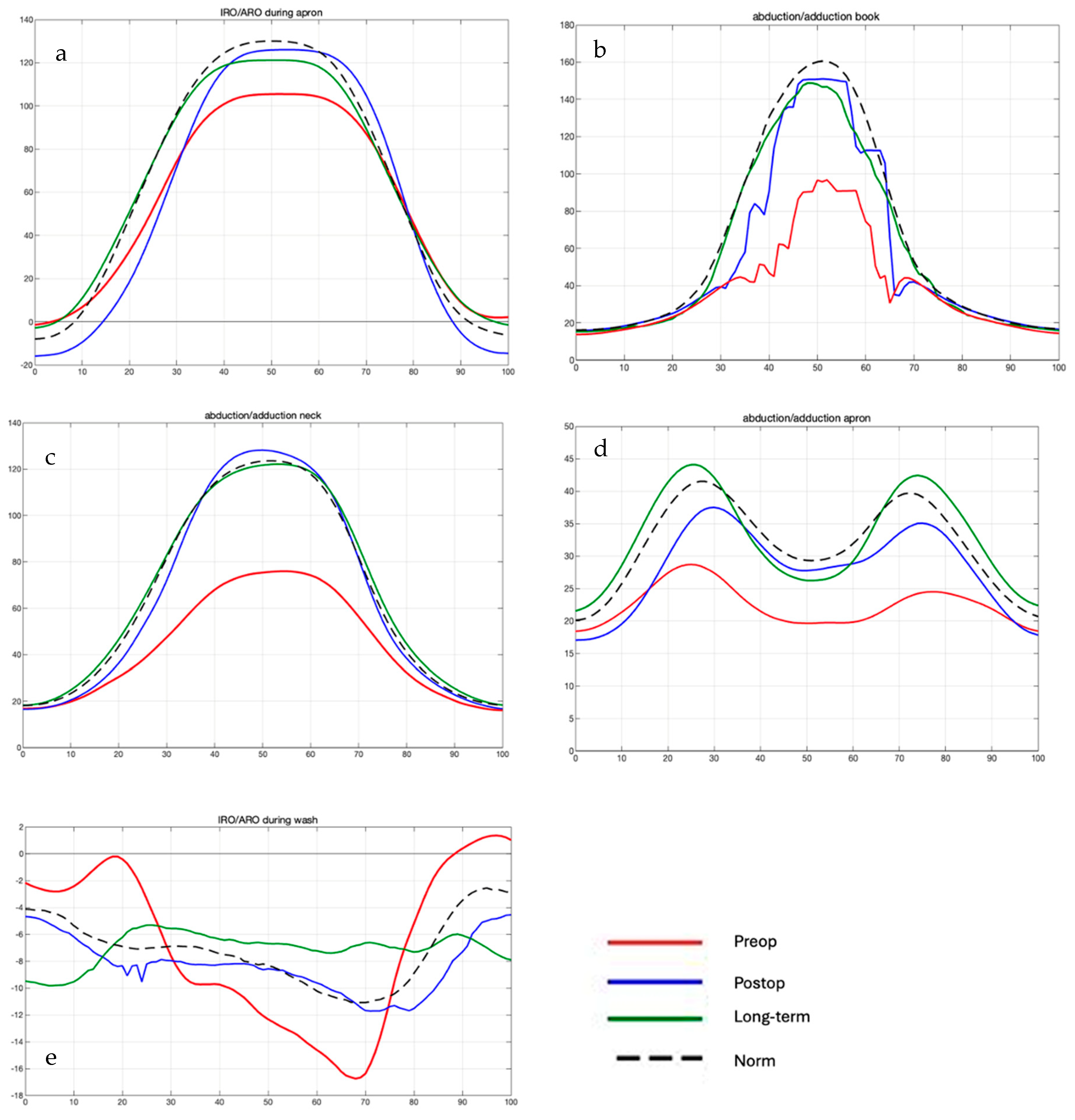
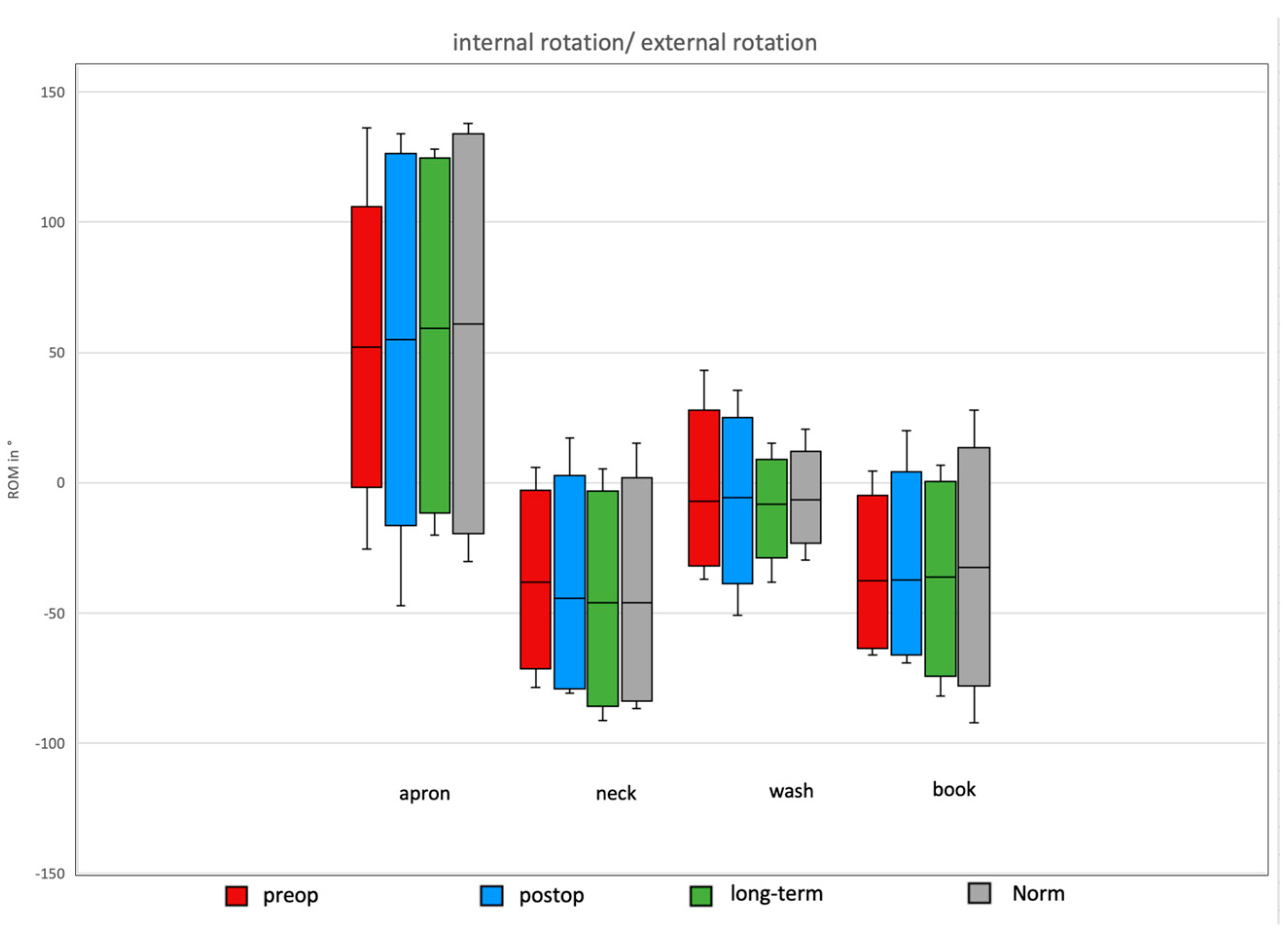
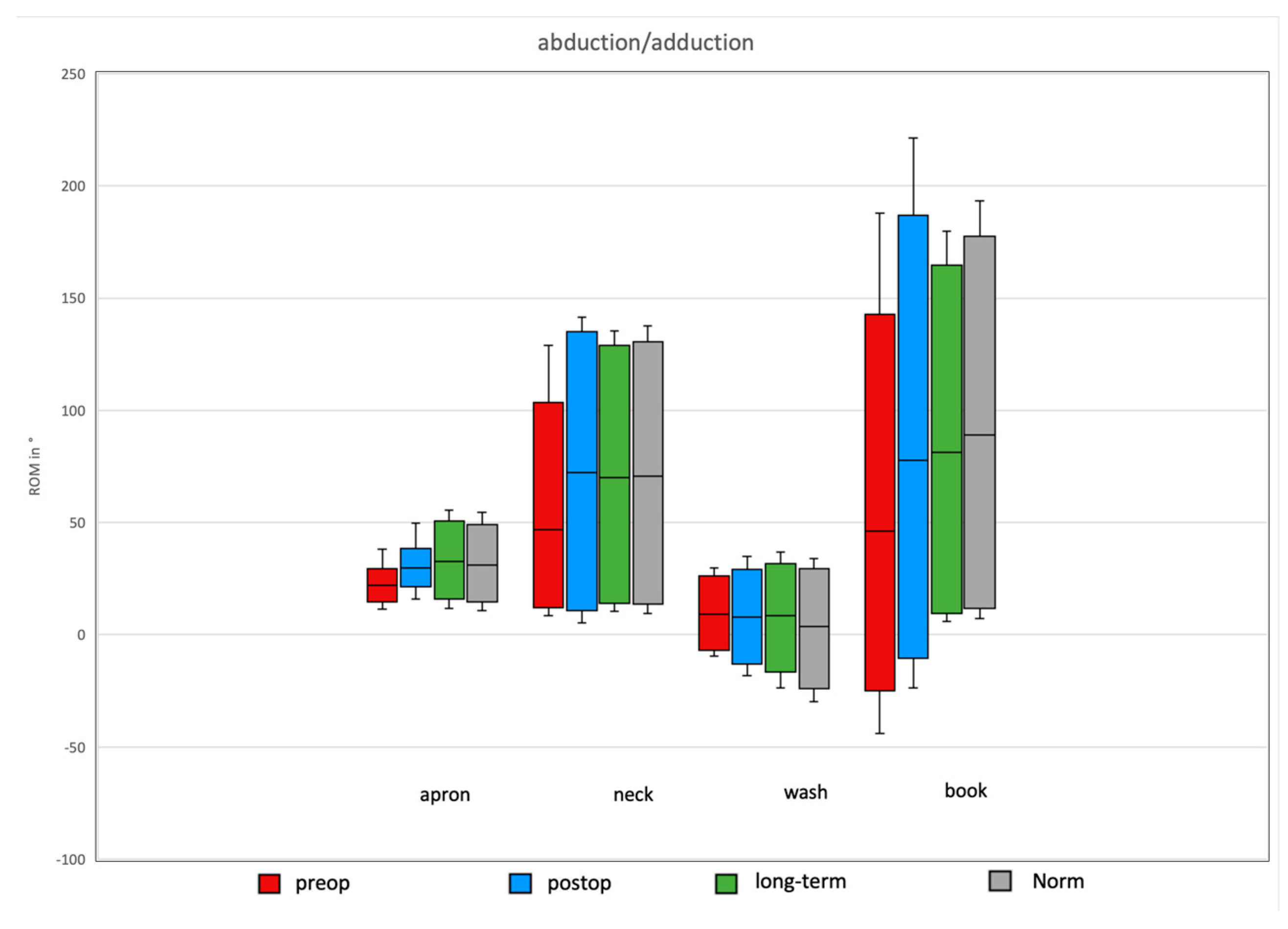
Comparison of Preoperative, Postoperative, and Long-Term Individual Ranges of Motion in Daily Activities After Bankart Repair to Normative Values
3.3. Daily Activity AROM in Ante-/Retroversion of the Shoulder After Bankart Repair
3.4. Daily Activity AROM in Abduction/Adduction of the Shoulder
3.5. Daily Activity AROM in External/Internal Rotation of the Shoulder
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muir, S.W.; Corea, C.L.; Beaupre, L. Evaluating change in clinical status: Reliability and measures of agreement for the assessment of glenohumeral range of motion. N. Am. J. Sports Phys. Ther. 2010, 5, 98–110. [Google Scholar] [PubMed]
- Shin, S.H.; Du, H.R.; Lee, O.-S.; Oh, J.H.; Kim, S.H. Within-day reliability of shoulder range of motion measurement with a smartphone. Man. Ther. 2012, 17, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.D.; MacDermid, J.C.; Chinchalkar, S.; Stevens, R.S.; King, G.J. Reliability of range-of-motion measurement in the elbow and forearm. J. Shoulder Elb. Surg. 1998, 7, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, M.J.; McHugh, M.P.; Johnson, C.P.; Tyler, T.F. Reliability of shoulder range of motion comparing a goniometer to a digital level. Physiother. Theory Pract. 2010, 26, 327–333. [Google Scholar] [CrossRef]
- Urban, V.; Kalberer, F.; Roos, M.; Dumont, C.E. Reliability of active range-of-motion measurement of the rotation in the forearm: Comparison of three measurement devices. Z Orthop. Ihre Grenzgeb. 2002, 140, 72–76. [Google Scholar] [CrossRef]
- Goodwin, J.; Clark, C.; Deakes, J.; Burdon, D.; Lawrence, C. Clinical methods of goniometry: A comparative study. Disabil. Rehabil. 1992, 14, 10–15. [Google Scholar] [CrossRef]
- Kolber, M.J.; Saltzman, S.B.; Beekhuizen, K.S.; Cheng, M.S. Reliability and minimal detectable change of inclinometric shoulder mobility measurements. Physiother. Theory Pract. 2009, 25, 572–581. [Google Scholar] [CrossRef]
