Cognitive Age Delta as a Marker of Healthy and Pathological Cognitive Aging: The Role of Lifestyle, Cognitive Reserve, and Vascular Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Design
2.2. Clinical and Neurological Evaluation
2.3. Neuropsychological Assessment
2.4. Cognitive Reserve
2.5. Lifestyle Variables
2.6. Syndromic Cognitive Diagnosis
2.7. Genetic Risk
2.8. CSF AD Biomarker
2.9. MRI Vascular Pathology
2.10. Biomarker Defined Classification of CU Participants
- CU Biomarker negative (CUA-V-) (n = 142): A−T−N− and V−.
- CU Amyloid pathology (CUA+) (n = 23): A+ (irrespective of T, N) and V-.
- CU Vascular pathology (CUV+) (n = 14): V+ with otherwise biomarker-negative ATN profile (A−T−N−).
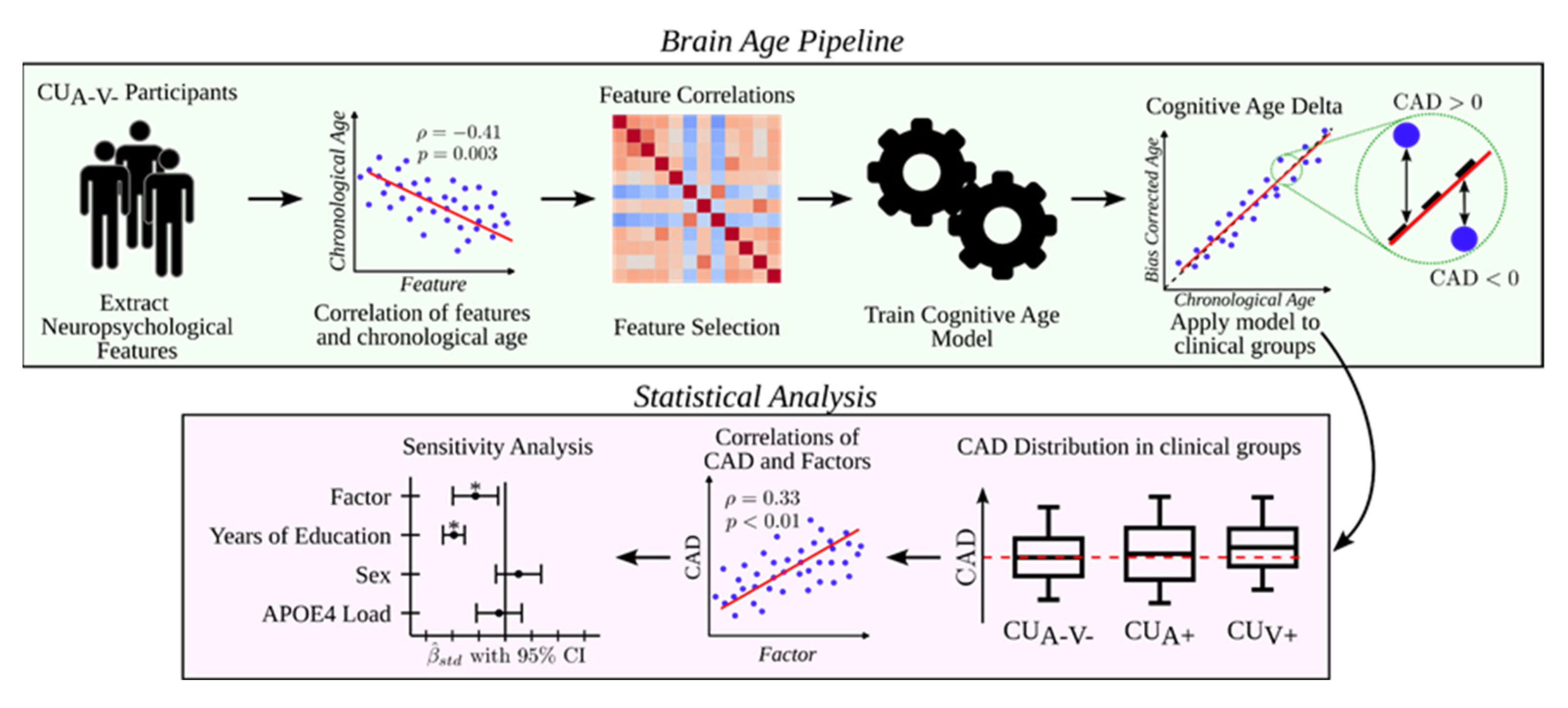
2.11. Cognitive Age Modeling and CAD
2.12. Statistical Analysis
3. Results
3.1. Sample Characteristics
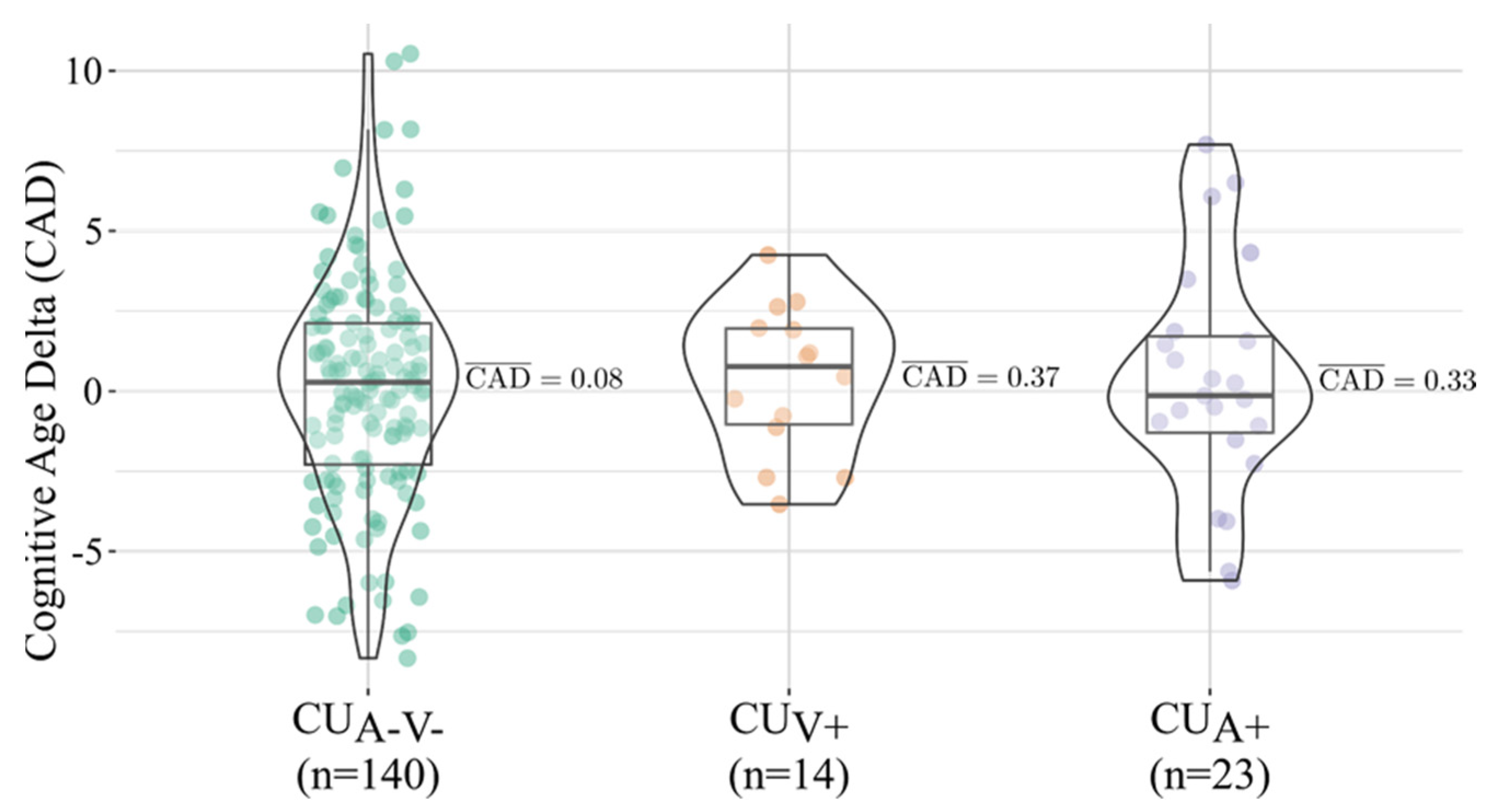
3.2. CAD Distribution and Group Comparisons
3.3. Associations Between CAD and Cognitive Reserve Related Variables
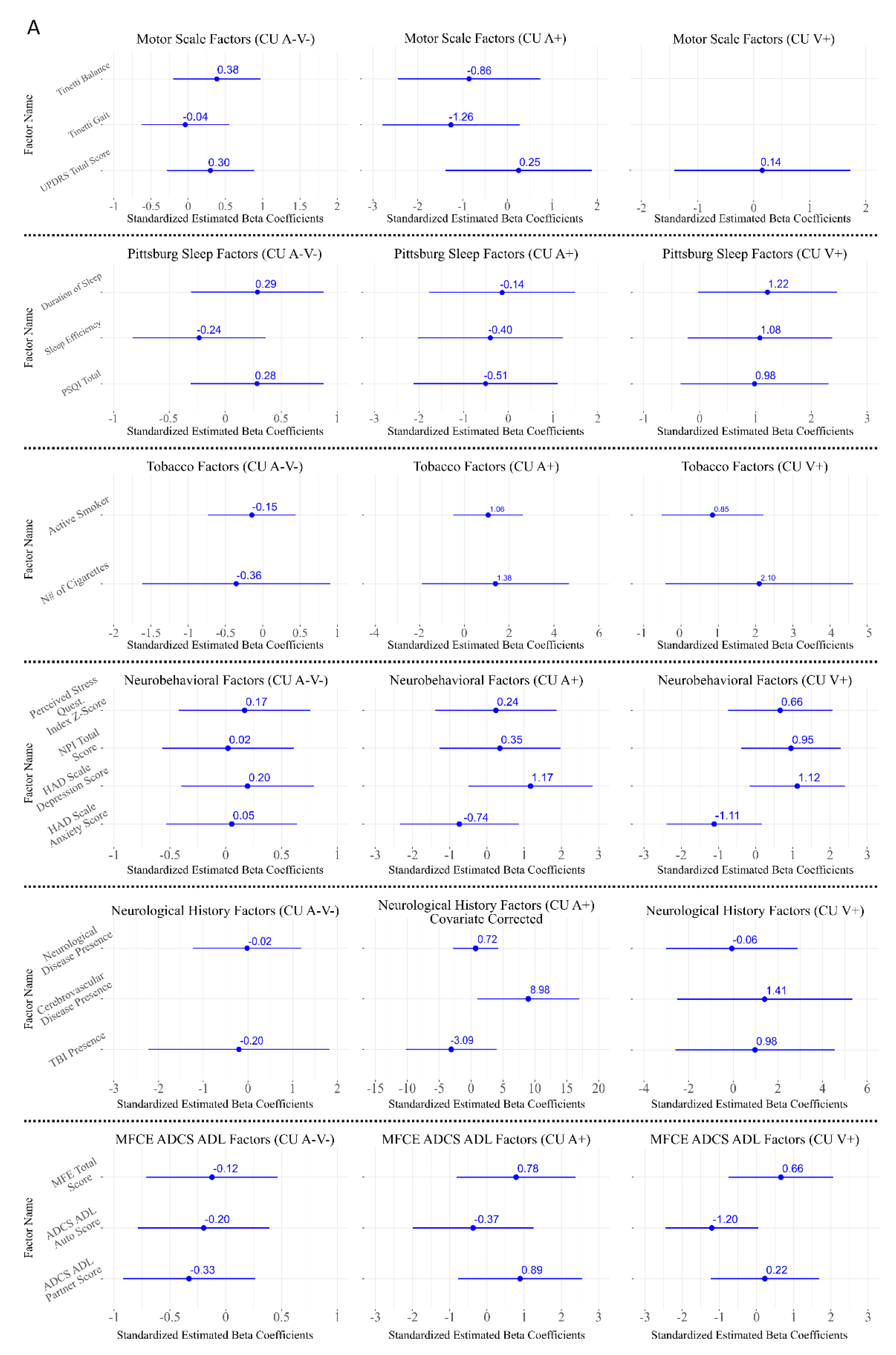
3.4. Associations Between CAD and Lifestyle Factors
3.5. Associations Between CAD and Neurological Assessment
3.6. Associations Between CAD and Cardiovascular Risk
3.7. Associations Between CAD and Neurobehavioral and Sleep-Related Factors
3.8. Associations Between CAD and CSF Biomarkers
4. Discussion
4.1. The Value of Cognitive Age Delta in the Biomarker Era
4.2. APOE and Sex Effects
4.3. CSF Biomarkers
4.4. Rigorous Biomarker Definition for Vascular Pathology
4.5. Differential Associations of Cognitive Reserve and Lifestyle Factors
4.6. Strengths
4.7. Limitations
4.8. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADCS-ADL | Alzheimer’s Disease Cooperative Study–Activities of Daily Living |
| AFAGI | Asociación de Familiares de Alzheimer y otras Demencias de Gipuzkoa |
| Aβ42 | Amyloid beta 1–42 peptide |
| APOE | Apolipoprotein E |
| ARIA | Amyloid-Related Imaging Abnormalities |
| AT(N) | Amyloid, Tau, and Neurodegeneration classification framework |
| A+/T+/N+/V+ | Positive for Amyloid/Tau/Neurodegeneration/Vascular burden |
| BMI | Body Mass Index |
| BNT | Boston Naming Test |
| CAD | Cognitive Age Delta |
| CITA | Center for Research and Memory Clinic, CITA-Alzheimer Foundation |
| CI | Confidence Interval |
| CMBs | Cerebral Microbleeds |
| cSS | Cortical Superficial Siderosis |
| CSF | Cerebrospinal Fluid |
| CR | Cognitive Reserve |
| CU | Cognitively Unimpaired |
| CUA−V−/ | Cognitively Unimpaired Amyloid- (A-T-N-) and Vascular– |
| CUA+ | Cognitively Unimpaired Amyloid+ (A+ and T and N+ or -) and Vascular– |
| CUV+ | Cognitively Unimpaired Vascular+ and Amyloid- (A-T-N-) |
| DNA | Deoxyribonucleic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ENRICA | Estudio de Nutrición y Riesgo Cardiovascular (Spanish Study on Nutrition and Cardiovascular Risk) |
| FCSRT | Free and Cued Selective Reminding Test |
| FDR | False Discovery Rate |
| FLAIR | Fluid-Attenuated Inversion Recovery |
| GAP | Gipuzkoa Alzheimer Project |
