Long-Term Real-World Outcomes of Mavacamten in Symptomatic Obstructive Hypertrophic Cardiomyopathy up to 108 Weeks
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Exposure and Outcomes Data
2.3. Statistical Analyses
3. Results
Baseline Characteristics
4. 108-Week Outcomes
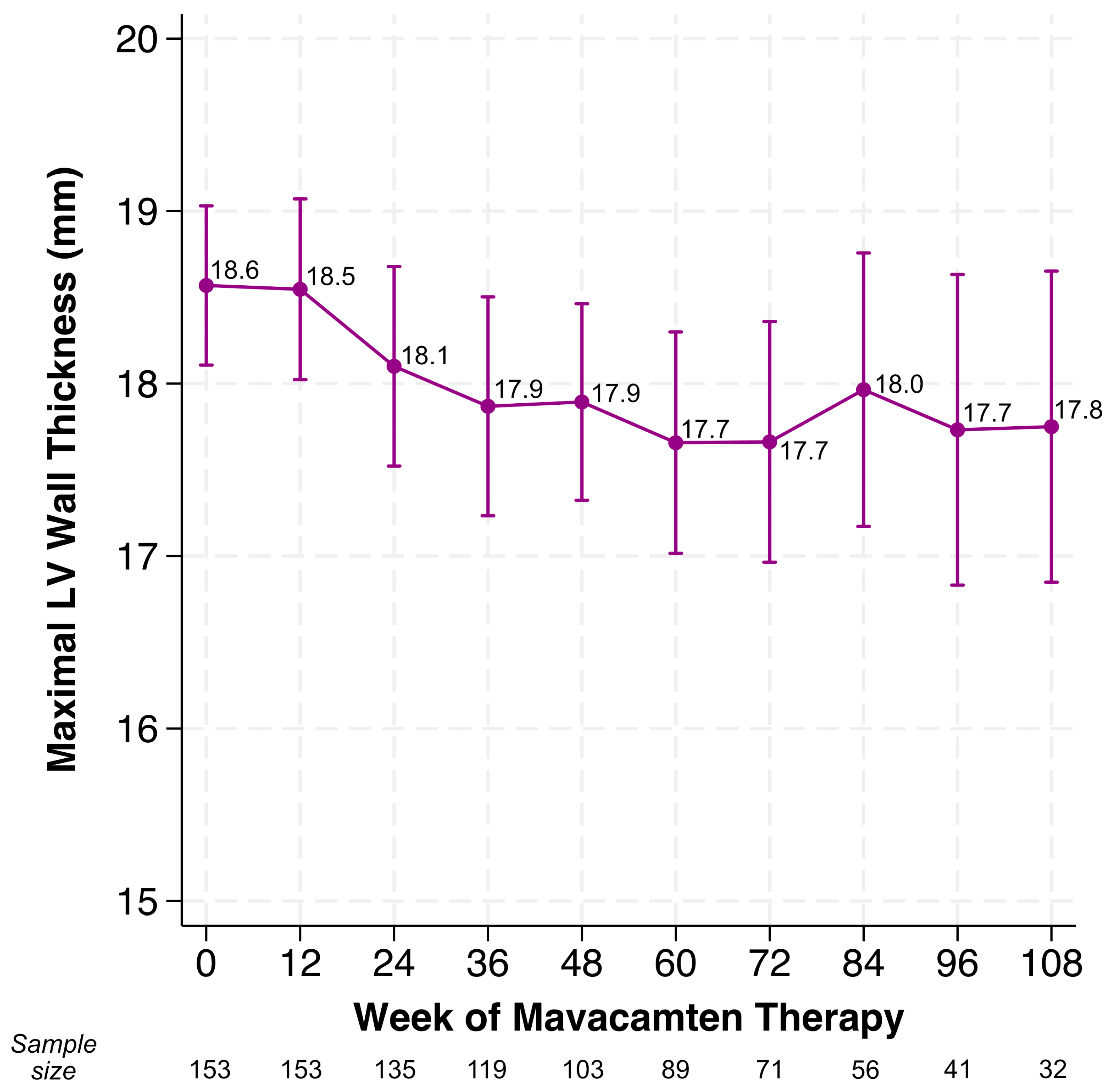
4.1. Changes in LVOT Gradients, LVEF, LV Wall Thickness
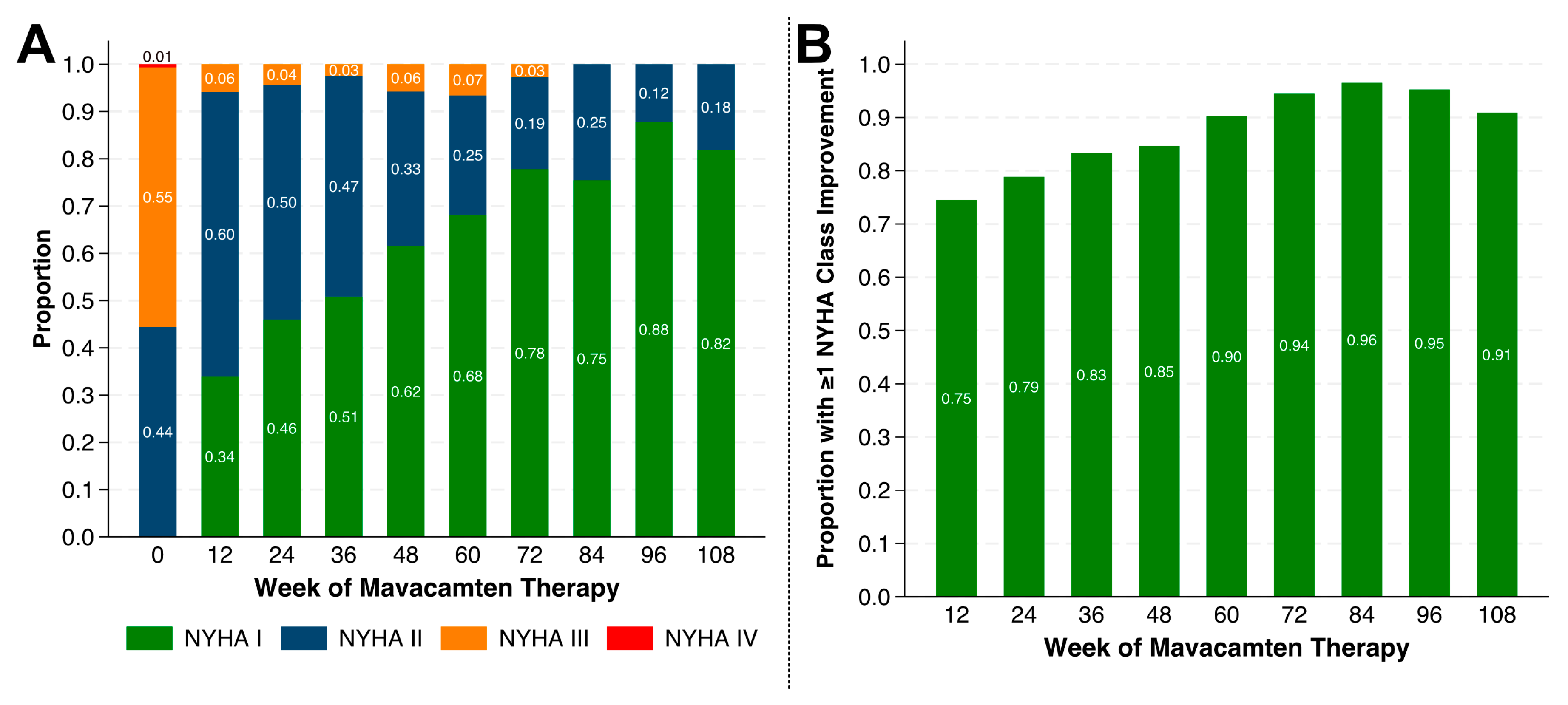
4.2. Changes in NYHA Class
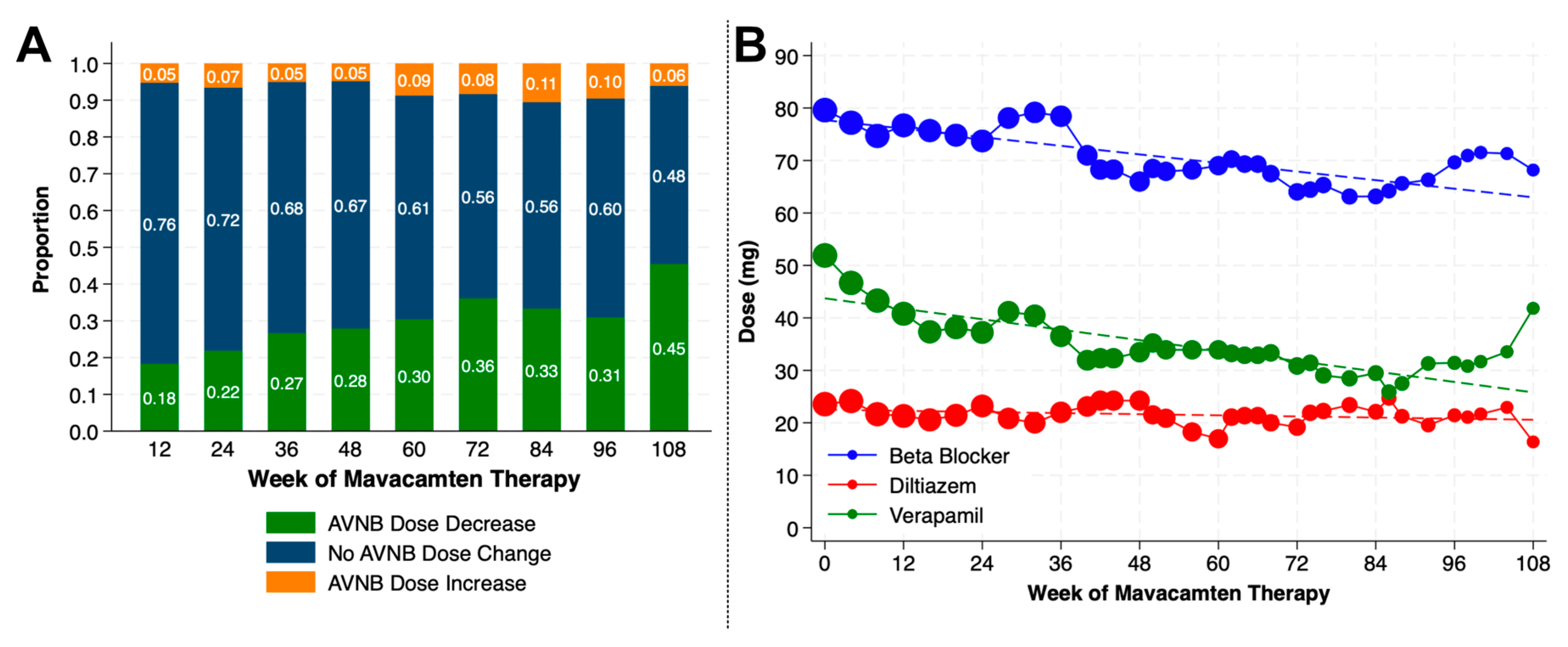
4.3. Changes in Background Cardiovascular Medications
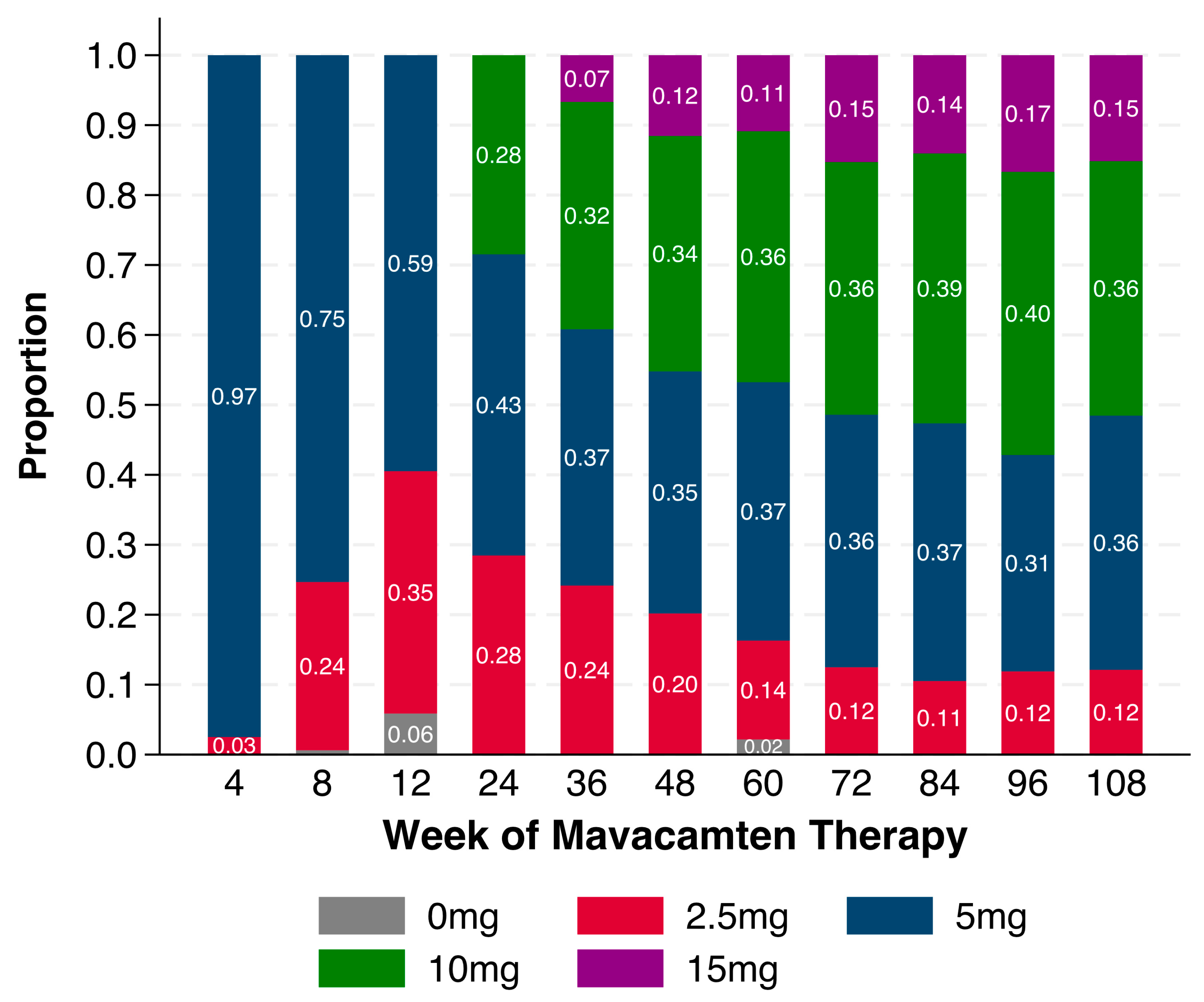
4.4. Mavacamten Dosing
4.5. Temporary Interruption for LVOT Gradient and LVEF
4.6. Permanent Discontinuation
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2024, 83, 2324–2405. [Google Scholar] [CrossRef]
- Cannon, R.O.; Rosing, D.R.; Maron, B.J.; Leon, M.B.; Bonow, R.O.; Watson, R.M.; Epstein, S.E. Myocardial ischemia in patients with hypertrophic cardiomyopathy: Contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation 1985, 71, 234–243. [Google Scholar] [CrossRef]