- Kolber, M.J.; Vega, F.; Widmayer, K.; Cheng, M.S. The reliability and minimal detectable change of shoulder mobility measurements using a digital inclinometer. Physiother. Theory Pract. 2011, 27, 176–184. [Google Scholar] [CrossRef]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Simon, J.; Doederlein, L.; McIntosh, A.S.; Metaxiotis, D.; Bock, H.G.; Wolf, S.I. The Heidelberg foot measurement method: Development, description and assessment. Gait Posture 2006, 23, 411–424. [Google Scholar] [CrossRef]
- Rau, G.; Disselhorst-Klug, C.; Schmidt, R. Movement biomechanics goes upwards: From the leg to the arm. J. Biomech. 2000, 33, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Raiss, P. Validierung eines neuen markerbasierten biomechanischen Modells in der 3-dimensionalen Bewegungsanalyse der oberen Extremität und Ermittlung des Bewegungsausmaßes bei Alltagsbewegungen. Z. Für Orthopädie Unfallchirurgie 2007, 125–127. [Google Scholar]
- Rab, G.; Petuskey, K.; Bagley, A. A method for determination of upper extremity kinematics. Gait Posture 2002, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Disselhorst-Klug, C.; Silny, J.; Rau, G. A marker-based measurement procedure for unconstrained wrist and elbow motions. J. Biomech. 1999, 32, 615–621. [Google Scholar] [CrossRef]
- Cappozzo, A.; Della Croce, U.; Leardini, A.; Chiari, L. Human movement analysis using stereophotogrammetry. Part 1: Theoretical background. Gait Posture 2005, 21, 186–196. [Google Scholar] [CrossRef]
- Hamming, D.; Braman, J.P.; Phadke, V.; LaPrade, R.F.; Ludewig, P.M. The accuracy of measuring glenohumeral motion with a surface humeral cuff. J. Biomech. 2012, 45, 1161–1168. [Google Scholar] [CrossRef]
- Rettig, O.; Fradet, L.; Kasten, P.; Raiss, P.; Wolf, S.I. A new kinematic model of the upper extremity based on functional joint parameter determination for shoulder and elbow. Gait Posture 2009, 30, 469–476. [Google Scholar] [CrossRef]
- Kasten, P.; Maier, M.; Wendy, P.; Rettig, O.; Raiss, P.; Wolf, S.; Loew, M. Can shoulder arthroplasty restore the range of motion in activities of daily living? A prospective 3D video motion analysis study. J. Shoulder Elb. Surg. 2010, 19 (Suppl. 2), 59–65. [Google Scholar] [CrossRef]
