Using the International Index of Erectile Function-15 in Comparative Analysis Between Transcutaneous Electrical Nerve Stimulation of the Pudendal Nerve and Low-Level Laser Therapy in the Treatment of Erectile Dysfunction After COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.1.1. Randomization of Participants
2.1.2. Participant Selection Criteria
- Europeans;
- Men;
- Age—from 21 to 60 years old;
- ED was first diagnosed after COVID-19, no later than one month after the onset of respiratory symptoms;
- Erectile dysfunction as a complication of COVID-19 was diagnosed by two urologists.
- Duration of ED—from 6 months to a year;
- All patients underwent vascular, nootropic, and vitamin therapy without sufficient effects;
- Mild or moderate erectile dysfunction with a score in the erectile function domain (EFD) of the International Index of Erectile Function-15 (IIEF-15) greater than 11 and less than 25 points;
- Hypoesthesia in the penis area from 3 to 7 points;
- Signed consent to participate.
- Epileptic seizures and other paroxysmal disorders;
- Mental disorders;
- Distal polyneuropathy;
- Central nervous system lesion;
- Endocrine disorders;
- Urologic disorders;
- Peyronie’s disease;
- Atherosclerosis and structural abnormalities of the penile arteries, internal pudendal arteries, and internal iliac artery;
- Veno-occlusive dysfunction;
- Active implantable medical devices.
2.1.3. Consent to Participate
2.1.4. Ethics Approval
2.1.5. Patient Demographics and Characteristics
2.2. Sample Size Calculation
2.3. Clinical Examuination and Outcomes Evaluation
2.3.1. Primary Endpoints
Erectile Function Domain by IIEF-15
Hypoesthesia
2.3.2. Secondary Endpoints
Orgasmic Function
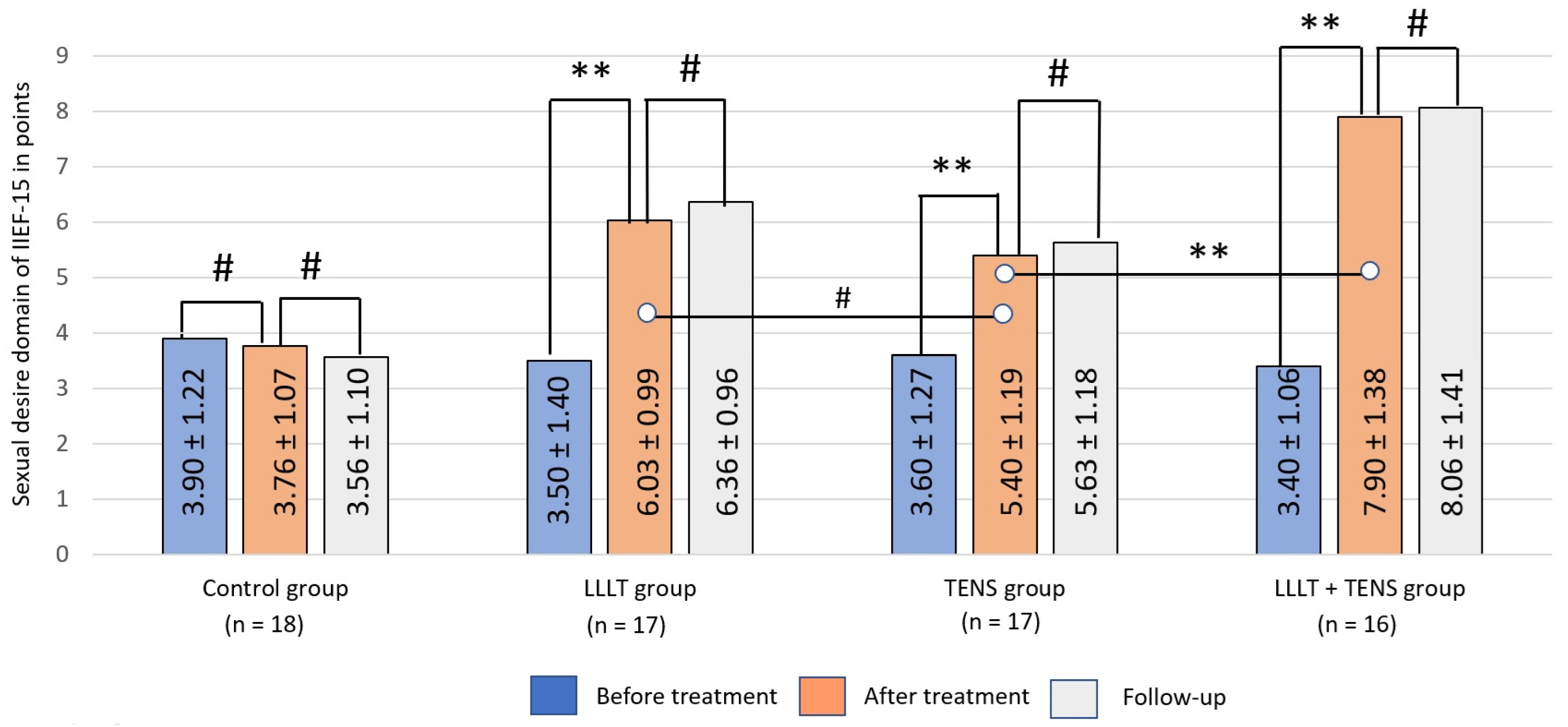
Sexual Desire
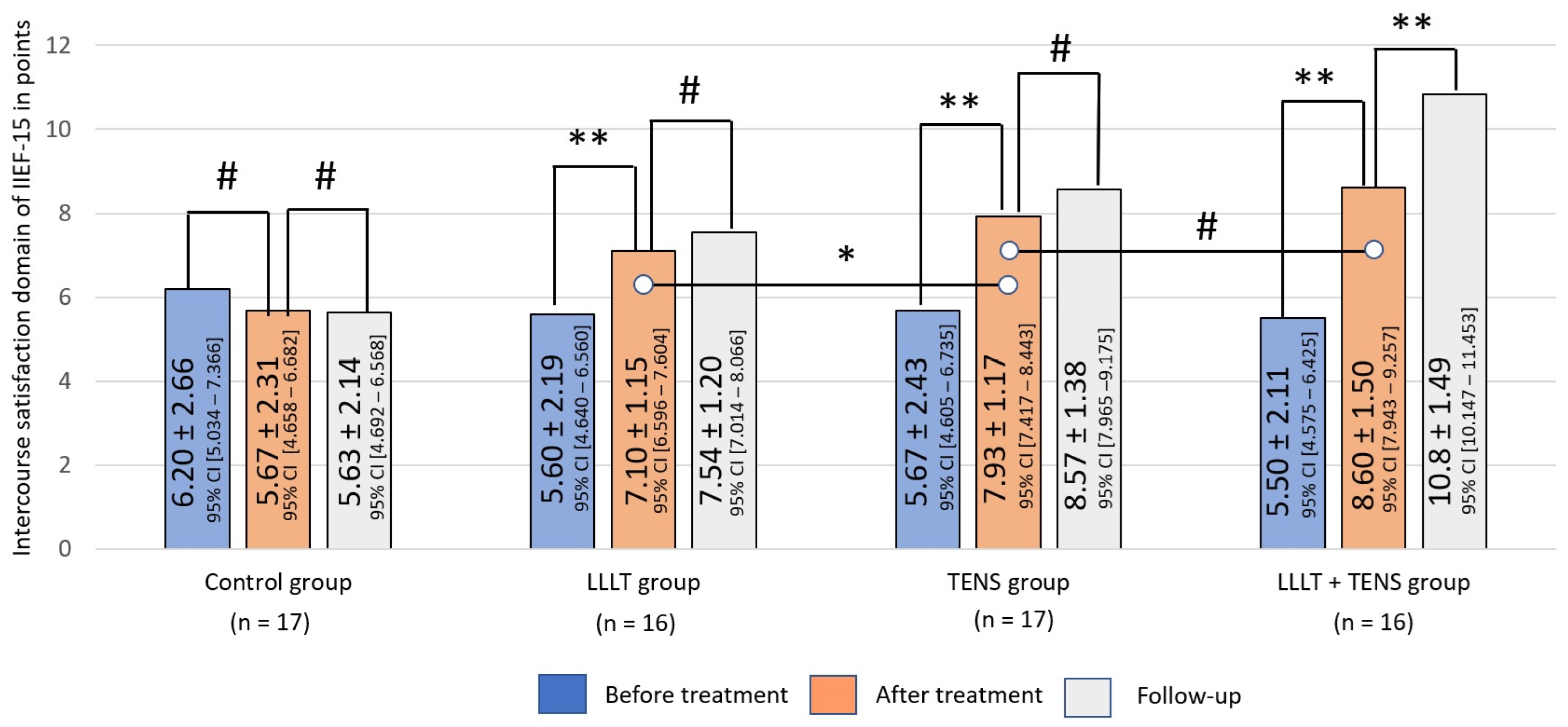
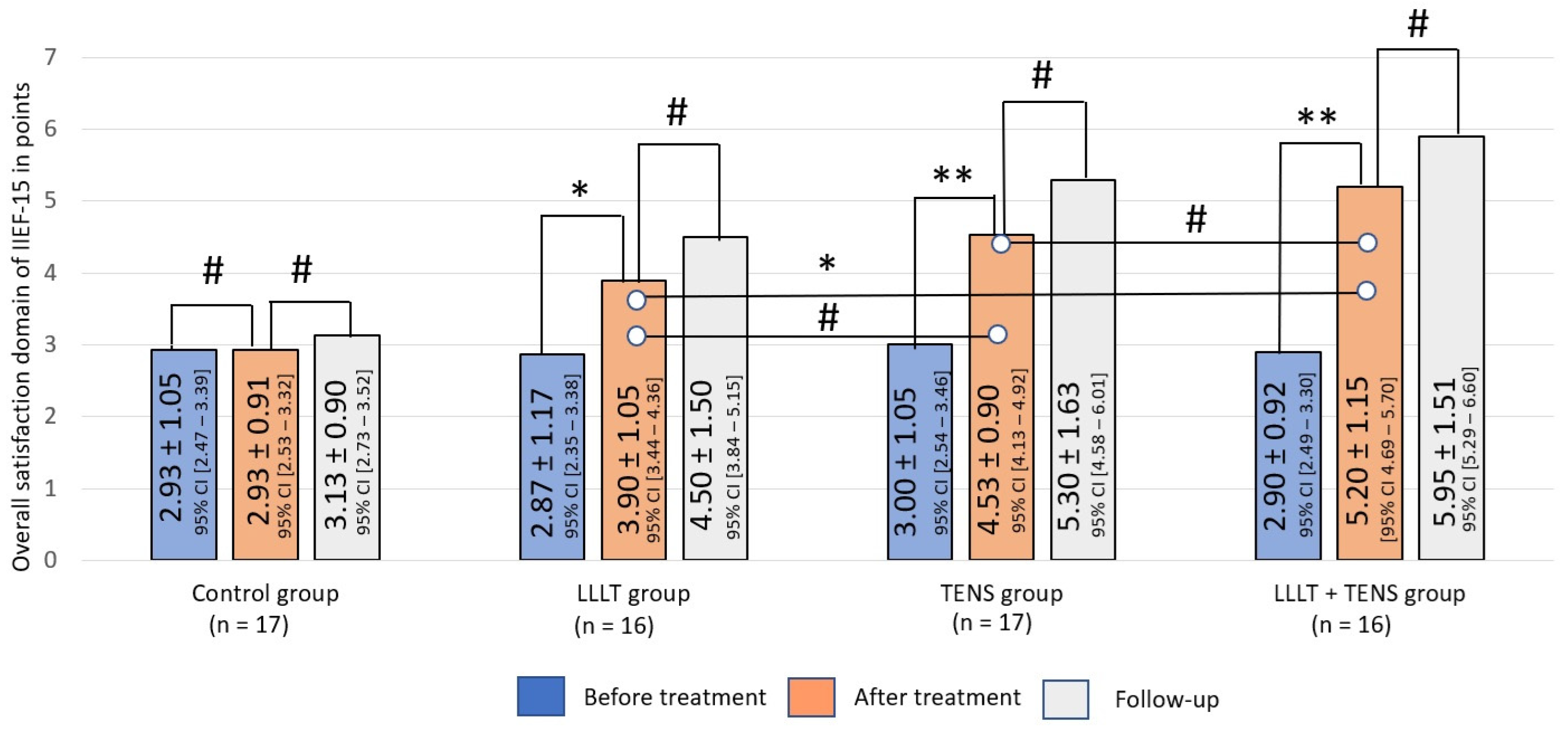
Sexual Satisfaction
2.3.3. Exploratory Endpoints
Electromyography Changes
Penile Doppler Ultrasound
2.4. Treatment Protocols
2.4.1. Transcutaneous Electrical Nerve Stimulation
2.4.2. Low-Level Laser Therapy
2.4.3. Combination of Transcutaneous Electrical Nerve Stimulation and Low-Level Laser Therapy
2.4.4. Sham TENS and LLLT
2.5. Statistical Analysis
3. Results
3.1. Primary Clinical Outcomes
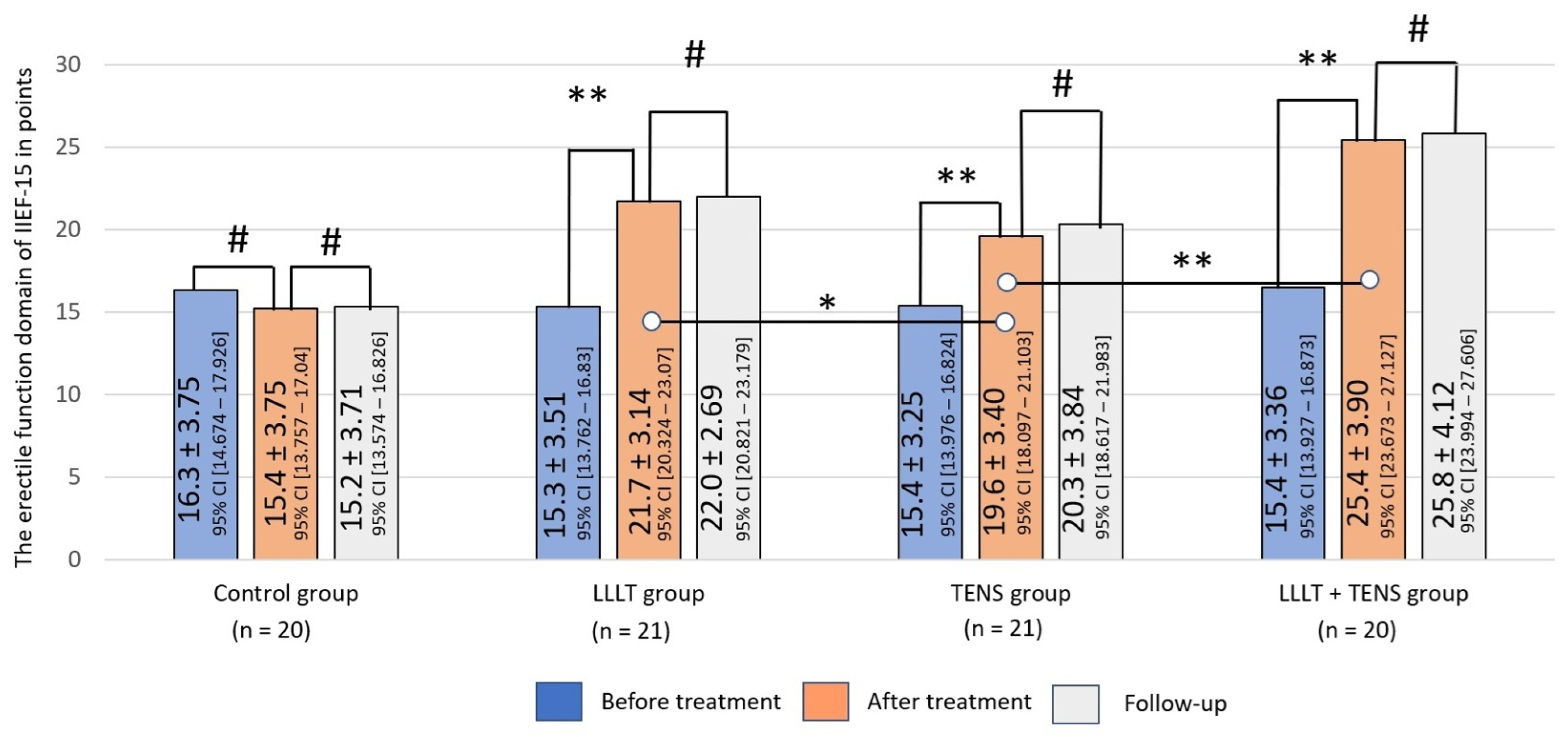
3.1.1. Erectile Dysfunction Domain by IIEF-15
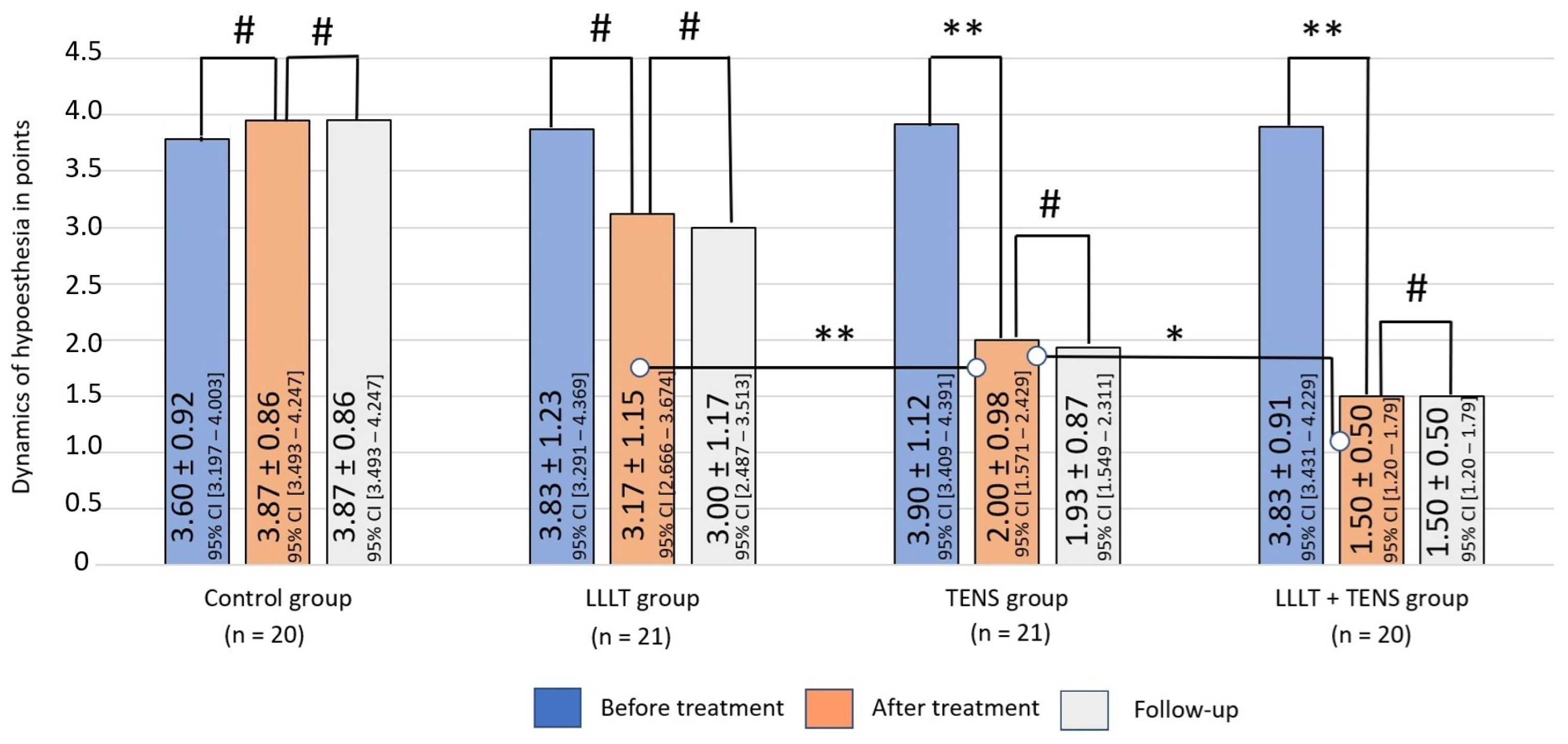
3.1.2. Hypoesthesia
3.2. Secondary Clinical Outcomes
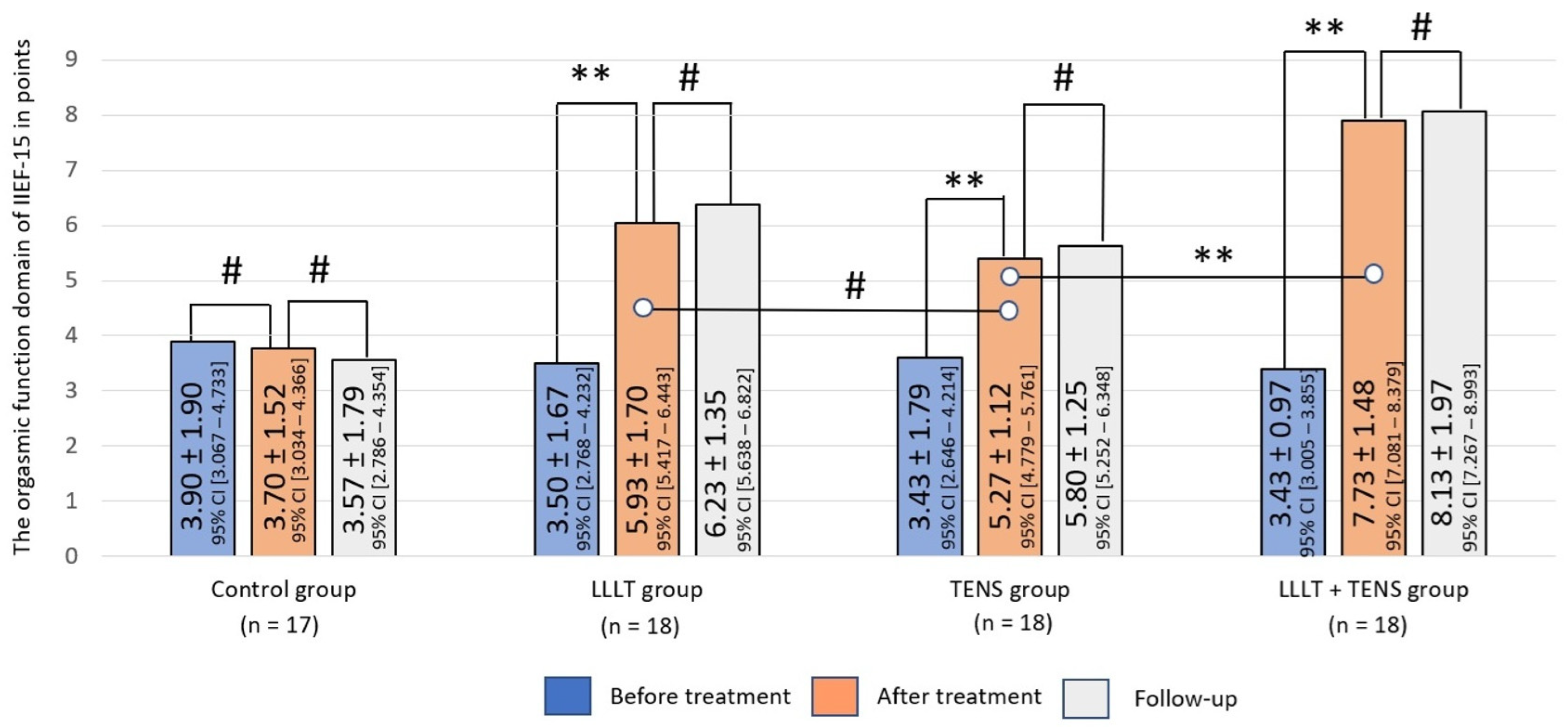
3.2.1. Orgasmic Function
3.2.2. Sexual Desire
3.2.3. Sexual Satisfaction
Intercourse Satisfaction
Overall Satisfaction
3.3. Exploratory Clinical Outcomes
Electromyography Examination
3.4. Side Effects
4. Discussion
4.1. Erectile Function
4.2. Orgasmic Function
4.3. Sexual Desire
4.4. Sexual Satisfaction
4.5. Prolonged Effect of LLLT and TENS
4.6. Combined Use of LLLT and TENS
4.7. Dynamics of Electromyography Changes
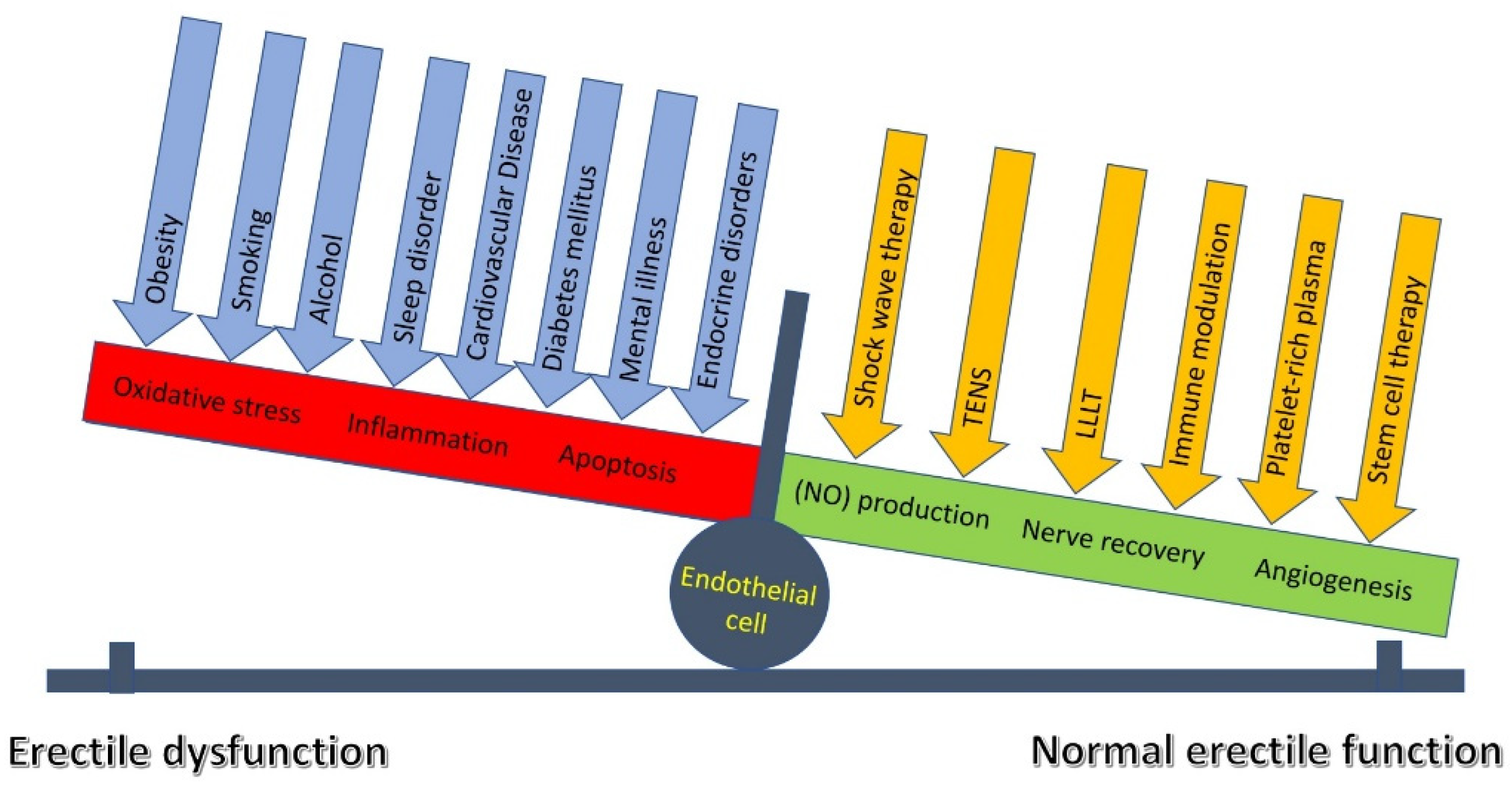
4.8. Endothelial Sensing and Signaling
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarker, R.; Roknuzzaman, A.S.M.; Hossain, M.J.; Bhuiyan, M.A.; Islam, M.R. The WHO declares COVID-19 is no longer a public health emergency of international concern: Benefits, challenges, and necessary precautions to come back to normal life. Int. J. Surg. 2023, 109, 2851–2852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarkesh, A.; Daei Sorkhabi, A.; Sheykhsaran, E.; Alinezhad, F.; Mohammadzadeh, N.; Hemmat, N.; Bannazadeh Baghi, H. Extrapulmonary Clinical Manifestations in COVID-19 Patients. Am. J. Trop. Med. Hyg. 2020, 103, 1783–1796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 28, 711616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galea, M.; Agius, M.; Vassallo, N. Neurological manifestations and pathogenic mechanisms of COVID-19. Neurol. Res. 2022, 44, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Uzundal, H.; Soydas, T.; Kutluhan, M.A.; Ozayar, A.; Okulu, E.; Kayigil, O. A possible mechanism of erectile dysfunction in coronavirus disease-19: Cavernosal smooth muscle damage: A pilot study. Rev. Int. Androl. 2023, 21, 100366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yazdanpanah, N.; Rezaei, N. Autoimmune complications of COVID-19. J. Med. Virol. 