| GLM | General Linear Model |
| GRE | Gradient-Recalled Echo |
| HADS | Hospital Anxiety and Depression Scale |
| HbA1c | Glycated Hemoglobin |
| HDL | High-Density Lipoprotein |
| IPAQ | International Physical Activity Questionnaire |
| IQR | Interquartile Range |
| IWG-3 | International Working Group Criteria, 3rd Revision |
| JLO | Judgment of Line Orientation Test |
| LDL | Low-Density Lipoprotein |
| M@T | Memory Alteration Test |
| MCI | Mild Cognitive Impairment |
| MMSE | Mini-Mental State Examination |
| MRI | Magnetic Resonance Imaging |
| MTA | Medial Temporal Atrophy scale |
| NPI | Neuropsychiatric Inventory |
| NPS | Neuropsychological Profile |
| OSA | Obstructive Sleep Apnea |
| PCR | Polymerase Chain Reaction |
| p-tau | Phosphorylated tau |
| t-tau | Total tau |
| PSQ | Perceived Stress Questionnaire |
| PSQI | Pittsburgh Sleep Quality Index |
| ROCF | Rey–Osterrieth Complex Figure Test |
| SVD | Small Vessel Disease |
| SWI | Susceptibility-Weighted Imaging |
| STRIVE/STRIVE-2 | Standards for Reporting Vascular Changes on Neuroimaging (1st and 2nd editions) |
| SUN | Seguimiento Universidad de Navarra |
| TMT-A/TMT-B | Trail Making Test Parts A and B |
| TOPF | Wechsler Test of Premorbid Functioning |
| UPDRS-III | Unified Parkinson’s Disease Rating Scale, Part III (motor section) |
| V | Vascular status (burden) |
| WAIS-III | Wechsler Adult Intelligence Scale, 3rd Edition |
| WMH | White Matter Hyperintensities |
Appendix A
| Standardized Coefficient (β) | Original Scale Coefficient (B) | |
|---|---|---|
| Digit Symbol WAIS-III | −1.7630 | −0.1156 |
| Stroop Color | −0.8945 | −0.0741 |
| Stroop Word-Color | −1.1618 | −0.1138 |
| 15 Object test | −0.6991 | −0.4875 |
| ROCF Recall 30 min | −1.3264 | −0.2507 |
| TMT-A | 0.1797 | 0.0172 |
| FCSRT Immediate Total Free Recall | −0.6966 | −0.1280 |
| TMT-B | −0.9684 | 0.0393 |
| Boston naming test | −0.1727 | −0.0519 |
| Phonological verbal fluency (“p”) | −0.0218 | −0.0046 |
| FCSRT Immediate Total Recall | −0.2325 | −0.0595 |
| Semantic verbal fluency (“animals”) | 0.0886 | 0.0150 |
| With CSF Data (n = 239) | Without CSF Data (n = 172) | p-Value | |
|---|---|---|---|
| Age, years, mean ± SD | 57 ± 7 | 57 ± 7 | 0.36 |
| Sex, female, n (%) | 125 (52.3%) | 103 (59.9%) | 0.08 |
| Years of education, mean ± SD | 14 ± 4 | 14 ± 4 | 0.71 |
| MCI syndromic diagnosis, n (%) | 39 (16.3%) | 23 (13.4%) | 0.25 |
| APOE ε4 carrier, n (%) | 60 (25.1%) | 44 (25.6%) | 0.39 |
References
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia Prevention, Intervention, and Care: 2024 Report of the Lancet Standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Safiri, S.; Ghaffari Jolfayi, A.; Fazlollahi, A.; Morsali, S.; Sarkesh, A.; Daei Sorkhabi, A.; Golabi, B.; Aletaha, R.; Motlagh Asghari, K.; Hamidi, S.; et al. Alzheimer’s Disease: A Comprehensive Review of Epidemiology, Risk Factors, Symptoms Diagnosis, Management, Caregiving, Advanced Treatments and Associated Challenges. Front. Med. 2024, 11, 1474043. [Google Scholar] [CrossRef]