- Maier, M.W.; Kasten, P.; Niklasch, M.; Dreher, T.; Zeifang, F.; Rettig, O.; Wolf, S.I. 3D motion capture using the HUX model for monitoring functional changes with arthroplasty in patients with degenerative osteoarthritis. Gait Posture 2014, 39, 7–11. [Google Scholar] [CrossRef]
- Miltner, O.; Williams, S.; Schmidt, R.; Siebert, C.H.; Rau, G.; Zilkens, K.W.; Disselhorst-Klug, C. Arm motion analysis: A new method and its clinical application. Z Orthop. Ihre Grenzgeb. 2003, 141, 171–176. [Google Scholar] [CrossRef]
- Omoumi, P.; Teixeira, P.; Lecouvet, F.; Chung, C.B. Glenohumeral joint instability. J. Magn. Reson. Imaging 2011, 33, 2–16. [Google Scholar] [CrossRef]
- Bigliani, L.U.; Pollock, R.G.; Soslowsky, L.J.; Flatow, E.L.; Pawluk, R.J.; Mow, V.C. Tensile properties of the inferior glenohumeral ligament. J. Orthop. Res. 1992, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, F.; Daum, P.; Bushe, C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability. Z. Orthop. Ihre Grenzgeb. 1994, 132, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Flatow, E.L.; Warner, J.I. Instability of the shoulder: Complex problems and failed repairs: Part, I. Relevant biomechanics, multidirectional instability, and severe glenoid loss. Instr. Course Lect. 1998, 47, 97–112. [Google Scholar] [PubMed]
- Leroux, T.; Wasserstein, D.; Veillette, C.; Khoshbin, A.; Henry, P.; Chahal, J.; Austin, P.; Mahomed, N.; Ogilvie-Harris, D. Epidemiology of primary anterior shoulder dislocation requiring closed reduction in Ontario, Canada. Am. J. Sports Med. 2014, 42, 442–450. [Google Scholar] [CrossRef]
- Hovelius, L. The natural history of primary anterior dislocation of the shoulder in the young. J. Orthop. Sci. 1999, 4, 307–317. [Google Scholar] [CrossRef]
- Hurley, E.T.; Manjunath, A.K.; Bloom, D.A.; Pauzenberger, L.; Mullett, H.; Alaia, M.J.; Strauss, E.J. Arthroscopic Bankart Repair Versus Conservative Management for First-Time Traumatic Anterior Shoulder Instability: A Systematic Review and Meta-analysis. Arthroscopy 2020, 36, 2526–2532. [Google Scholar] [CrossRef]