- Maron, B.A.; Kleiner, D.E.; Arons, E.; Wertheim, B.M.; Sharma, N.S.; Haley, K.J.; Samokhin, A.O.; Rowin, E.J.; Maron, M.S.; Rosing, D.R.; et al. Evidence of Advanced Pulmonary Vascular Remodeling in Obstructive Hypertrophic Cardiomyopathy with Pulmonary Hypertension. Chest 2023, 163, 678–686. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003, 348, 295–303. [Google Scholar] [CrossRef]
- Spertus, J.A.; Fine, J.T.; Elliott, P.; Ho, C.Y.; Olivotto, I.; Saberi, S. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): Health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Green, E.M.; Wakimoto, H.; Anderson, R.L.; Evanchik, M.J.; Gorham, J.M.; Harrison, B.C.; Henze, M.; Kawas, R.; Oslob, J.D.; Rodriguez, H.M.; et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 2016, 351, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Owens, A.; Geske, J.B.; Wolski, K.; Naidu, S.S.; Smedira, N.G.; Cremer, P.C.; Schaff, H.; McErlean, E.; Sewell, C.; et al. Myosin Inhibition in Patients with Obstructive Hypertrophic Cardiomyopathy Referred for Septal Reduction Therapy. J. Am. Coll. Cardiol. 2022, 80, 95–108. [Google Scholar] [CrossRef]