- Nianogo, R.A.; Rosenwohl-Mack, A.; Yaffe, K.; Carrasco, A.; Hoffmann, C.M.; Barnes, D.E. Risk Factors Associated with Alzheimer Disease and Related Dementias by Sex and Race and Ethnicity in the US. JAMA Neurol. 2022, 79, 584. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Salthouse, T.A. Trajectories of Normal Cognitive Aging. Psychol. Aging 2019, 34, 17–24. [Google Scholar] [CrossRef]
- Prince, J.B.; Davis, H.L.; Tan, J.; Muller-Townsend, K.; Markovic, S.; Lewis, D.M.G.; Hastie, B.; Thompson, M.B.; Drummond, P.D.; Fujiyama, H.; et al. Cognitive and Neuroscientific Perspectives of Healthy Ageing. Neurosci. Biobehav. Rev. 2024, 161, 105649. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and Investigating Cognitive Reserve, Brain Reserve, and Brain Maintenance. Alzheimer’s Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Garcia Condado, J.; Cortes, J.M. NeuropsychBrainAge: A Biomarker for Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12493. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Villain, N.; Schneider, L.; Fox, N.; Campbell, N.; Galasko, D.; Kivipelto, M.; Jessen, F.; Hanseeuw, B.; Boada, M.; et al. Alzheimer Disease as a Clinical-Biological Construct—An International Working Group Recommendation. JAMA Neurol. 2024, 81, 1304–1311. [Google Scholar] [CrossRef]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Bos, I.; Vos, S.J.B.; Jansen, W.J.; Vandenberghe, R.; Gabel, S.; Estanga, A.; Ecay-Torres, M.; Tomassen, J.; den Braber, A.; Lleó, A.; et al. Amyloid-β, Tau, and Cognition in Cognitively Normal Older Individuals: Examining the Necessity to Adjust for Biomarker Status in Normative Data. Front. Aging Neurosci. 2018, 10, 193. [Google Scholar] [CrossRef]
- Borland, E.; Stomrud, E.; van Westen, D.; Hansson, O.; Palmqvist, S. The Age-Related Effect on Cognitive Performance in Cognitively Healthy Elderly Is Mainly Caused by Underlying AD Pathology or Cerebrovascular Lesions: Implications for Cutoffs Regarding Cognitive Impairment. Alzheimers Res. Ther. 2020, 12, 30. [Google Scholar] [CrossRef]
- López-Martos, D.; Brugulat-Serrat, A.; Cañas-Martínez, A.; Canals-Gispert, L.; Marne, P.; Gramunt, N.; Suárez-Calvet, M.; Milà-Alomà, M.; Minguillon, C.; Fauria, K.; et al. Reference Data for Attentional, Executive, Linguistic, and Visual Processing Tests Obtained from Cognitively Healthy Individuals with Normal Alzheimer’s Disease Cerebrospinal Fluid Biomarker Levels. J. Alzheimer’s Dis. 2023, 95, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Hall, C.B.; Katz, M.J.; Derby, C.A.; Lipnicki, D.M.; Crawford, J.D.; Guaita, A.; Vaccaro, R.; Davin, A.; Kim, K.W.; et al. Education, Occupational Complexity, and Incident Dementia: A COSMIC Collaborative Cohort Study. J. Alzheimer’s Dis. 2022, 85, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dhana, K.; Huang, Y.; Huang, L.; Tao, Y.; Liu, X.; Melo van Lent, D.; Zheng, Y.; Ascherio, A.; Willett, W.; et al. Association of the Mediterranean Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet With the Risk of Dementia. JAMA Psychiatry 2023, 80, 630. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.S.; Zuidersma, M.; Oude Voshaar, R.C.; Zuidema, S.U.; van den Heuvel, E.R.; Stolk, R.P.; Smidt, N. Social Relationships and Risk of Dementia: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. Ageing Res. Rev. 2015, 22, 39–57. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Levälahti, E.; Laatikainen, T.; Lindström, J.; Peltonen, M.; Solomon, A.; Ahtiluoto, S.; Antikainen, R.; Hänninen, T.; et al. Recruitment and Baseline Characteristics of Participants in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)—A Randomized Controlled Lifestyle Trial. Int. J. Environ. Res. Public Health 2014, 11, 9345–9360. [Google Scholar] [CrossRef]
- Estanga, A.; Ecay-Torres, M.; Ibañez, A.; Izagirre, A.; Villanua, J.; Garcia-Sebastian, M.; Iglesias Gaspar, M.T.; Otaegui-Arrazola, A.; Iriondo, A.; Clerigue, M.; et al. Beneficial Effect of Bilingualism on Alzheimer’s Disease CSF Biomarkers and Cognition. Neurobiol. Aging 2017, 50, 144–151. [Google Scholar] [CrossRef]
- Ecay-Torres, M.; Estanga, A.; Tainta, M.; Izagirre, A.; Garcia-Sebastian, M.; Villanua, J.; Clerigue, M.; Iriondo, A.; Urreta, I.; Arrospide, A.; et al. Increased CAIDE Dementia Risk, Cognition, CSF Biomarkers, and Vascular Burden in Healthy Adults. Neurology 2018, 91, e217–e226. [Google Scholar] [CrossRef]
- Tinetti, M.E. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Galasko, D.; Bennett, D.A.; Sano, M.; Marson, D.; Kaye, J.; Edland, S.D. ADCS Prevention Instrument Project: Assessment of Instrumental Activities of Daily Living for Community-Dwelling Elderly Individuals in Dementia Prevention Clinical Trials. Alzheimer Dis. Assoc. Disord. 2006, 20, S152–S169. [Google Scholar] [CrossRef]
- Montejo, P.; Montenegro, M.; Sueiro, M.J.; Huertas, E. Cuestionario de Fallos de Memoria de La Vida Cotidiana (MFE). Análisis de Factores Con Población Española. An. Psicol. 2014, 30, 320–328. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Levenstein, S.; Prantera, C.; Varvo, V.; Scribano, M.L.; Berto, E.; Luzi, C.; Andreoli, A. Development of the Perceived Stress Questionnaire: A New Tool for Psychosomatic Research. J. Psychosom. Res. 1993, 37, 19–32. [Google Scholar] [CrossRef]
- Rodríguez-Artalejo, F.; Graciani, A.; Guallar-Castillón, P.; León-Muñoz, L.M.; Zuluaga, M.C.; López-García, E.; Gutiérrez-Fisac, J.L.; Taboada, J.M.; Aguilera, M.T.; Regidor, E.; et al. Justificación y Métodos Del Estudio Sobre Nutrición y Riesgo Cardiovascular En España (ENRICA). Rev. Esp. Cardiol. 2011, 64, 876–882. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Rami, L.; Molinuevo, J.L.; Sanchez-Valle, R.; Bosch, B.; Villar, A. Screening for Amnestic Mild Cognitive Impairment and Early Alzheimer’s Disease with M@T (Memory Alteration Test) in the Primary Care Population. Int. J. Geriatr. Psychiatry 2007, 22, 294–304. [Google Scholar] [CrossRef]
