The Effect of Long-Term Non-Invasive Ventilation on Tracheostomy-Free Survival and Hospitalizations in Types 2 and 3 Spinal Muscular Atrophy Patients
Abstract
1. Introduction
2. Methods
2.1. Participants
- (A)
- At the time the decision was made to initiate NIV:
- (B)
- During the follow-up period:
- -
- The reasons why it had become necessary to discontinue LT-NIV, classified as one of the following: (a) death due to cardio-respiratory acute illness; (b) tracheostomy due to cardio-respiratory acute illness, NIV complications, or full-time ventilator dependence; or (c) lack of motivation.
- -
- If complications associated with NIV use had arisen. Complications were classified as follows: (a) major complications, including those that are potentially life-threatening or lead to the need for intubation and/or tracheostomy; or (b) minor complications, defined as mild or transient medical problems related to features specific to NIV, such as the interface or airflow [26].
- -
- If there had been any hospitalizations (in our own or other hospitals) linked to respiratory problems. The conditions associated with hospitalization/s due to respiratory complications were classified as upper respiratory tract infection (URTI), pneumonia, aspiration pneumonia, pneumothorax, pulmonary thromboembolism, and abuse of sedatives. The diagnosis of URTI was based on the presence of one or more of the following signs or symptoms: fever, throat irritation or sore throat, and hoarseness [27]. The diagnosis of pneumonia was made in accordance with current guidelines [28].
- -
- If a therapy that enhanced the production of SMN protein had been prescribed. In our case, Nusinersen (Spinraza, Biogen, Cambridge, MA, USA) and/or Risdiplam (Evrysdi, Genentech/Roche, South San Francisco, CA, USA) were considered.
- -
- The patient’s FVC values from last PFT carried out before tracheostomy tube was placed, the study period came to an end, or the patient died.
2.2. Interventions
2.2.1. Non-Invasive Ventilation
2.2.2. Other Interventions
2.3. Study Endpoint and Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal muscular atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Bertini, E.; Iannaccone, S.T. Childhood spinal muscular atrophy: Controversies and challenges. Lancet Neurol. 2012, 11, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Carmela Pera, M.; Scoto, M.; Finkel, R.; Muntoni, F. Spinal muscular atrophy—Insights and challenges in the treatment era. Nat. Rev. Neurol. 2020, 16, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.A.; Racca, F.; Ottonello, G.; Vianello, A.; Berardinelli, A.; Crescimanno, G.; Casiraghi, J.L.; Agosto, C.; Banfi, P.; Baranello, G.; et al. 1st Italian SMA Family Association Consensus Meeting: Management and recommendations for respiratory involvement in spinal muscular atrophy (SMA) types I–III, Rome, Italy, 30–31 January 2015. Neuromuscul. Disord. 2015, 25, 979–989. [Google Scholar] [CrossRef]