2022, 94, 54–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, M.J.A.; Ribeiro, L.R.; Gouveia, M.I.M.; Marcelino, B.D.R.; Santos, C.S.D.; Lima, K.V.B.; Lima, L.N.G.C. Hyperinflammatory Response in COVID-19: A Systematic Review. Viruses 2023, 15, 553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kichloo, A.; Dettloff, K.; Aljadah, M.; Albosta, M.; Jamal, S.; Singh, J.; Wani, F.; Kumar, A.; Vallabhaneni, S.; Khan, M.Z. COVID-19 and Hypercoagulability: A Review. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620962853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashraf, U.M.; Abokor, A.A.; Edwards, J.M.; Waigi, E.W.; Royfman, R.S.; Hasan, S.A.; Smedlund, K.B.; Hardy, A.M.G.; Chakravarti, R.; Koch, L.G. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genom. 2021, 53, 51–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodler, S.; von Büren, J.; Buchner, A.; Stief, C.; Elkhanova, K.; Wülfing, C.; Jungmann, S. Epidemiology and Treatment Barriers of Patients with Erectile Dysfunction Using an Online Prescription Platform: A Cross-Sectional Study. Sex. Med. 2020, 8, 370–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadibrata, E.; Sutyarso, S.; Busman, H.; Sumardi, S. Erectile Dysfunction and Ejaculatory Dysfunction in Covid-19 Recovered Patient: Temporary or Persistent? Urol. J. 2025, 21, 8113. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.; Mollaioli, D.; Ciocca, G.; Colonnello, E.; Limoncin, E.; Balercia, G.; Jannini, E.A. “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology 2021, 9, 1053–1059. [Google Scholar] [CrossRef]
- Kato, H.; Ichihara, N.; Saito, H.; Fujitani, S.; Ota, K.; Takahashi, Y.; Harada, T.; Hattori, T.; Komeya, M.; Hosozawa, M.; et al. Prevalence of erectile dysfunction as long-COVID symptom in hospitalized Japanese patients. Sci. Rep. 2025, 15, 6279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kresch, E.; Achua, J.; Saltzman, R.; Khodamoradi, K.; Arora, H.; Ibrahim, E.; Kryvenko, O.N.; Almeida, V.W.; Firdaus, F.; Hare, J.M.; et al. COVID-19 Endothelial Dysfunction Can Cause Erectile Dysfunction: Histopathological, Immunohistochemical, and Ultrastructural Study of the Human Penis. World J. Men’s Health 2021, 39, 466–469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, T.C.; Edwards, N.C.; Bhattacharyya, S.K.; Nitschelm, K.D.; Burnett, A.L. The Epidemic of COVID-19-Related Erectile Dysfunction: A Scoping Review and Health Care Perspective. Sex. Med. Rev. 2022, 10, 286–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, J.; Younus, F.; Iftikhar, I.; Usman, M. Love in the time of COVID-19: A scoping review on male sexual health. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 496–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sansone, A.; Mollaioli, D.; Limoncin, E.; Ciocca, G.; Bắc, N.H.; Cao, T.N.; Hou, G.; Yuan, J.; Zitzmann, M.; Giraldi, A.; et al. The Sexual Long COVID (SLC): Erectile Dysfunction as a Biomarker of Systemic Complications for COVID-19 Long Haulers. Sex. Med. Rev. 2022, 10, 271–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pizzol, D.; Shin, J.I.; Trott, M.; Ilie, P.C.; Ippoliti, S.; Carrie, A.M.; Ghayda, R.A.; Lozano, J.M.O.; Muyor, J.M.; Butler, L.; et al. Social environmental impact of COVID-19 and erectile dysfunction: An explorative review. J. Endocrinol. Investig. 2022, 45, 483–487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seehusen, F.; Clark, J.J.; Sharma, P.; Bentley, E.G.; Kirby, A.; Subramaniam, K.; Wunderlin-Giuliani, S.; Hughes, G.L.; Patterson, E.I.; Michael, B.D.; et al. Neuroinvasion and Neurotropism by SARS-CoV-2 Variants in the K18-hACE2 Mouse. Viruses 2022, 14, 1020. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.F.; Yan, L.-M.; Chin, A.W.H.; Fung, K.; Choy, K.-T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.A.P.M.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Laksono, B.M.; de Vrij, F.M.S.; Kushner, S.A.; Harschnitz, O.; van Riel, D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022, 45, 358–368. [Google Scholar] [CrossRef]
- Jacob, F.; Pather, S.R.; Huang, W.-K.; Zhang, F.; Hao Wong, S.Z.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem. Cell 2020, 27, 937–950. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dean, R.C.; Lue, T.F. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol. Clin. N. Am. 2005, 32, 379–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacDonald, S.M.; Burnett, A.L. Physiology of Erection and Pathophysiology of Erectile Dysfunction. Urol. Clin. N. Am. 2021, 48, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Polat, S.; Karkin, K.; Gürlen, G.; Aydamirov, M. Can anxiety and depression serve as primary factors associated with erectile dysfunction after coronavirus disease? Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2314–2319. [Google Scholar] [CrossRef] [PubMed]