- Buschke, H. Cued Recall in Amnesia. J. Clin. Neuropsychol. 1984, 6, 433–440. [Google Scholar] [CrossRef]
- Pena-Casanova, J.; Gramunt-Fombuena, N.; Quinones-Ubeda, S.; Sanchez-Benavides, G.; Aguilar, M.; Badenes, D.; Molinuevo, J.L.; Robles, A.; Barquero, M.S.; Payno, M.; et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for the Rey-Osterrieth Complex Figure (Copy and Memory), and Free and Cued Selective Reminding Test. Arch. Clin. Neuropsychol. 2009, 24, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. Test de Copia y de Reproduccion de Memoria de Figuras Geometricas Complejas, 9th ed.; TEA Ediciones S.A.: Madrid, Spain, 2009. [Google Scholar]
- Goodglass, H. Evaluacion de La Afasia y Trastornos Relacionados, 3rd ed.; Editorial Médica Paramericana: Madrid, Spain, 2005. [Google Scholar]
- Pena-Casanova, J.; Quinones-Ubeda, S.; Gramunt-Fombuena, N.; Aguilar, M.; Casas, L.; Molinuevo, J.L.; Robles, A.; Rodriguez, D.; Barquero, M.S.; Antunez, C.; et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for Boston Naming Test and Token Test. Arch. Clin. Neuropsychol. 2009, 24, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Pena-Casanova, J.; Quinones-Ubeda, S.; Gramunt-Fombuena, N.; Quintana-Aparicio, M.; Aguilar, M.; Badenes, D.; Cerulla, N.; Molinuevo, J.L.; Ruiz, E.; Robles, A.; et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for Verbal Fluency Tests. Arch. Clin. Neuropsychol. 2009, 24, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Benton, A.; Hannay, H.J.; Varney, N.R. Visual Perception of Line Direction in Patients with Unilateral Brain Disease. Neurology 1975, 25, 907. [Google Scholar] [CrossRef]
- Pillon, B.; Dubois, B.; Bonnet, A.-M.; Esteguy, M.; Guimaraes, J.; Vigouret, J.-M.; Lhermitte, F.; Agid, Y. Cognitive Slowing in Parkinson’s Disease Fails to Respond to Levodopa Treatment. Neurology 1989, 39, 762. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-III. Escala de Inteligencia de Wechsler Para Adultos-III, 2nd ed.; TEA Ediciones S.A.: Madrid, Spain, 2001. [Google Scholar]
- Reitan, R.; Wolfson, D. The Halstead—Reitan Neuropsychological Test Battery. Theory and Clinical Interpretation, 2nd ed.; Neuropsychology Press: Tucson, AZ, USA, 1985. [Google Scholar]
- Golden, C. Stroop: Test de Colores y Palabras, 5th ed.; TEA Ediciones S.A.: Madrid, Spain, 2007. [Google Scholar]
- Pena-Casanova, J.; Blesa, R.; Aguilar, M.; Gramunt-Fombuena, N.; Gomez-Anson, B.; Oliva, R.; Molinuevo, J.L.; Robles, A.; Barquero, M.S.; Antunez, C.; et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Methods and Sample Characteristics. Arch. Clin. Neuropsychol. 2009, 24, 307–319. [Google Scholar] [CrossRef]
- Rami, L.; Valls-Pedret, C.; Bartrés-Faz, D.; Caprile, C.; Solé-Padullés, C.; Castellvi, M.; Olives, J.; Bosch, B.; Molinuevo, J.L. Cognitive Reserve Questionnaire. Scores Obtained in a Healthy Elderly Population and in One with Alzheimer’s Disease. Rev. Neurol. 2011, 52, 195–201. [Google Scholar]