- Jakobsen, B.W.; Johannsen, H.V.; Suder, P.; Sojbjerg, J.O. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: A randomized study with 10-year follow-up. Arthroscopy 2007, 23, 118–123. [Google Scholar] [CrossRef]
- Lichtenberg, S. Kapitel 16—Arthroskopische Operationen bei Instabilität. In Schulterchirurgie (Fünfte Ausgabe); Habermeyer, P., Lichtenberg, S., Loew, M., Magosch, P., Martetschläger, F., Tauber, M., Eds.; Urban & Fischer: Munich, Germany, 2017; pp. 443–468. ISBN 978-3-437-22342-6. [Google Scholar]
- Marquardt, B.; Loew, M. Schulterluxation, rezidivierend und habituell. Obere Extrem. 2010, 5, 60–65. [Google Scholar] [CrossRef]
- Castagna, A.; Markopoulos, N.; Conti, M.; Delle Rose, G.; Papadakou, E.; Garofalo, R. Arthroscopic bankart suture-anchor repair: Radiological and clinical outcome at minimum 10 years of follow-up. Am. J. Sports Med. 2010, 38, 2012–2016. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Jia, Y.; Zhang, G.; Zhou, J.; Wu, D.; Jiang, J.; Yun, X. Risk factors for recurrence after Bankart repair: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 113. [Google Scholar] [CrossRef]
- Fradet, L.; Liefhold, B.; Rettig, O.; Bruckner, T.; Akbar, M.; Wolf, S.I. Proposition of a protocol to evaluate upper-extremity functional deficits and compensation mechanisms: Application to elbow contracture. J. Orthop. Sci. 2015, 20, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.W.; Caspers, M.; Zeifang, F.; Dreher, T.; Klotz, M.C.; Wolf, S.I.; Kasten, P. How does reverse shoulder replacement change the range of motion in activities of daily living in patients with cuff tear arthropathy? A prospective optical 3D motion analysis study. Arch. Orthop. Trauma. Surg. 2014, 134, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Rettig, O.; Krautwurst, B.; Maier, M.W.; Wolf, S.I. Definition of anatomical zero positions for assessing shoulder pose with 3D motion capture during bilateral abduction of the arms. BMC Musculoskelet. Disord. 2015, 16, 383. [Google Scholar] [CrossRef]
- Wu, G.; van der Helm, F.C.; Veeger, H.E.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X.; et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef]