- Desai, M.Y.; Owens, A.; Wolski, K.; Geske, J.B.; Saberi, S.; Wang, A.; Sherrid, M.; Cremer, P.C.; Lakdawala, N.K.; Tower-Rader, A.; et al. Mavacamten in Obstructive Hypertrophic Cardiomyopathy Patients Referred for Septal Reduction: Health Status Analysis Through Week 56 in VALOR-HCM Trial. J. Am. Coll. Cardiol. 2024, 84, 1041–1045. [Google Scholar] [CrossRef]
- Hegde, S.M.; Lester, S.J.; Solomon, S.D.; Michels, M.; Elliott, P.M.; Nagueh, S.F.; Choudhury, L.; Zemanek, D.; Zwas, D.R.; Jacoby, D.; et al. Effect of Mavacamten on Echocardiographic Features in Symptomatic Patients with Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 2518–2532. [Google Scholar] [CrossRef]
- Saberi, S.; Cardim, N.; Yamani, M.; Schulz-Menger, J.; Li, W.; Florea, V.; Sehnert, A.J.; Kwong, R.Y.; Jerosch-Herold, M.; Masri, A.; et al. Mavacamten Favorably Impacts Cardiac Structure in Obstructive Hypertrophic Cardiomyopathy: EXPLORER-HCM Cardiac Magnetic Resonance Substudy Analysis. Circulation 2021, 143, 606–608. [Google Scholar] [CrossRef]