- Fernández Ballesteros, R.; Zamarron Cassinello, M.D.; Diez Nicolas, J.; De Juan Espinosa, M.; Montero, P.; Lopez Bravo, M.; Hernandez Torres, A. Estudio Longitudinal Sobre Envejecimiento Activo (ELEA). Estudios I+D+I; IMSERSO: Madrid, Spain, 2006; Volume 37. [Google Scholar]
- Blázquez, L.; De Juan, D.; Ruiz-Martínez, J.; Emparanza, J.I.; Sáenz, A.; Otaegui, D.; Sistiaga, A.; Martínez-Lage, P.; Lamet, I.; Samaranch, L.; et al. Genes Related to Iron Metabolism and Susceptibility to Alzheimer’s Disease in Basque Population. Neurobiol. Aging 2007, 28, 1941–1943. [Google Scholar] [CrossRef]
- Duits, F.H.; Martinez-Lage, P.; Paquet, C.; Engelborghs, S.; Lleó, A.; Hausner, L.; Molinuevo, J.L.; Stomrud, E.; Farotti, L.; Ramakers, I.H.G.B.; et al. Performance and Complications of Lumbar Puncture in Memory Clinics: Results of the Multicenter Lumbar Puncture Feasibility Study. Alzheimer’s Dement. 2016, 12, 154–163. [Google Scholar] [CrossRef]
- Alcolea, D.; Martínez-Lage, P.; Sánchez-Juan, P.; Olazarán, J.; Antúnez, C.; Izagirre, A.; Ecay-Torres, M.; Estanga, A.; Clerigué, M.; Guisasola, M.C.; et al. Amyloid Precursor Protein Metabolism and Inflammation Markers in Preclinical Alzheimer Disease. Neurology 2015, 85, 626–633. [Google Scholar] [CrossRef]
- Landau, S.M.; Lu, M.; Joshi, A.D.; Pontecorvo, M.; Mintun, M.A.; Trojanowski, J.Q.; Shaw, L.M.; Jagust, W.J. Comparing Positron Emission Tomography Imaging and Cerebrospinal Fluid Measurements of Β-amyloid. Ann. Neurol. 2013, 74, 826–836. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An Unbiased Descriptive Classification Scheme for Alzheimer Disease Biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.; Alavi, A.; Hurtig, H.; Zimmerman, R. MR Signal Abnormalities at 1.5 T in Alzheimer’s Dementia and Normal Aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Scheltens, P.; Leys, D.; Barkhof, F.; Huglo, D.; Weinstein, H.C.; Vermersch, P.; Kuiper, M.; Steinling, M.; Wolters, E.C.; Valk, J. Atrophy of Medial Temporal Lobes on MRI in “Probable” Alzheimer’s Disease and Normal Ageing: Diagnostic Value and Neuropsychological Correlates. J. Neurol. Neurosurg. Psychiatry 1992, 55, 967–972. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging Standards for Research into Small Vessel Disease and Its Contribution to Ageing and Neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.-E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S.; et al. Neuroimaging Standards for Research into Small Vessel Disease-Advances since 2013. Lancet Neurol. 2023, 22, 602–618. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Selkoe, D.J.; Schindler, S.E.; Aisen, P.; Apostolova, L.G.; Atri, A.; Greenberg, S.M.; Hendrix, S.B.; Petersen, R.C.; Weiner, M.; et al. Donanemab: Appropriate Use Recommendations. J. Prev. Alzheimers Dis. 2025, 12, 100150. [Google Scholar] [CrossRef]
- Lövdén, M.; Fratiglioni, L.; Glymour, M.M.; Lindenberger, U.; Tucker-Drob, E.M. Education and Cognitive Functioning Across the Life Span. Psychol. Sci. Public Interest 2020, 21, 6–41. [Google Scholar] [CrossRef]