- Harirugsakul, K.; Wainipitapong, S.; Phannajit, J.; Paitoonpong, L.; Tantiwongse, K. Erectile dysfunction after COVID-19 recovery: A follow-up study. PLoS ONE 2022, 17, 0276429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.; Li, C.; Song, J. COVID-19 infection may reduce serum testosterone levels and increase the risk of erectile dysfunction: A two-sample Mendelian randomization study. Investig. Clin. Urol. 2025, 66, 152–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbagallo, F.; Calogero, A.E.; Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; Aversa, A.; La Vignera, S. The testis in patients with COVID-19: Virus reservoir or immunization resource? Transl. Androl. Urol. 2020, 9, 1897–1900. [Google Scholar] [CrossRef]
- Çayan, S.; Uğuz, M.; Saylam, B.; Akbay, E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: A cohort study. Aging Male 2021, 23, 1493–1503. [Google Scholar] [CrossRef]
- Minhas, S.; Boeri, L.; Capogrosso, P.; Cocci, A.; Corona, G.; Dinkelman-Smit, M.; Falcone, M.; Jensen, C.F.; Gül, M.; Kalkanli, A.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2025 Update on Male Infertility. Eur. Urol. 2025, 87, 601–616. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropolous, K.; Gül, M.; et al. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 25, 333–357. [Google Scholar] [CrossRef]
- Burnett, A.L.; Nehra, A.; Breau, R.H.; Culkin, D.J.; Faraday, M.M.; Hakim, L.S.; Heidelbaugh, J.; Khera, M.; McVary, K.T.; Miner, M.M.; et al. Erectile Dysfunction: AUA Guideline. J. Urol. 2018, 200, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Sharifi, R. Non-invasive Management Options for Erectile Dysfunction When a Phosphodiesterase Type 5 Inhibitor Fails. Drugs Aging 2018, 35, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chen, Y.; Yan, J.; Christensen, G.; Belhadj, S.; Tolone, A.; Paquet-Durand, F. The role of cGMP-signalling and calcium-signalling in photoreceptor cell death: Perspectives for therapy development. Pflug. Arch. 2021, 473, 1411–1421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matieli, L.; Berezovsky, A.; Salomao, S.R.; Allemann, N.; Martins, E.N.; Hirai, F.E.; Ota-Arakaki, J.; Morales, M.S.; de Freitas, D. Ocular toxicity assessment of chronic sildenafil therapy for pulmonary arterial hypertension. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1167–1174. [Google Scholar] [CrossRef]
- Barroso, F.; Ribeiro, J.C.; Miranda, E.P. Phosphodiesterase Type 5 Inhibitors and Visual Side Effects: A Narrative Review. J. Ophthalmic. Vis. Res. 2021, 16, 248–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonanni, M.; Rehak, L.; Massaro, G.; Benedetto, D.; Matteucci, A.; Russo, G.; Esperto, F.; Federici, M.; Mauriello, A.; Sangiorgi, G.M. Autologous Immune Cell-Based Regenerative Therapies to Treat Vasculogenic Erectile Dysfunction: Is the Immuno-Centric Revolution Ready for the Prime Time? Biomedicines 2022, 10, 1091. [Google Scholar] [CrossRef]
- Epifanova, M.V.; Gvasalia, B.R.; Durashov, M.A.; Artemenko, S.A. Platelet-Rich Plasma Therapy for Male Sexual Dysfunction: Myth or Reality? Sex. Med. Rev. 2020, 8, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Furtado, T.P.; Saffati, G.; Furtado, M.H.; Khera, M. Stem cell therapy for erectile dysfunction: A systematic review. Sex. Med. Rev. 2023, 12, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, V.A.; Lisco, G.; De Tullio, A.; Guastamacchia, E.; Triggiani, V.; Jirillo, E. The pathogenic role of the immune system in erectile dysfunction and Peyronie’s disease: Focusing on immunopathophysiology and potential therapeutic strategies. Sex. Med. Rev. 2024, 12, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Chang, M.L.; Wang, Y.C.; Chen, W.H.; Wu, C.C.; Yeh, S.D. Revisiting the Regenerative Therapeutic Advances Towards Erectile Dysfunction. Cells 2020, 9, 1250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, S.; Sun, S.; Guo, F.; Liu, H. Efficacy of platelet-rich