- Veldhoen, E.S.; Wijngaarde, C.A.; Hulzebos, E.H.; Wösten-van Asperen, R.M.; Wadman, R.I.; van Eijk, R.P.; Asselman, F.L.; Stam, M.; Otto, L.A.; Cuppen, I.; et al. Natural history of respiratory muscle strength in spinal muscular atrophy: A prospective national cohort study. Orphanet J. Rare Dis. 2022, 17, 70. [Google Scholar] [CrossRef]
- Schroth, M.K. Special considerations in the respiratory management of Spinal Muscular Atrophy. Pediatrics 2009, 123 (Suppl. S4), 245–249. [Google Scholar] [CrossRef]
- Testa, M.B.; Pavone, M.; Bertini, E.; Petrone, A.; Pagani, M.; Cutrera, R. Sleep-disordered breathing in spinal muscular atrophy types 1 and 2. Am. J. Phys. Med. Rehabil. 2005, 84, 666–670. [Google Scholar] [CrossRef]
- LoMauro, A.; Romei, M.; Priori, R.; Laviola, M.; D’Angelo, M.G.; Aliverti, A. Alterations of thoraco-abdominal volumes and asynchronies in patients with spinal muscle atrophy type III. Respir. Physiol. Neurobiol. 2014, 197, 1–8. [Google Scholar] [CrossRef]
- Bach, J.R.; Wang, T.-G. Noninvasive long-term ventilatory support for individuals with Spinal Muscular Atrophy and functional bulbar musculature. Arch. Phys. Med. Rehabil. 1995, 76, 213–217. [Google Scholar] [CrossRef]
- Mellies, U.; Ragette, R.; Dohna Schwake, C.; Boehm, H.; Voit, T.; Teschler, H. Long-term noninvasive ventilation in children and adolescents with neuromuscular disorders. Eur. Respir. J. 2003, 22, 631–636. [Google Scholar] [CrossRef]
- Petrone, A.; Pavone, M.; Testa, M.B.; Petreschi, F.; Bertini, E.; Cutrera, R. Noninvasive ventilation in children with spinal muscular atrophy types 1 and 2. Am. J. Phys. Med. Rehabil. 2007, 86, 216–221. [Google Scholar] [CrossRef]
- Ioos, C.; Leclair-Richard, D.; Mrad, S.; Barois, A.; Estournet-Mathiaud, B. Respiratory capacity course in patients with infantile spinal muscular atrophy. Chest 2004, 126, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Meyer, O.H.; Simonds, A.K.; Schroth, M.K.; Graham, R.J.; Kirschner, J.; Iannaccone, S.T.; Crawford, T.O.; Woods, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 2018, 28, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Finkel, R.S.; Bertini, E.S.; Schroth, M.; Simonds, A.; Wong, B.; Aloysius, A.; Morrison, L.; Main, M.; Crawford, T.O.; et al. Consensus statement for standard of care in spinal muscular atrophy. J. Child. Neurol. 2022, 22, 1027–1049. [Google Scholar] [CrossRef] [PubMed]
- Kersting, C.; Kneer, M.; Barzel, A. Patient-relevant outcomes: What are we talking about? A scoping review to improve conceptual clarity. BMC Health Serv. Res. 2020, 20, 596. [Google Scholar] [CrossRef]
- Katz, S.; Selvadurai, H.; Keilty, K.; Mitchell, M.; MacLusky, I. Outcome of non-invasive positive pressure ventilation in paediatric neuromuscular disease. Arch. Dis. Child. 2004, 89, 121–124. [Google Scholar] [CrossRef]
- Dohna-Schwake, C.; Podlewski, P.; Voit, T.; Mellies, U. Non-invasive ventilation reduces respiratory tract infections in children with neuromuscular disorders. Pediatr. Pulmonol. 2008, 43, 67–71. [Google Scholar] [CrossRef]