- Klukowska, A.M.; Vandertop, W.P.; Schröder, M.L.; Staartjes, V.E. Calculation of the minimum clinically important difference (MCID) using different methodologies: Case study and practical guide. Eur. Spine J. 2024, 33, 3388–3400. [Google Scholar] [CrossRef]
- Sedaghat, A.R. Understanding the Minimal Clinically Important Difference (MCID) of Patient-Reported Outcome Measures. Otolaryngol. Head. Neck Surg. 2019, 161, 551–560. [Google Scholar] [CrossRef]
- Lim, K.T.; Tan, W.P.M.; Tan, A.H.C. Survivorship and outcomes of arthroscopic bankart repair for anterior shoulder dislocations: A minimum of 2 year follow-up. Shoulder Elb. 2024, 17, 574–589. [Google Scholar] [CrossRef]
- Moroder, P.; Danzinger, V.; Maziak, N.; Plachel, F.; Pauly, S.; Scheibel, M.; Minkus, M. Characteristics of functional shoulder instability. J. Shoulder Elbow Surg. 2020, 29, 68–78. [Google Scholar] [CrossRef]
- Itoigawa, Y.; Hooke, A.W.; Sperling, J.W.; Steinmann, S.P.; Zhao, K.D.; Itoi, E.; An, K.-N. Bankart repair alone in combined Bankart and superior labral anterior-posterior lesions preserves range of motion without compromising joint stability. JSES Int. 2020, 4, 63–67. [Google Scholar] [CrossRef]
- Meyer, A.; Klouche, S.; Bauer, T.; Rousselin, B.; Hardy, P. Residual inferior glenohumeral instability after arthroscopic Bankart repair: Radiological evaluation and functional results. Orthop. Traumatol. Surg. Res. 2011, 97, 590–594. [Google Scholar] [CrossRef]
- Livett, M.F.; Williams, D.; Potter, H.; Cairns, M. Functional cortical changes associated with shoulder instability—A systematic review. Shoulder Elb. 2022, 14, 452–464. [Google Scholar] [CrossRef]
- Shitara, H.; Shimoyama, D.; Sasaki, T.; Hamano, N.; Ichinose, T.; Yamamoto, A.; Kobayashi, T.; Osawa, T.; Iizuka, H.; Hanakawa, T.; et al. The Neural Correlates of Shoulder Apprehension: A Functional MRI Study. PLoS ONE 2015, 10, e0137387. [Google Scholar] [CrossRef]
- van Iersel, T.P.; van Spanning, S.H.; Verweij, L.P.E.; Priester-Vink, S.; van Deurzen, D.F.P.; van den Bekerom, M.P.J. Why do patients with anterior shoulder instability not return to sport after surgery? A systematic review of 63 studies comprising 3545 patients. JSES Int. 2023, 7, 376–384. [Google Scholar] [CrossRef]