plasma in the treatment of erectile dysfunction: A meta-analysis of controlled and single-arm trials. PLoS ONE 2024, 19, e0313074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Li, Z.; Han, Z.; Chen, Y.; Xu, Y.; Xu, J.; Hu, H.; Ou, N.; Zheng, X.; Yin, Y.; et al. The critical role of PDGFRa + Sca1 + fibroblasts in angiogenesis and vascular repair in the corpus cavernosum. Stem Cell Res. Ther. 2025, 16, 313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fridayana, F.R.; Rho, B.Y.; Yin, G.N.; Ry, J.K. A narrative review of the distribution and role of multipotent fibroblasts in penile tissue: Implications for regenerative medicine and erectile dysfunction. Transl. Androl. Urol. 2025, 14, 2769–2780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suçeken, F.Y.; Coşer, Ş.; Kurtuluş, D.; Akgül, M.; Kül, K.; Sevim, M.; Ekşi, M.; Küçük, E.V.; Aras, B. Transcutaneous electrical nerve stimulation (TENS) therapy in rehabilitating erectile dysfunction after bilateral nerve sparing robotic assisted radical prostatectomy. World J. Urol. 2025, 43, 337. [Google Scholar] [CrossRef] [PubMed]
- Carboni, C.; Fornari, A.; Bragante, K.C.; Averbeck, M.A.; Vianna da Rosa, P.; Mea Plentz, R.D. An initial study on the effect of functional electrical stimulation in erectile dysfunction: A randomized controlled trial. Int. J. Impot. Res. 2018, 30, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.M.; Liu, K.F.; Hu, W.T.; Zhu, P.Y.; Xu, B.; Wang, Z.H.; Gong, Y.Z. Transcutaneous neuromuscular electrical stimulation for the treatment of erectile dysfunction: A clinical observation. Zhonghua Nan Ke Xue 2022, 28, 691–695. (In Chinese) [Google Scholar] [PubMed]
- Yang, L.; Liu, G.; Jiang, D.; Lin, G.; Ren, Z.; Fan, H.; Yang, B.; Mu, L.; Lue, T.F.; He, D. Effect of near-infrared laser treatment on improving erectile function in rats with diabetes mellitus. Andrology 2023, 11, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, J.; Fan, Y.; Wu, M. Influence of 980-nm Diode Laser Vaporization on Sexual Function: A Short-Term Follow-Up Study. J. Endourol. 2018, 32, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Mussttaf, R.A.; Jenkins, D.F.L.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Allameh, F.; Razzaghi, M.; Rayegani, S.M.; Fallah-Karkan, M.; Ranjbar, A.; Rahavian, A.; Javadi, A.; Ghiasy, S.; Razzaghi, Z. Laser Therapy for Peyronie’s Disease: A Randomized Control Double-Blind Pilot Study. J. Lasers Med. Sci. 2019, 10, S37–S42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, X.; Jiang, W.; Guo, X.; Du, C.; Wang, X.; Tian, Y.; An, H.; Wang, J.; Luo, Y.; Guo, Y.; et al. Laser boosting the influx of calcium ions to enhance gasdermin e-dependent pyroptosis driven by a dual-layer polydopamine nanoagonist. Chem. Eng. J. 2023, 476, 146748. [Google Scholar] [CrossRef]
- Shannon, E.K.; Stevens, A.; Edrington, W.; Zhao, Y.; Jayasinghe, A.K.; Page-McCaw, A.; Hutson, M.S. Multiple Mechanisms Drive Calcium Signal Dynamics around Laser-Induced Epithelial Wounds. Biophys. J. 2017, 113, 1623–1635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiu, W.-T.; Chang, H.-A.; Lin, Y.-H.; Lin, Y.-S.; Chang, H.-T.; Lin, H.-H.; Huang, S.-C.; Tang, M.-J.; Shen, M.-R. Bcl-2 regulates store-operated Ca2+ entry to modulate ER stress-induced apoptosis. Cell Death Discov. 2018, 4, 37. [Google Scholar] [CrossRef]
- Andrew, P.J.; Mayer, B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999, 43, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ankri, R.; Friedman, H.; Savion, N.; Kotev-Emeth, S.; Breitbart, H.; Lubart, R. Visible light induces nitric oxide (NO) formation in sperm and endothelial cells. Lasers Surg. Med. 2010, 42, 348–352. [Google Scholar] [CrossRef]
- Dabbous, O.A.; Soliman, M.M.; Mohamed, N.H.; Elseify, M.Y.; Elsheikh, M.S.; Alsharkawy, A.A.A.; al Aziz, M.M.A. Evaluation of the improvement effect of laser acupuncture biostimulation in asthmatic children by exhaled inflammatory biomarker level of nitric oxide. Lasers Med. Sci. 2017, 32, 53–59. [Google Scholar] [CrossRef]