- Ganguli, M.; Snitz, B.E.; Lee, C.-W.; Vanderbilt, J.; Saxton, J.A.; Chang, C.-C.H. Age and Education Effects and Norms on a Cognitive Test Battery from a Population-Based Cohort: The Monongahela-Youghiogheny Healthy Aging Team. Aging Ment. Health 2010, 14, 100–107. [Google Scholar] [CrossRef]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer Disease: Risk, Mechanisms and Therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Wisdom, N.M.; Callahan, J.L.; Hawkins, K.A. The Effects of Apolipoprotein E on Non-Impaired Cognitive Functioning: A Meta-Analysis. Neurobiol. Aging 2011, 32, 63–74. [Google Scholar] [CrossRef]
- Fortea, J.; Pegueroles, J.; Alcolea, D.; Belbin, O.; Dols-Icardo, O.; Vaqué-Alcázar, L.; Videla, L.; Gispert, J.D.; Suárez-Calvet, M.; Johnson, S.C.; et al. APOE4 Homozygozity Represents a Distinct Genetic Form of Alzheimer’s Disease. Nat. Med. 2024, 30, 1284–1291. [Google Scholar] [CrossRef]
- de Lange, A.G.; Anatürk, M.; Rokicki, J.; Han, L.K.M.; Franke, K.; Alnæs, D.; Ebmeier, K.P.; Draganski, B.; Kaufmann, T.; Westlye, L.T.; et al. Mind the Gap: Performance Metric Evaluation in Brain-age Prediction. Hum. Brain Mapp. 2022, 43, 3113–3129. [Google Scholar] [CrossRef]
- de Lange, A.-M.G.; Cole, J.H. Commentary: Correction Procedures in Brain-Age Prediction. Neuroimage Clin. 2020, 26, 102229. [Google Scholar] [CrossRef]
- Butler, E.R.; Chen, A.; Ramadan, R.; Le, T.T.; Ruparel, K.; Moore, T.M.; Satterthwaite, T.D.; Zhang, F.; Shou, H.; Gur, R.C.; et al. Pitfalls in Brain Age Analyses. Hum. Brain Mapp. 2021, 42, 4092–4101. [Google Scholar] [CrossRef]
- Garcia Condado, J.G.; Tellaetxe-Elorriaga, I.; Cortes, J.M.; Erramuzpe, A. AgeML: Age Modeling with Machine Learning. IEEE J. Biomed. Health Inform. 2025, 29, 3772–3781. [Google Scholar] [CrossRef]
- More, S.; Antonopoulos, G.; Hoffstaedter, F.; Caspers, J.; Eickhoff, S.B.; Patil, K.R. Brain-Age Prediction: A Systematic Comparison of Machine Learning Workflows. Neuroimage 2023, 270, 119947. [Google Scholar] [CrossRef]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An Epigenetic Biomarker of Aging for Lifespan and Healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Charykova, I.; Gorkom, K.N.-V.; Zaki, N.; Almansoori, T.M.; Baylis, G.; Ljubisavljevic, M.; Belghali, M. Predicting Age from Behavioral Test Performance for Screening Early Onset of Cognitive Decline. Front. Aging Neurosci. 2021, 13, 661514. [Google Scholar] [CrossRef]
- Anatürk, M.; Kaufmann, T.; Cole, J.H.; Suri, S.; Griffanti, L.; Zsoldos, E.; Filippini, N.; Singh-Manoux, A.; Kivimäki, M.; Westlye, L.T.; et al. Prediction of Brain Age and Cognitive Age: Quantifying Brain and Cognitive Maintenance in Aging. Hum. Brain Mapp. 2021, 42, 1626–1640. [Google Scholar] [CrossRef]