- Cremer, P.C.; Geske, J.B.; Owens, A.; Jaber, W.A.; Harb, S.C.; Saberi, S.; Wang, A.; Sherrid, M.; Naidu, S.; Schaff, H.; et al. Myosin Inhibition and Left Ventricular Diastolic Function in Patients with Obstructive Hypertrophic Cardiomyopathy Referred for Septal Reduction Therapy: Insights From the VALOR-HCM Study. Circ. Cardiovasc. Imaging 2022, 15, e014986. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Oręziak, A.; Masri, A.; Barriales-Villa, R.; Abraham, T.P.; Owens, A.T.; Jensen, M.K.; Wojakowski, W.; Seidler, T.; Hagege, A.; et al. Long-term effect of mavacamten in obstructive hypertrophic cardiomyopathy. Eur. Heart J. 2024, 45, 5071–5083. [Google Scholar] [CrossRef]
- Desai, M.Y.; Hajj-Ali, A.; Rutkowski, K.; Ospina, S.; Gaballa, A.; Emery, M.; Asher, C.; Xu, B.; Thamilarasan, M.; Popovic, Z.B. Real-world experience with mavacamten in obstructive hypertrophic cardiomyopathy: Observations from a tertiary care center. Prog. Cardiovasc. Dis. 2024, 86, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Reza, N.; Dubey, A.; Carattini, T.; Marzolf, A.; Hornsby, N.; de Feria, A.; Mahmud, N.; Schuler, P.; Owens, A.T. Real-World Experience and 36-Week Outcomes of Patients with Symptomatic Obstructive Hypertrophic Cardiomyopathy Treated with Mavacamten. JACC Heart Fail. 2024, 12, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Boyle, T.A.; Reza, N.; Hyman, M.; Supple, G.; See, V.Y.; Marzolf, A.; Hornsby, N.; de Feria, A.; Wang, T.; Margulies, K.B.; et al. Atrial Fibrillation in Patients Receiving Mavacamten for Obstructive Hypertrophic Cardiomyopathy: Real-World Incidence, Management, and Outcomes. JACC Clin. Electrophysiol. 2024, 11, 411–413. [Google Scholar] [CrossRef]
- Abood, Z.; Jan, M.F.; Ashraf, M.; Kroboth, S.; Sanders, H.; Schweitzer, M.; Misicka, A.; Ollerman, E.; Jahangir, A.; Galazka, P.; et al. Mavacamten in real-life practice: Initial experience at a hypertrophic cardiomyopathy centre. ESC Heart Fail. 2025, 12, 672–676. [Google Scholar] [CrossRef]