- Patel, D.V.; Halls, J.; Patel, U. Investigation of erectile dysfunction. Br. J. Radiol. 2012, 85, S69–S78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moskvin, S.; Askhadulin, E.; Kochetkov, A. Low-Level Laser Therapy in Prevention of the Development of Endothelial Dysfunction and Clinical Experience of Treatment and Rehabilitation of COVID-19 Patients. Rehabil. Res. Pract. 2021, 2021, 6626932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Zamil, M.; Minenko, I.A.; Kulikova, N.G.; Alade, M.; Petrova, M.M.; Pronina, E.A.; Romanova, I.V.; Narodova, E.A.; Nasyrova, R.F.; Shnayder, N.A. Clinical Experience of High Frequency and Low Frequency TENS in Treatment of Diabetic Neuropathic Pain in Russia. Healthcare 2022, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.; Kulikova, N.G.; Minenko, I.A.; Shurygina, I.P.; Petrova, M.M.; Mansur, N.; Kuliev, R.R.; Blinova, V.V.; Khripunova, O.V.; Shnayder, N.A. Comparative Analysis of High-Frequency and Low-Frequency Transcutaneous Electrical Stimulation of the Right Median Nerve in the Regression of Clinical and Neurophysiological Manifestations of Generalized Anxiety Disorder. J. Clin. Med. 2024, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.; Minenko, I.A.; Kulikova, N.G.; Mansur, N.; Nuvakhova, M.B.; Khripunova, O.V.; Shurygina, I.P.; Topolyanskaya, S.V.; Trefilova, V.V.; Petrova, M.M.; et al. Efficiency of Direct Transcutaneous Electroneurostimulation of the Median Nerve in the Regression of Residual Neurological Symptoms after Carpal Tunnel Decompression Surgery. Biomedicines 2023, 11, 2396. [Google Scholar] [CrossRef]
- Alarcón, J.B.; Chuhuaicura, P.B.; Sluka, K.A.; Vance, C.G.T.; Fazan, V.P.S.; Godoy, K.A.; Fuentes, R.E.; Dias, F.J. Transcutaneous Electrical Nerve Stimulation in Nerve Regeneration: A Systematic Review of In Vivo Animal Model Studies. Neuromodulation 2022, 25, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Ryu, O.; Cho, K.; Kim, D.; Lim, J.; Hong, S.H.; Cho, Y.S. The effect of charge-balanced transcutaneous electrical nerve stimulation on rodent facial nerve regeneration. Sci. Rep. 2022, 12, 1388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Zamil, M.; Kulikova, N.G.; Minenko, I.A.; Mansur, N.; Zalozhnev, D.M.; Uzdenov, M.B.; Dzhanibekova, A.A.; Gochiyayev, A.A.; Shnayder, N.A. Functional Recovery and Regenerative Effects of Direct Transcutaneous Electrical Nerve Stimulation in Treatment of Post-COVID-19 Guillain–Barré and Acute Transverse Myelitis Overlap Syndrome: A Clinical Case. J. Funct. Morphol. Kinesiol. 2024, 9, 40. [Google Scholar] [CrossRef]
- Al-Zamil, M.; Minenko, I.A.; Shnayder, N.A.; Petrova, M.M.; Babochkina, Z.M.; Kaskaeva, D.S.; Lim, V.G.; Khripunova, O.V.; Shurygina, I.P.; Garganeeva, N.P. Recovery and Protective Effect of Direct Transcutaneous Electrical Nerve Stimulation in the Treatment of Acute and Subacute Fibular Tunnel Syndrome. J. Clin. Med. 2025, 14, 4247. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.; Kulikova, N.G.; Shnayder, N.A.; Korchazhkina, N.B.; Petrova, M.M.; Mansur, N.; Smekalkina, L.V.; Babochkina, Z.M.; Vasilyeva, E.S.; Zhhelambekov, I.V. Spatial Distribution Dynamics of Sensory Disturbances in the Treatment of Obesity-Related Meralgia Paresthetica Using Transcutaneous Electrical Nerve Stimulation. J. Clin. Med. 2025, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Green, M.; Tram, J.; Wang, E.; Murphy, M.; Abd-Elsayed, A.A.; Chakravarthy, K. Latest Advancements in Transcutaneous Electrical Nerve Stimulation (TENS) and Electronic Muscle Stimulation (EMS): Revisiting an Established Therapy with New Possibilities. J. Pain Res. 2025, 18, 137–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishikawa, M.; Miura, H.; Tamura, Y.; Murakami, A. Effect of Electrical Muscle Stimulation on Vascular Endothelial Function during Prolonged Sitting. Phys. Ther. Res. 2022, 25, 127–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sherry, J.E.; Oehrlein, K.M.; Hegge, K.S.; Morgan, B.J. Effect of burst-mode transcutaneous electrical nerve stimulation on peripheral vascular resistance. Phys. Ther. 2001, 81, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.J.; Ribeiro, J.P.; Cipriano, G., Jr.; Umpierre, D.; Cahalin, L.P.; Moraes, R.S.; Chiappa, G.R. Effect of transcutaneous electrical nerve stimulation on muscle metaboreflex in healthy young and older subjects. Eur. J. Appl. Physiol. 2012, 112, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Puzin, M.N. Application of TENS in the treatment of erectile dysfunction. Clin. Neurol. 2024, 1, 9–11. Available online: https://elibrary.ru/item.asp?id=82823883 (accessed on 12 January 2024).