- Delgrande, D.; Lonjon, G.; Hardy, P.; Schoch, B.; Werthel, J.D. Long-term results of arthroscopic Bankart repairs for anterior instability of the shoulder in patients aged thirty years or older. Int. Orthop. 2021, 45, 1583–1589. [Google Scholar] [CrossRef]
- Kartus, C.; Kartus, J.; Matis, N.; Forstner, R.; Resch, H. Long-term independent evaluation after arthroscopic extra-articular Bankart repair with absorbable tacks. A clinical and radiographic study with a seven to ten-year follow-up. J. Bone Joint Surg. Am. 2007, 89, 1442–1448. [Google Scholar] [CrossRef]
- Tingart, M.; Bäthis, H.; Bouillon, B.; Neugebauer, E.; Tiling, T. Die operative Therapie der traumatischen SchulterluxationGibt es evidenzbasierte Indikationen für die arthroskopische Bankart-Operation? Der Unfallchirurg 2001, 104, 894–901. [Google Scholar] [CrossRef]
- Sahin, E.; Dilek, B.; Baydar, M.; Gundogdu, M.; Ergin, B.; Manisali, M.; Akalin, E.; Gulbahar, S. Shoulder proprioception in patients with subacromial impingement syndrome. J. Back. Musculoskelet. Rehabil. 2017, 30, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Lubiatowski, P.; Ogrodowicz, P.; Wojtaszek, M.; Romanowski, L. Bilateral shoulder proprioception deficit in unilateral anterior shoulder instability. J. Shoulder Elbow Surg. 2019, 28, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Keener, J.D.; Patterson, B.M.; Orvets, N.; Chamberlain, A.M. Degenerative Rotator Cuff Tears: Refining Surgical Indications Based on Natural History Data. J. Am. Acad. Orthop. Surg. 2019, 27, 156–165. [Google Scholar] [CrossRef]

| Movement | Preop | SD-Preop | Postop | SD-Postop | Mean Difference, 95% CI | SD Difference | p-Values | MCID |
|---|---|---|---|---|---|---|---|---|
| Flexion Shoulder | 89.99 | ±62.37 | 148.46 | ±8.71 | 58.47 [−19.7; 136.7] | ±62.98 | 0.10 | 31.2 |
| Extension Shoulder | −38.49 | ±16.88 | −41.07 | ±13.88 | 2.58 [−24.5; 29.7] | ±21.85 | 0.81 | 8.4 |
| Abduction | 76.12 | ±61.47 | 145.66 | ±15.63 | 69.53 [−9.3; 148.3] | ±63.43 | 0.07 | 30.7 |
| ARO | 2.54 | ±41.58 | 18.98 | ±38.87 | 16.44 [−54.3; 87.2] | ±56.92 | 0.55 | 20.8 |