- Kim, D.S.; Chu, E.L.; Keamy-Minor, E.E.; Paranjpe, I.D.; Tang, W.L.; Tang, W.L.; O’Sullivan, J.W.; O’Sullivan, J.W.; Desai, Y.B.; Desai, Y.B.; et al. One-year real-world experience with mavacamten and its physiologic effects on obstructive hypertrophic cardiomyopathy. Front. Cardiovasc. Med. 2024, 11, 1429230. [Google Scholar] [CrossRef]
- Ramonfaur, D.; Gasperetti, A.; Blake, V.E.; Rivers, B.; Kassamali, A.A.; Kasper, E.K.; Barouch, L.A.; Wu, K.C.; Madrazo, J.A.; Carrick, R.T. Eighteen-Month Real-World Experience Using Mavacamten for Treatment of Obstructive Hypertrophic Cardiomyopathy in a Racially Diverse Population. J. Am. Heart Assoc. 2024, 13, e034069. [Google Scholar] [CrossRef]
- Bristol Myers Squibb, Inc. CAMZYOS U.S. Prescribing Information. Available online: https://packageinserts.bms.com/pi/pi_camzyos.pdf (accessed on 7 August 2022).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- CAMZYOSTM (Mavacamten) REMS Program. Available online: https://www.camzyosrems.com/ (accessed on 17 December 2023).