- Young, H.K.; Lowe, A.; Fitzgerald, D.A.; Seton, C.; Waters, K.A.; Kenny, E.; Hynan, L.S.; Iannaccone, S.T.; North, K.N.; Ryan, M.M. Outcome of noninvasive ventilation in children with neuromuscular disease. Neurology 2007, 68, 198–201. [Google Scholar] [CrossRef]
- Bach, J.R. A Comparison of long-term ventilatory support alternatives from the perspective of the patient and care giver. Chest 1993, 104, 1702–1706. [Google Scholar] [CrossRef]
- Ambrosino, N.; Vianello, A. Where to perform long-term ventilation. Respir. Care Clin. N. Am. 2002, 8, 463–478. [Google Scholar] [CrossRef]
- AlBalawi, M.M.; Castro-Codesal, M.; Featherstone, R.; Sebastianski, M.; Vandermeer, B.; Alkhaledi, B.; Bedi, P.K.; Abusido, T.; MacLean, J.E. Outcomes of long-term noninvasive ventilation use in children with neuromuscular disease. Systematic review and meta-analysis. Ann. Am. Thorac. Soc. 2022, 19, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Pontier-Marchandise, S.; Texereau, J.; Prigent, A.; Gonzalez-Bermejo, J.; Rabec, C.; Gagnadoux, F.; Letierce, A.; Winck, J.C. Home NIV treatment quality in patients with chronic respiratory failure having participated to the French nationwide telemonitoring experimental program (The TELVENT study). Respir. Med. Res. 2023, 84, 101028. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef]
- Mazzone, E.S.; Mayhew, A.; Montes, J.; Ramsey, D.; Fanelli, L.; Dunaway Young, S.; Salazar, R.; De Sanctis, R.; Pasternak, A.; Glanzman, A.; et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve 2017, 55, 869–874. [Google Scholar] [CrossRef]
- Choudhry, M.N.; Ahmad, Z.; Verma, R. Adolescent Idiopathic Scoliosis. Open Orthop. J. 2016, 10, 143–154. [Google Scholar] [CrossRef]
- Carron, M.; Freo, U.; BaHammam, A.S.; Dellweg, D.; Guarracino, F.; Cosentini, R.; Feltracco, P.; Vianello, A.; Ori, C.; Esquinas, A. Complications of non-invasive ventilation techniques: A comprehensive qualitative review of randomized trials. Br. J. Anaesth. 2013, 110, 896–914. [Google Scholar] [CrossRef]
- Vianello, A.; Corrado, A.; Arcaro, G.; Gallan, F.; Ori, C.; Minuzzo, M.; Bevilacqua, M. Mechanical insufflation-exsufflation improves outcomes for neuromuscular disease patients with respiratory tract infections. Am. J. Phys. Med. Rehabil. 2005, 84, 83–88. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- O’Donohue, W.J.; Giovannoni, R.M.; Goldberg, A.I.; Keens, T.G.; Make, B.J.; Plummer, A.L.; Prentice, W.S. Long-term Mechanical Ventilation: Guidelines for management in the home and at alternate community sites: Report of the ad hoc committee, respiratory care section, American College of Chest Physicians. Chest 1986, 90 (Suppl. S1), 1S–37S. [Google Scholar] [CrossRef]
- Hirji, K.F.; Mehta, C.R.; Patel, N.R. Computing distributions for exact logistic regression. JASA 1987, 82, 1110–1117. [Google Scholar] [CrossRef]