- Neijenhuijs, K.I.; Holtmaat, K.; Aaronson, N.K.; Holzner, B.; Terwee, C.B.; Cuijpers, P.; Verdonck-de Leeuw, I.M. The International Index of Erectile Function (IIEF)-A Systematic Review of Measurement Properties. J. Sex. Med. 2019, 16, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Malladi, P.; Simeoni, S.; Panicker, J.N. The Role of Pelvic Neurophysiology Testing in the Assessment of Patients with Voiding Dysfunction. Curr. Bladder Dysfunct. Rep. 2020, 15, 229–239. [Google Scholar] [CrossRef]
- Lau, B.M.; Lee, J.D.; Sanchez-Vidaña, D.I.; Chan, J.M.; Lau, W.W.; So, K.F. The Neural correlates of COVID-19-induced erectile dysfunction in males. Ageing Neur. Dis. 2023, 3, 8. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, T.; Yang, P.; Gao, R.; Shen, L.; Gao, P.; Gao, J.; Liu, X.; Jiang, H.; Zhang, X. The neurological decline and psychological factors caused by coronavirus disease 2019 may be predictors of erectile dysfunction. Andrology 2024, 12, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.K.; Zalozhnev, D.M.; Vasilieva, E.S.; Markosyan, T.G.; Kozyrkova, N.V.; Maksimova, E.A.; Surkova, M.A.; Korchazhkina, N.B. The effectiveness of low-level laser therapy in the treatment of patients with post-COVID-19 erectile dysfunction. Russ. J. Physiother. Balneol. Rehabil. 2023, 22, 429–437. [Google Scholar] [CrossRef]
- Asfandiyarov, F.R.; Kruglov, V.A.; Vybornov, S.V.; Seidov, K.S.; Nersesyan, A.Y.; Kruglova, E.Y. Post-COVID-19 transient hypogonadism and erectile dysfunction. Exp. Clin. Urol. 2021, 3, 112–118. [Google Scholar] [CrossRef]
- Gök, A.; Altan, M.; Doğan, A.E.; Eraslan, A.; Uysal, F.Ş.; Öztürk, U.; Saguner, A.M.; İmamoğlu, M.A. Does Post-COVID-19 Erectile Dysfunction Improve over Time? J. Clin. Med. 2023, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Moskvin, S.V.; Ivanchenko, L.P. Chronobiological approach to the treatment of patients with erectile dysfunction using a combination of local negative pressure and laser illumination. Urologiia 2014, 3, 48–53. (In Russian). Available online: https://journals.eco-vector.com/1728-2985/article/view/280195 (accessed on 5 August 2024). [PubMed]
- Chu, Y.H.; Chen, S.Y.; Hsieh, Y.L.; Teng, Y.H.; Cheng, Y.J. Low-level laser therapy prevents endothelial cells from TNF-α/cycloheximide-induced apoptosis. Lasers Med. Sci. 2018, 33, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.Y.; de Sousa, M.V.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. Photochem. Photobiol. 2015, 91, 411–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.Y.; Nagata, K.; Tedford, C.E.; McCarthy, T.; Hamblin, M.R. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J. Biophotonics 2013, 6, 829–838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tunç, S.; Altuntaş, Ş.L.; Atmaca, M.; Çakıcı, Ç.; Yiğitbaşı, T.; Liou, Y.C.; Chang, W.A. The effect of low-level laser therapy on the oxidative stress level and quality of life in patients with Hashimoto’s thyroiditis. Free Radic. Res. 2024, 58, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magied, N.; Elkady, A.A.; Abdel Fattah, S.M. Effect of Low-Level Laser on Some Metals Related to Redox State and Histological Alterations in the Liver and Kidney of Irradiated Rats. Biol. Trace Elem. Res. 2020, 194, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; He, Y.; Ni, W.; Zhang, Y.; Zhu, Y.; Cao, M.; He, R.; Yao, M. LLLT accelerates experimental wound healing under microgravity conditions via PI3K/AKT-CCR2 signal axis. Front. Bioeng. Biotechnol. 2024, 12, 1387474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019, 50, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Agas, D.; Hanna, R.; Benedicenti, S.; De Angelis, N.; Sabbieti, M.G.; Amaroli, A. Photobiomodulation by Near-Infrared 980-nm Wavelengths Regulates Pre-Osteoblast Proliferation and Viability through the PI3K/Akt/Bcl-2 Pathway. Int. J. Mol. Sci. 2021, 22, 7586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moskvin, S.V. Low-Level Laser Therapy in Russia: History, Science and Practice. J. Lasers Med. Sci. 2017, 8, 56–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Araki, S.; Osuka, K.; Takata, T.; Tsuchiya, Y.; Watanabe, Y. Coordination between Calcium/Calmodulin-Dependent Protein Kinase II and Neuronal Nitric Oxide Synthase in Neurons. Int. J. Mol. Sci. 2020, 21, 7997. [Google Scholar] [CrossRef]
- Benza, R.L.; Grünig, E.; Sandner, P.; Stasch, J.P.; Simonneau, G. The nitric oxide-soluble guanylate cyclase-cGMP pathway in pulmonary hypertension: From PDE5 to soluble guanylate cyclase. Eur. Respir. Rev. 2024, 33, 230183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Decaluwé, K.; Pauwels, B.; Boydens, C.; Thoonen, R.; Buys, E.S.; Brouckaert, P.; Van de Voorde, J. Erectile Dysfunction in Heme-Deficient Nitric Oxide-Unresponsive Soluble Guanylate Cyclase Knock-In Mice. J. Sex. Med. 2017, 14, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Zikopoulos, A.; Dimitriadis, F.; Sheshi, D.; Politis, M.; Moustakli, E.; Symeonidis, E.N.; Chrisofos, M.; Sofikitis, N.; Zachariou, A. Oxidative Stress and Erectile Dysfunction: Pathophysiology, Impacts, and Potential Treatments. Curr. Issues Mol. Biol. 2024, 46, 8807–8834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simmers, P.; Gishto, A.; Vyavahare, N.; Kothapalli, C.R. Nitric oxide stimulates matrix synthesis and deposition by adult human aortic smooth muscle cells within three-dimensional cocultures. Tissue Eng. Part A 2015, 21, 1455–1470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tahmasbi, F. Application of transcutaneous electrical nerve stimulation (TENS) for restoring sexual function after gender-affirming genital reconstruction: A hypothesis. Ther. Adv. Urol. 2025, 17, 17562872251358125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tahmasbi, F.; Rahimi-Mamaghani, A.; Soleimanzadeh, F.; Salehi-Pourmehr, H.; Mohammad-Rahimi, M. Application of peripheral electrical stimulation for treatment of erectile dysfunction: A systematic review and meta-analysis. Sex. Med. Rev. 2025, 13, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.K.; Zalozhnev, D.M.; Kulikova, N.G.; Vasilieva, E.S. The combination of electrotherapy with pelvic floor muscle training in the treatment of a patient with postcovid erectile dysfunction. Clinical case. Russ. J. Physiother. Balneol. Rehabil. 2023, 22, 307–314. [Google Scholar] [CrossRef]
- Jin, M.Y.; D’Souza, R.S.; Abd-Elsayed, A.A. Efficacy of Neuromodulation Interventions for the Treatment of Sexual Dysfunction: A Systematic Review. Neuromodulation 2023, 26, 1518–1534. [Google Scholar] [CrossRef] [PubMed]