| IRO | 38.75 | ±23.34 | 54.86 | ±18.85 | 16.11 [−21.2; 53.4] | ±30.00 | 0.29 | 11.7 |
| Pro-/Supination | 117.13 | ±17.74 | 124.67 | ±15.94 | 7.55 [−22.1; 37.1] | ±23.85 | 0.51 | 8.9 |
| Ext/Flex Elbow | 125.13 | ±31.44 | 144.07 | ±5.18 | 18.93 [−20.7; 58.5] | ±31.86 | 0.26 | 15.7 |
| Movement | Post-Op | SD-Post-Op | Long-Term | SD-Long-Term | Mean Difference, 95% CI | SD Difference | p-Values |
|---|---|---|---|---|---|---|---|
| Flexion Shoulder | 148.46 | ±8.71 | 157.40 | ±14.95 | 8.94 [–5.7, 23.6] | ±17.30 | 0.20 |
| Extension Shoulder | −41.07 | ±13.88 | −51.45 | ±10.00 | –10.38 [–27.9, 7.1] | ±17.11 | 0.19 |
| Abduction | 145.66 | ±15.63 | 152.28 | ±15.16 | 6.62 [–13.7, 26.9] | ±21.78 | 0.47 |
| ARO | 18.98 | ±38.87 | 14.37 | ±22.87 | –4.61 [–53.9, 44.7] | ±45.10 | 0.82 |
| IRO | 54.86 | ±18.85 | 25.21 | ±7.80 | –29.65 [–54.3, –5.0] | ±20.40 | <0.05 |
| Pro-/Supination | 124.67 | ±15.94 | 126.24 | ±14.81 | 1.57 [–18.8, 22.0] | ±21.76 | 0.86 |
| Ext/Flex elbow | 144.07 | ±5.18 | 146.38 | ±5.01 | 2.31 [–4.4, 9.0] | ±7.20 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetto, P.; Liewald, R.; Spranz, D.M.; Tsitlakidis, S. Dynamic Kinematic Assessment with 3D Motion Analysis After Arthroscopic Bankart Repair: A Mid- to Long-Term Study. J. Clin. Med. 2025, 14, 8204. https://doi.org/10.3390/jcm14228204
Hetto P, Liewald R, Spranz DM, Tsitlakidis S. Dynamic Kinematic Assessment with 3D Motion Analysis After Arthroscopic Bankart Repair: A Mid- to Long-Term Study. Journal of Clinical Medicine. 2025; 14(22):8204. https://doi.org/10.3390/jcm14228204
Chicago/Turabian StyleHetto, Pit, Raissa Liewald, David M. Spranz, and Stefanos Tsitlakidis. 2025. "Dynamic Kinematic Assessment with 3D Motion Analysis After Arthroscopic Bankart Repair: A Mid- to Long-Term Study" Journal of Clinical Medicine 14, no. 22: 8204. https://doi.org/10.3390/jcm14228204
APA StyleHetto, P., Liewald, R., Spranz, D. M., & Tsitlakidis, S. (2025). Dynamic Kinematic Assessment with 3D Motion Analysis After Arthroscopic Bankart Repair: A Mid- to Long-Term Study. Journal of Clinical Medicine, 14(22), 8204. https://doi.org/10.3390/jcm14228204