- Han, Y.J.; Park, J.D.; Lee, B.; Choi, Y.H.; Suh, D.I.; Lim, B.C.; Chae, J.H. Home mechanical ventilation in childhood-onset hereditary neuromuscular diseases: 13 years’ experience at a single center in Korea. PLoS ONE 2015, 10, e0122346. [Google Scholar] [CrossRef] [PubMed]
- Patout, M.; Lhuillier, E.; Kaltsakas, G.; Benattia, A.; Dupuis, J.; Arbane, G.; Declercq, P.L.; Ramsay, M.; Marino, P.; Molano, L.C.; et al. Long-term survival following initiation of home non-invasive ventilation: A European study. Thorax 2020, 75, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, E.S.; Wijngaarde, C.A.; Verweij-van den Oudenrijn, L.P.; Asselman, F.L.; Wösten-van Asperen, R.M.; Hulzebos, E.H.J.; van der Ent, K.; Cuppen, I.; Gaytant, M.A.; van Eijk, R.P.; et al. Relative hyperventilation in non-ventilated patients with spinal muscular atrophy. Eur. Respir. J. 2020, 56, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cancelinha, C.; Madureira, N.; Mação, P.; Pleno, P.; Silva, T.; Estêvão, M.H.; Félix, M. Long-term ventilation in children: Ten years later. Rev. Port. Pneumol. 2015, 21, 16–21. [Google Scholar] [CrossRef]
- Racca, F.; Vianello, A.; Mongini, T.; Ruggeri, P.; Versaci, A.; Vita, G.L.; Vita, G. Practical approach to respiratory emergencies in neurological diseases. Neurol. Sci. 2020, 41, 497–508. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Martínez-Vizcaíno, V.; Cavero-Redondo, I.; Martínez-García, I.; Moreno-Herráiz, N.; Álvarez-Bueno, C.; Saz-Lara, A. Efficacy of risdiplam in spinal muscular atrophy: A systematic review and meta-analysis. Pharmacotherapy 2024, 44, 97–105. [Google Scholar] [CrossRef]
- Domènech-Clar, R.; Nauffal-Manzur, D.; Perpiñá-Tordera, M.; Compte-Torrero, L.; Macián-Gisbert, V. Home mechanical ventilation for restrictive thoracic diseases: Effects on patient quality-of-life and hospitalizations. Respir. Med. 2003, 97, 1320–1327. [Google Scholar] [CrossRef]
| All Cases (n = 18) | Group A (n = 12) | Group B (n = 6) | p-Value | |
|---|---|---|---|---|
| Age (years), median (range) | 16.5 (2–55) | 14.0 (2–45) | 26.5 (12–55) | 0.122 |
| Gender (females/males), N | 6/12 | 5/7 | 1/5 | 0.600 |
| BMI (kg/m2), median (range) | 15.8 (8.2–30.9) | 14.4 (8.2–22.2) | 20.7 (8.2–30.9) | 0.345 |
| Smokers, n (%) | 2 (12.6) | 1 (8.3) | 1 (16.7) | 0.542 |
| SMA type (2/3) | 10/8 | 8/4 | 2/4 | 0.321 |
| RULM score, median (range) | 7 (0–32) | 8 (0–32) | 7 (7–7) | 0.999 |
| Pts previously administered spinal fusion, N. (%) | 5 (27.8) | 4 (33.3) | 1 (16.7) | 0.999 |
| Pts previously administered PEG, N. (%) | 5 (27.8) | 3 (25.0) | 2 (33.3) | 0.999 |
| Pts with comorbidities, N. (%) | 2 (11.1) | 2 (16.6) | 0 (0) | 0.529 |
| ACCI, median (range) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0.345 |
| Pts with severe scoliosis, N. (%) | 8 (44.4) | 7 (58.3) | 1 (16.6) | 0.510 |
| FVC, L median (range) | 0.45 (0.15–3.34) | 0.38 (0.18–3.34) | 1.13 (0.15–3.03) | 0.272 |
| FVC, % median (range) | 31 (8–81) | 20 (8–81) | 37 (10–71) | 0.689 |