- Nagueh, S.F.; Phelan, D.; Abraham, T.; Alicia Armour, R.D.C.S.; Desai, M.Y.; Dragulescu, A.; Gilliland, Y.; Lester, S.J.; Maldonado, Y.; Mohiddin, S.; et al. Recommendations for Multimodality Cardiovascular Imaging of Patients with Hypertrophic Cardiomyopathy: An Update from the American Society of Echocardiography, in Collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2022, 35, 533–569. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.S.; et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Day, S.M.; Ashley, E.A.; Michels, M.; Colan, S.D.; Jacoby, D.; Marchionni, N.; Vincent-Tompkins, J.; Ho, C.Y.; et al. Association of Obesity with Adverse Long-term Outcomes in Hypertrophic Cardiomyopathy. JAMA Cardiol. 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Fordham, M.; Marzolf, A.B.; Hornsby, N.; de Feria, A.; Owens, A.T.; Reza, N. Real World Experience of Glucagon-Like Peptide 1 Receptor Agonists in Patients with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2024, 83, 13. [Google Scholar] [CrossRef]
- Geske, J.B.; Ong, K.C.; Siontis, K.C.; Hebl, V.B.; Ackerman, M.J.; Hodge, D.O.; Miller, V.M.; Nishimura, R.A.; Oh, J.K.; Schaff, H.V.; et al. Women with hypertrophic cardiomyopathy have worse survival. Eur. Heart J. 2017, 38, 3434–3440. [Google Scholar] [CrossRef]
- Olivotto, I.; Maron, M.S.; Adabag, A.S.; Casey, S.A.; Vargiu, D.; Link, M.S.; Udelson, J.E.; Cecchi, F.; Maron, B.J. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 480–487. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, M.S.; Wells, S.; Patel, P.P.; Koethe, B.C.; Maron, B.J. Impact of Sex on Clinical Course and Survival in the Contemporary Treatment Era for Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e012041. [Google Scholar] [CrossRef] [PubMed]
- Cresci, S.; Bach, R.G.; Saberi, S.; Owens, A.T.; Spertus, J.A.; Hegde, S.M.; Lakdawala, N.K.; Nilles, E.K.; Wojdyla, D.M.; Sehnert, A.J.; et al. Effect of Mavacamten in Women Compared with Men with Obstructive Hypertrophic Cardiomyopathy: Insights From EXPLORER-HCM. Circulation 2024, 149, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.; Rowin, E.J.; Bhatt, V.; Maron, M.S.; Maron, B.J. Association Between Race and Clinical Profile of Patients Referred for Hypertrophic Cardiomyopathy. Circulation 2018, 137, 1973–1975. [Google Scholar] [CrossRef]

| Variable | (N = 163) |
|---|---|
| Age at mavacamten start (years) | |
| mean (±SD) | 62.8 (13.7) |
| Sex, n (%) | |
| Female | 91 (55.8) |
| Male | 72 (44.2) |
| Race, n (%) | |
| White | 136 (83.4) |
| Asian | 8 (4.9) |
| Black or African American | 6 (3.7) |
| Other | 6 (3.7) |
| Not Reported | 7 (4.3) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 4 (2.5) |
| Insurance type, n (%) | |
| Commercial (private) | 91 (55.8) |
| Medicare/Medicare Advantage | 71 (43.6) |
| Medicaid | 1 (0.6) |
| Care supports, n (%) | |
| Has a care partner | 136 (83.4) |
| Lives alone | 30 (18.4) |
| Distance from treating center, n (%) | |
| ≤10 miles | 31 (19.0) |
| 11–50 miles | 100 (61.3) |
| 51–200 miles | 31 (19.0) |
| >200 miles | 1 (0.6) |
| Family history of HCM, n (%) | 40 (24.5) |
| Genetic testing performed, n (%) | 124 (76.1) |
| Pathogenic or likely pathogenic variant present | 26 (16.0) |
| MYBPC3 | 16 (9.8) |