- Pescatori, E.S.; Calabro, A.; Artibani, W.; Pagano, F.; Triban, C.; Italiano, G. Electrical stimulation of the dorsal nerve of the penis evokes reflex tonic erections of the penile body and reflex ejaculatory responses in the spinal rat. J. Urol. 1993, 149, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Shendy, W.; El Semary, M.; Battecha, K.; Abdel-Azim, M.; Mourad, H.; El Gohary, A. Efficacy of transcutaneous electrical nerve stimulation versus biofeedback training on bladder and erectile dysfunction in patients with spinal cord injury. Egypt. J. Neurol. Psychiatry Neurosurg. 2015, 52, 194. [Google Scholar] [CrossRef]
- Lavoisier, P.; Roy, P.; Dantony, E.; Watrelot, A.; Ruggeri, J.; Dumoulin, S. Pelvic-Floor Muscle Rehabilitation in Erectile Dysfunction and Premature Ejaculation. Phys. Ther. 2014, 94, 1731–1743. [Google Scholar] [CrossRef]
- Ovchinnikov, R.; Pyatnitskiy, I. Pelvic Floor Muscle Anatomy and its Contribution to Penile Erection in Olive Baboons. Urol. Res. Pract. 2024, 50, 173–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toda, N.; Ayajiki, K.; Okamura, T. Nitric oxide and penile erectile function. Pharmacol. Ther. 2005, 106, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Rampin, O. Contrôle nerveux de l’érection [Neural control of erection]. J. Soc. Biol. 2004, 198, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F. Neurophysiology of Erection and Ejaculation. J. Sex. Med. 2011, 8, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Koca Kutlu, A.; Ceçen, D.; Gürgen, S.G.; Sayın, O.; Cetin, F. A Comparison Study of Growth Factor Expression following Treatment with Transcutaneous Electrical Nerve Stimulation, Saline Solution, Povidone-Iodine, and Lavender Oil in Wounds Healing. Evid. Based Complement Alternat. Med. 2013, 2013, 361832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bevilacqua, M.; Dominguez, L.J.; Barrella, M.; Barbagallo, M. Induction of vascular endothelial growth factor release by transcutaneous frequency modulated neural stimulation in diabetic polyneuropathy. J. Endocrinol. Investig. 2007, 30, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Cebalo, N.; Negovetić Vranić, D.; Bašić Kes, V. The Effect of Transcutaneous Electric Nerve Stimulation (TENS) on Anxiety and Fear in Children Aged 9-14 Years. Acta Stomatol. Croat 2020, 54, 412–419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cebalo, N.; Cebalo, J.; Budak, L.; Vranić, L.; Jurišić Kvesić, A.; Bašić Kes, V.; Negovetić Vranić, D. Use of transcutaneous electrical nerve stimulation (TENS) to reduce anxiety in pediatric dentistry. Dent. Med. Probl. 2025, 62, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Qiao, M.; Ma, Y.; Luo, Y.; Fang, J.; Yang, Y. The efficacy and safety of transcutaneous auricular vagus nerve stimulation in the treatment of depressive disorder: A systematic review and meta-analysis of randomized controlled trials. J. Affect. Disord. 2023, 337, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xie, T.; Peng, J.; Zhou, X.; Long, J.; Yang, M.; Zhu, H.; Yang, J. Factors associated with anxiety and depression in patients with erectile dysfunction: A cross-sectional study. BMC Psychol. 2023, 11, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krassioukov, A.; Elliott, S. Neural Control and Physiology of Sexual Function: Effect of Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2017, 23, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Min, S.; Xu, J.; Ren, C.; Cai, Z.; Li, H.; Wang, Z. The correlation between premature ejaculation and a high incidence of erectile dysfunction and its research progress: A narrative review. Transl. Androl. Urol. 2024, 13, 2338–2350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ševčíková, A.; Gottfried, J.; Gocieková, V.; Gore-Gorszewska, G.; Blinka, L.; Kotík, J. The Role of Non-penetrative Partnered Sex Activities in the Associations Among Erectile Difficulties, Sex and Relationship Satisfaction in Men Aged 50. Int. J. Sex. Health 2023, 35, 30–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, V.; Dolendo, I.; Uloko, M.; Hsieh, T.C.; Patel, D. Male delayed orgasm and anorgasmia: A practical guide for sexual medicine providers. Int. J. Impot. Res. 2024, 36, 186–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasan, P.; A Rijadi, S.; Purnomo, S.; Kainama, H. The Possible Application of low Reactive-Level Laser Therapy (Lllt) in the Treatment of Male Infertility: A preliminary report. Laser Ther. 2004, 14, 65–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moussa, M.; Chakra, M.A.; Dabboucy, B.; Fares, Y.; Dellis, A.; Papatsoris, A. Transcutaneous dorsal penile nerve stimulation for the treatment of premature ejaculation: A novel technique. Asian J. Urol. 2022, 9, 337–339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corona, G.; Maggi, M. The role of testosterone in male sexual function. Rev. Endocr. Metab. Disord. 2022, 23, 1159–1172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montejo, A.L.; Montejo, L.; Baldwin, D.S. The impact of severe mental disorders and psychotropic medications on sexual health and its implications for clinical management. World Psychiatry 2018, 17, 3–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bravi, C.A.; Tin, A.; Montorsi, F.; Mulhall, J.P.; Eastham, J.A.; Vickers, A.J. Erectile Function and Sexual Satisfaction: The Importance of Asking About Sexual Desire. J. Sex. Med. 2020, 17, 349–352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moskvin, S.V.; Apolikhin, O.I. Effectiveness of low level laser therapy for treating male infertility. BioMedicine 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuby, H.; Hertzberg, E.; Maltz, L.; Oron, U. Long-term safety of low-level laser therapy at different power densities and single or multiple applications to the bone marrow in mice. Photomed. Laser Surg. 2013, 31, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Stausholm, M.B.; Naterstad, I.F.; Alfredo, P.P.; Couppé, C.; Fersum, K.V.; Leal-Junior, E.C.P.; Lopes-Martins, R.Á.B.; Joensen, J.; Bjordal, J.M. Short- and Long-Term Effectiveness of Low-Level Laser Therapy Combined with Strength Training in Knee Osteoarthritis: A Randomized Placebo-Controlled Trial. J. Clin. Med. 2022, 11, 3446. [Google Scholar] [CrossRef]