| PaO2 (mmHg), median (range) | 76.2 (67.0–88.2) | 76.8 (67.0–88.2) | 76.2 (73.0–82.0) | 0.999 |
| PaCO2 (mmHg), median (range) | 39.2 (32.9–70.0) | 38.0 (32.9–41.5) | 43.0 (38.0–70.0) | 0.088 |
| Arterial pH, median (range) | 7.42 (7.39–7.50) | 7.42 (7.39–7.50) | 7.41 (7.39–7.45) | 0.563 |
| All Cases (n = 18) | Group A (n = 12) | Group B (n = 6) | p-Value | |
|---|---|---|---|---|
| Nusinersen, n (%) | 2 (11.1) | 1 (8.3) | 1 (16.6) | 0.999 |
| Risdiplam, n (%) | 0.114 | |||
| -First line treatment | 3 (16.6) | 3 (25.0) | 0 (0) | |
| -Switch from Nusinersen | 2 (11.1) | 2 (16.6) | 0 (0) |
| All Cases (n = 18) | Group A (n = 12) | Group B (n = 6) | p-Value | |
|---|---|---|---|---|
| LT-NIV initiation (elective/post-acute), n (%) | 15/3 | 12/0 | 3/3 | 0.025 |
| Daily NIV use at the end of follow-up, n (%) | 0.303 | |||
| • Nocturnal | 4 (22.2) | 4 (33.3) | 0 (0) | |
| • Intermediate | 8 (44.4) | 4 (33.3) | 4 (66.6) | |
| • Life support | 6 (33.3) | 4 (33.3) | 2 (33.3) | |
| Complications, n (%) | 0.245 | |||
| • Nasal skin breakdown | 2 (11.1) | 1 (8.3) | 1 (16.7) | |
| • Nasal congestion | 1 (5.5) | 0 (0) | 1 (16.7) | |
| • Acute gastric distension | 1 (5.5) | 1 (8.3) | 0 (0) | |
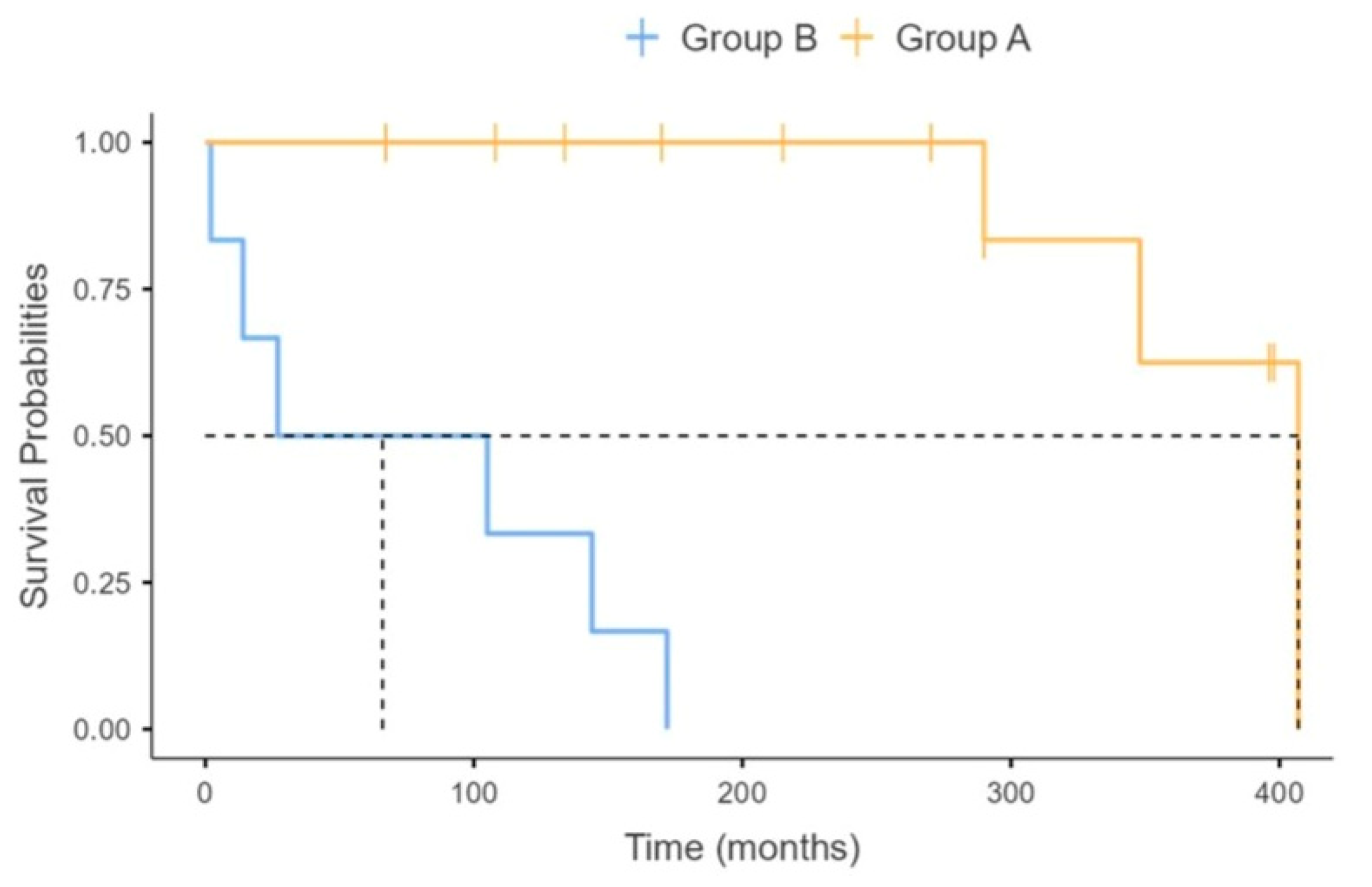
| Tracheostomy-free survival (months), median (range) | 171 (2–407) | 280 (67–407) | 66 (2–172) | 0.010 |
| Hospitalization rate (episodes/yr), median (range) | 0.09 (0.00–1.44) | 0.07 (0.00–0.18) | 0.35 (0.06–1.44) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vianello, A.; Guarnieri, G.; Bertagna De Marchi, L.; Molena, B.; Arcaro, G.; Capece, G.; Sogus, E.; Lionello, F.; Pegoraro, E. The Effect of Long-Term Non-Invasive Ventilation on Tracheostomy-Free Survival and Hospitalizations in Types 2 and 3 Spinal Muscular Atrophy Patients. J. Clin. Med. 2025, 14, 8171. https://doi.org/10.3390/jcm14228171
Vianello A, Guarnieri G, Bertagna De Marchi L, Molena B, Arcaro G, Capece G, Sogus E, Lionello F, Pegoraro E. The Effect of Long-Term Non-Invasive Ventilation on Tracheostomy-Free Survival and Hospitalizations in Types 2 and 3 Spinal Muscular Atrophy Patients. Journal of Clinical Medicine. 2025; 14(22):8171. https://doi.org/10.3390/jcm14228171
Chicago/Turabian StyleVianello, Andrea, Gabriella Guarnieri, Leonardo Bertagna De Marchi, Beatrice Molena, Giovanna Arcaro, Giuliana Capece, Elena Sogus, Federico Lionello, and Elena Pegoraro. 2025. "The Effect of Long-Term Non-Invasive Ventilation on Tracheostomy-Free Survival and Hospitalizations in Types 2 and 3 Spinal Muscular Atrophy Patients" Journal of Clinical Medicine 14, no. 22: 8171. https://doi.org/10.3390/jcm14228171
APA StyleVianello, A., Guarnieri, G., Bertagna De Marchi, L., Molena, B., Arcaro, G., Capece, G., Sogus, E., Lionello, F., & Pegoraro, E. (2025). The Effect of Long-Term Non-Invasive Ventilation on Tracheostomy-Free Survival and Hospitalizations in Types 2 and 3 Spinal Muscular Atrophy Patients. Journal of Clinical Medicine, 14(22), 8171. https://doi.org/10.3390/jcm14228171