| MYH7 | 8 (4.9) |
| TNNT2 | 1 (0.6) |
| TPN11 | 1 (0.6) |
| Duration of HCM diagnosis (years) | |
| mean (±SD) | 8.9 (7.8) |
| Medical history, n (%) | |
| Hypertension | 99 (60.7) |
| Coronary artery disease | 26 (16.0) |
| Atrial fibrillation | 38 (23.3) |
| Atrial fibrillation/flutter ablation | 12 (7.4) |
| Implantable cardioverter defibrillator | 44 (27.0) |
| Prior septal reduction therapy | 14 (8.6) |
| Alcohol septal ablation | 4 (2.5) |
| Septal myectomy | 10 (6.1) |
| New York Heart Association class, n (%) | |
| II | 73 (44.8) |
| III | 88 (54.0) |
| IV | 2 (1.2) |
| Vital signs | |
| BMI (kg/m2), mean (±SD) | 30.9 (6.0) |
| Systolic blood pressure (mmHg), mean (±SD) | 124.2 (14.8) |
| Diastolic blood pressure (mmHg), mean (±SD) | 72.7 (9.1) |
| Heart rate (beats per minute), mean (±SD) | 67.8 (12.4) |
| Cardiac rhythm, n (%) | |
| Sinus | 145 (89.0) |
| Atrial-sensed, ventricular paced | 4 (2.5) |
| Atrial-paced, ventricular-sensed | 10 (6.1) |
| Atrial-paced, ventricular-paced | 3 (1.8) |
| Ventricular-paced | 1 (0.6) |
| Echocardiographic parameters | |
| LVEF (%), mean (±SD) | 68.0 (5.7) |
| Maximal left ventricular wall thickness (mm), mean (±SD) | 18.6 (2.9) |
| LVOT gradient at rest (mm Hg), mean (±SD) | 53.0 (36.7) |
| LVOT gradient with Valsalva (mm Hg), mean (±SD) | 79.7 (33.2) |
| Mitral regurgitation severity, n (%) | |
| Trace | 21 (12.9) |
| Mild | 68 (41.7) |
| Moderate | 63 (38.7) |
| Severe | 11 (6.8) |
| Background HCM therapy, n (%) | |
| Beta blocker alone | 91 (55.8) |
| Nondihydropyridine calcium channel blocker alone | 32 (19.6) |
| Beta blocker and nondihydropyridine calcium channel blocker | 27 (16.6) |
| Beta blocker and disopyramide | 5 (3.1) |
| None | 8 (4.9) |
| Starting mavacamten dose (mg), n (%) | |
| 2.5 mg | 4 (2.5) |
| 5.0 mg | 159 (97.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, N.; Dubey, A.; Mahmud, N.; Austin, M.A.; Duqueney, E.; Patel, P.; Boakye, E.; Marzolf, A.; Hornsby, N.; de Feria, A.; et al. Long-Term Real-World Outcomes of Mavacamten in Symptomatic Obstructive Hypertrophic Cardiomyopathy up to 108 Weeks. J. Clin. Med. 2025, 14, 8181. https://doi.org/10.3390/jcm14228181
Reza N, Dubey A, Mahmud N, Austin MA, Duqueney E, Patel P, Boakye E, Marzolf A, Hornsby N, de Feria A, et al. Long-Term Real-World Outcomes of Mavacamten in Symptomatic Obstructive Hypertrophic Cardiomyopathy up to 108 Weeks. Journal of Clinical Medicine. 2025; 14(22):8181. https://doi.org/10.3390/jcm14228181
Chicago/Turabian StyleReza, Nosheen, Anandkumar Dubey, Nadim Mahmud, Melissa A. Austin, Estherland Duqueney, Parth Patel, Ellen Boakye, Amy Marzolf, Nicole Hornsby, Alejandro de Feria, and et al. 2025. "Long-Term Real-World Outcomes of Mavacamten in Symptomatic Obstructive Hypertrophic Cardiomyopathy up to 108 Weeks" Journal of Clinical Medicine 14, no. 22: 8181. https://doi.org/10.3390/jcm14228181
APA StyleReza, N., Dubey, A., Mahmud, N., Austin, M. A., Duqueney, E., Patel, P., Boakye, E., Marzolf, A., Hornsby, N., de Feria, A., Carattini, T., Schuler, P., & Owens, A. T. (2025). Long-Term Real-World Outcomes of Mavacamten in Symptomatic Obstructive Hypertrophic Cardiomyopathy up to 108 Weeks. Journal of Clinical Medicine, 14(22), 8181. https://doi.org/10.3390/jcm14228181