- Lawrence, J.; Sorra, K. Photobiomodulation as Medicine: Low-Level Laser Therapy (LLLT) for Acute Tissue Injury or Sport Performance Recovery. J. Funct. Morphol. Kinesiol. 2024, 9, 181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maldaner, D.R.; Azzolin, V.F.; Barbisan, F.; Mastela, M.H.; Teixeira, C.F.; Dihel, A.; Duarte, T.; Pellenz, N.L.; Lemos, L.F.C.; Negretto, C.M.U.; et al. In vitro effect of low-level laser therapy on the proliferative, apoptosis modulation, and oxi-inflammatory markers of premature-senescent hydrogen peroxide-induced dermal fibroblasts. Lasers Med. Sci. 2019, 34, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.d.S.; de Oliveira, H.A.; Teixeira, I.L.A.; Antonio, E.L.; Silva, F.A.; Sunemi, S.; Leal-Junior, E.C.; Carvalho, P.d.T.C.d.; Tucci, P.J.F.; Serra, A.J. Low-level laser therapy prevents muscle apoptosis induced by a high-intensity resistance exercise in a dose-dependent manner. Lasers Med. Sci. 2020, 35, 1867–1870. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Edvinsson, L.; Laher, I. Smoking and Endothelial Dysfunction. Curr. Vasc. Pharmacol. 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leite, L.N.; do Vale, G.T.; Simplicio, J.A.; De Martinis, B.S.; Carneiro, F.S.; Tirapelli, C.R. Ethanol-induced erectile dysfunction and increased expression of pro-inflammatory proteins in the rat cavernosal smooth muscle are mediated by NADPH oxidase-derived reactive oxygen species. Eur. J. Pharmacol. 2017, 804, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Podlutsky, A.; Wolin, M.S.; Losonczy, G.; Pacher, P.; Ungvari, Z. Oxidative stress and accelerated vascular aging: Implications for cigarette smoking. Front. Biosci. 2009, 14, 3128–3144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Su, M.; Liu, G.; Wu, X.; Feng, X.; Tang, D.; Jiang, H.; Zhang, X. Chronic sleep deprivation induces erectile dysfunction through increased oxidative stress, apoptosis, endothelial dysfunction, and corporal fibrosis in a rat model. J. Sex. Med. 2024, 21, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, F.; Colalillo, G.; Finazzi Agrò, E.; Miano, R.; Fuschi, A.; Asimakopoulos, A.D. Endothelial Dysfunction, Erectile Deficit and Cardiovascular Disease: An Overview of the Pathogenetic Links. Biomedicines 2022, 10, 1848. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, E.C.; Oh, C.K.; Lee, S.; Yu, H.S.; Lim, J.S.; Kim, H.W.; Walsh, T.; Kim, M.H.; Jung, J.H.; et al. Testosterone replacement in men with sexual dysfunction. Cochrane Database Syst. Rev. 2024, 2024, CD013071. [Google Scholar] [CrossRef]
- Hostnik, B.; Tonin, G.; Janež, A.; Klen, J. Erectile Dysfunction in Diabetes Mellitus: A Comprehensive Narrative Review of Pathophysiology, Genetic Association Studies and Therapeutic Approaches. Endocrinol. Diabetes Metab. 2025, 8, e70099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Xu, W.; Wang, T.; Wang, S.; Liu, J.; Jiang, H. Effect of weight loss on erectile function in men with overweight or obesity: A meta-analysis of randomised controlled trials. Andrologia 2022, 54, e14250. [Google Scholar] [CrossRef] [PubMed]
- Velurajah, R.; Brunckhorst, O.; Waqar, M.; McMullen, I.; Ahmed, K. Erectile dysfunction in patients with anxiety disorders: A systematic review. Int. J. Impot. Res. 2022, 34, 177–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


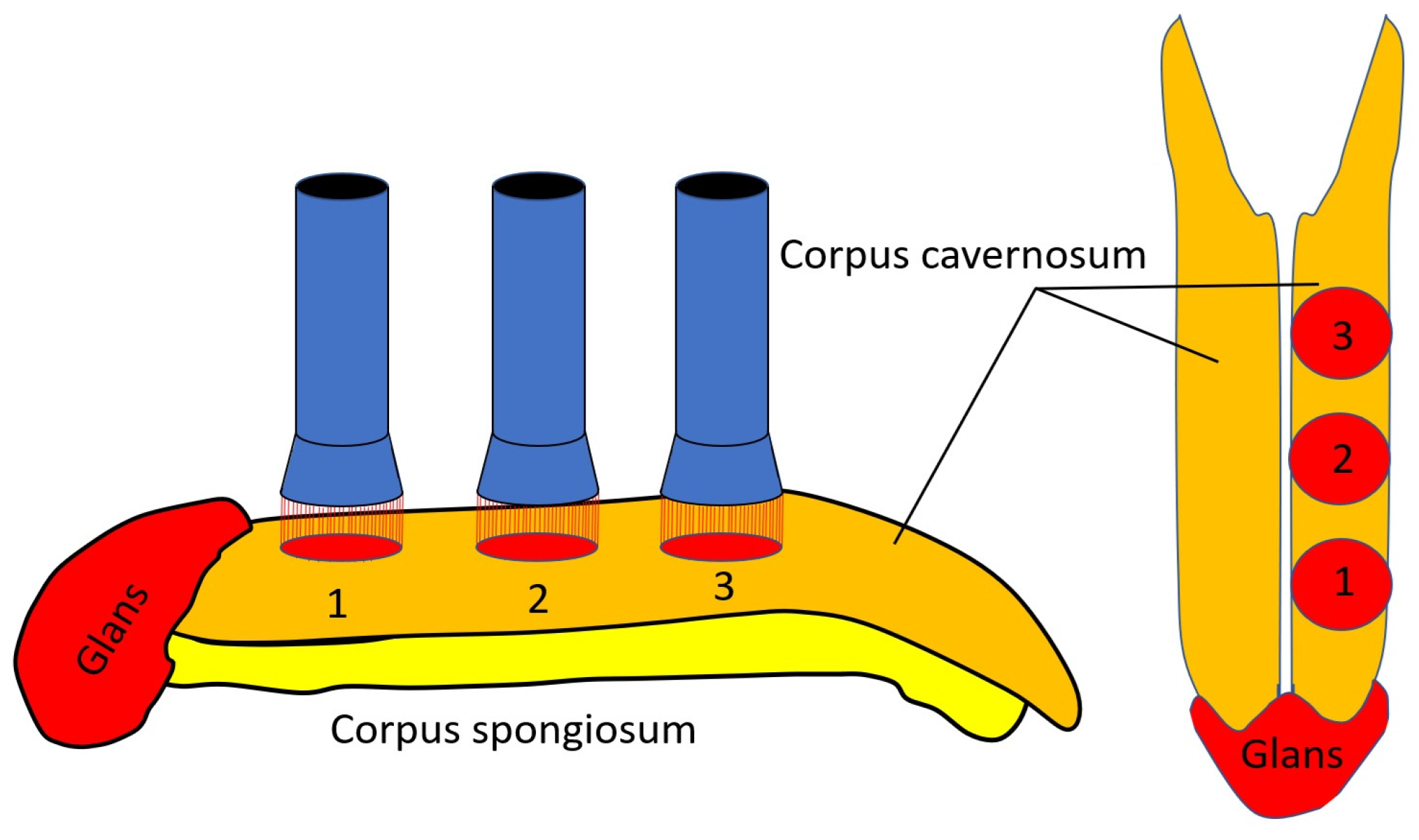
| Characteristic | Control Group | LLLT Group | TENS Group | LLLT + TENS Group | F * | p ** |
|---|---|---|---|---|---|---|
| Number of the participants, n | 20 | 21 | 21 | 20 | ||
| Age, years | 41.2 ± 7.7 | 41.2 ± 6.9 | 41.8 ± 6.5 | 41.5 ± 6.0 | 0.04 | 0.99 |
| Disease duration, months | 8.70 ± 1.9 | 8.90 ± 1.8 | 8.80 ± 1.6 | 8.60 ± 1.6 | 0.11 | 0.95 |
| Duration of completed pharmacotherapy, months | 2.53 ± 0.77 | 2.50 ± 0.63 | 2.70 ± 0.65 | 2.63 ± 0.67 | 0.37 | 0.78 |
| The erectile function domain, points | 16.3 ± 3.75 | 15.3 ± 3.51 | 15.4 ± 3.25 | 16.5 ± 3.36 | 0.22 | 0.87 |
| Hypoesthesia, points | 3.60 ± 0.92 | 3.83 ± 1.23 | 3.90 ± 1.12 | 3.83 ± 0.91 | 0.32 | 0.99 |
| Characteristic | Control Group | LLLT Group | TENS Group | LLLT + TENS Group | F * | p ** |
|---|---|---|---|---|---|---|
| Number of the participants, n | 9 | 8 | 13 | 8 | ||
| Polyphasic MUP (%) | 46.1 ± 10.5 | 39.4 ± 18.6 | 38.8 ± 10.4 | 24.4 ± 10.5 | 4.364 | 0.011 |
| Minimum (%) | 30 | 15 | 20 | 15 | ||
| Maximum (%) | 65 | 50 | 55 | 45 | ||
| Median (%) | 45 | 45 | 40 | 20 | ||
| Increase in MUP latency (%) | 37.8 ± 8.08 | 29.4 ± 12.4 | 23.6 ± 7.53 | 29.4 ± 10.2 | 4.041 | 0.015 |
| Minimum (%) | 25 | 15 | 10 | 15 | ||
| Maximum (%) | 50 | 50 | 35 | 45 | ||
| Median (%) | 35 | 27.5 | 25 | 27.5 | ||
| Increase in MUP amplitude | 11.7 ± 4.08 | 8.75 ± 5.99 | 15.0 ± 4.11 | 11.9 ± 5.21 | 2.917 | 0.048 |
| Minimum (%) | 5 | 0 | 10 | 0 | ||
| Maximum (%) | 20 | 15 | 20 | 20 | ||
| Median (%) | 10 | 10 | 15 | 12.5 |
| Characteristic | Before Treatment | After Treatment | t * | p ** | Cohen’s d |
|---|---|---|---|---|---|
| Number of the participants, n | 12 | 12 | |||
| Polyphasic MUP (%) | 33.8 ± 7.42 | 41.0 ± 8.51 | 2.20 | 0.03 | 0.90 |
| Minimum (%) | 25 | 25 | |||
| Maximum (%) | 50 | 55 | |||
| Median (%) | 35 | 37.5 | |||
| Increase in MUP latency (%) | 25.0 ± 7.38 | 35.4 ± 6.89 | 3.56 | 0.001 | 1.45 |
| Minimum (%) | 15 | 20 | |||
| Maximum (%) | 40 | 45 | |||
| Median (%) | 25 | 35 | |||
| Increase in MUP amplitude | 11.7 ± 4.08 | 18.7 ± 5.99 | 3.34 | 0.003 | 1.36 |
| Minimum (%) | 10 | 0 | |||
| Maximum (%) | 20 | 25 | |||
| Median (%) | 12.5 | 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zamil, M.; Kulikova, N.G.; Zalozhnev, D.M.; Shnayder, N.A.; Petrova, M.M.; Garganeeva, N.P.; Zhukova, N.G.; Tutinina, O.V.; Naprienko, M.V.; Smekalkina, L.V. Using the International Index of Erectile Function-15 in Comparative Analysis Between Transcutaneous Electrical Nerve Stimulation of the Pudendal Nerve and Low-Level Laser Therapy in the Treatment of Erectile Dysfunction After COVID-19. J. Clin. Med. 2025, 14, 8193. https://doi.org/10.3390/jcm14228193
Al-Zamil M, Kulikova NG, Zalozhnev DM, Shnayder NA, Petrova MM, Garganeeva NP, Zhukova NG, Tutinina OV, Naprienko MV, Smekalkina LV. Using the International Index of Erectile Function-15 in Comparative Analysis Between Transcutaneous Electrical Nerve Stimulation of the Pudendal Nerve and Low-Level Laser Therapy in the Treatment of Erectile Dysfunction After COVID-19. Journal of Clinical Medicine. 2025; 14(22):8193. https://doi.org/10.3390/jcm14228193
Chicago/Turabian StyleAl-Zamil, Mustafa, Natalia G. Kulikova, Denis M. Zalozhnev, Natalia A. Shnayder, Marina M. Petrova, Natalia P. Garganeeva, Natalia G. Zhukova, Olga V. Tutinina, Margarita V. Naprienko, and Larisa V. Smekalkina. 2025. "Using the International Index of Erectile Function-15 in Comparative Analysis Between Transcutaneous Electrical Nerve Stimulation of the Pudendal Nerve and Low-Level Laser Therapy in the Treatment of Erectile Dysfunction After COVID-19" Journal of Clinical Medicine 14, no. 22: 8193. https://doi.org/10.3390/jcm14228193
APA StyleAl-Zamil, M., Kulikova, N. G., Zalozhnev, D. M., Shnayder, N. A., Petrova, M. M., Garganeeva, N. P., Zhukova, N. G., Tutinina, O. V., Naprienko, M. V., & Smekalkina, L. V. (2025). Using the International Index of Erectile Function-15 in Comparative Analysis Between Transcutaneous Electrical Nerve Stimulation of the Pudendal Nerve and Low-Level Laser Therapy in the Treatment of Erectile Dysfunction After COVID-19. Journal of Clinical Medicine, 14(22), 8193. https://doi.org/10.3390/jcm14228193