- Chun, M.Y.; Jang, H.; Kim, S.-J.; Park, Y.H.; Yun, J.; Lockhart, S.N.; Weiner, M.; De Carli, C.; Moon, S.H.; Choi, J.Y.; et al. Emerging Role of Vascular Burden in AT(N) Classification in Individuals with Alzheimer’s and Concomitant Cerebrovascular Burdens. J. Neurol. Neurosurg. Psychiatry 2024, 95, 44–51. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Kwak, S. Rethinking Neuropsychological Test Validity in Dementia Assessment: A Critical Review in the Age of Neuroimaging and Digital Markers. Front. Hum. Neurosci. 2025, 19, 1578648. [Google Scholar] [CrossRef]
- Kim, J.; Na, H.K.; Byun, J.; Shin, J.; Kim, S.; Lee, B.H.; Na, D.L. Tracking Cognitive Decline in Amnestic Mild Cognitive Impairment and Early-Stage Alzheimer Dementia: Mini-Mental State Examination versus Neuropsychological Battery. Dement. Geriatr. Cogn. Disord. 2017, 44, 105–117. [Google Scholar] [CrossRef]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in Alzheimer’s Disease and Other Neurological Disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef]
- Altmann, A.; Tian, L.; Henderson, V.W.; Greicius, M.D. Sex Modifies the APOE-related Risk of Developing Alzheimer Disease. Ann. Neurol. 2014, 75, 563–573. [Google Scholar] [CrossRef]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Schumacher Dimech, A.; Santuccione Chadha, A.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex Differences in Alzheimer Disease—The Gateway to Precision Medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef]
- Lin, Y.; Lee, W.; Fuh, J. The Role of Cognitive Reserve in White Matter Hyperintensities: From Cognitive Aging to Alzheimer’s Spectrum. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2025, 17, e70167. [Google Scholar] [CrossRef]
- Martínez Camblor, L.; Peña Suárez, J.M.; Martínez-Cachero García, M.; Santamarta Liébana, E.; Rodríguez Castro, J.; Saiz Ayala, A. Microhemorragias Cerebrales. Utilidad de Las Secuencias de Susceptibilidad Magnética (SWI). Radiologia 2023, 65, 362–375. [Google Scholar] [CrossRef]
- Bartrés-Faz, D.; Arenaza-Urquijo, E.M. Structural and Functional Imaging Correlates of Cognitive and Brain Reserve Hypotheses in Healthy and Pathological Aging. Brain Topogr. 2011, 24, 340–357. [Google Scholar] [CrossRef]
- Stern, Y.; Albert, M.; Barnes, C.A.; Cabeza, R.; Pascual-Leone, A.; Rapp, P.R. A Framework for Concepts of Reserve and Resilience in Aging. Neurobiol. Aging 2023, 124, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, P.; Weigand, S.D.; Przybelski, S.A.; Knopman, D.S.; Smith, G.E.; Trojanowski, J.Q.; Shaw, L.M.; Decarli, C.S.; Carmichael, O.; Bernstein, M.A.; et al. Cognitive Reserve and Alzheimer’s Disease Biomarkers Are Independent Determinants of Cognition. Brain 2011, 134, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Rentz, D.M.; Locascio, J.J.; Becker, J.A.; Moran, E.K.; Eng, E.; Buckner, R.L.; Sperling, R.A.; Johnson, K.A. Cognition, Reserve, and Amyloid Deposition in Normal Aging. Ann. Neurol. 2010, 67, 353–364. [Google Scholar] [CrossRef]
- Valenzuela, M.J.; Sachdev, P. Brain Reserve and Dementia: A Systematic Review. Psychol. Med. 2006, 36, 441–454. [Google Scholar] [CrossRef]
- Corbo, I.; Marselli, G.; Di Ciero, V.; Casagrande, M. The Protective Role of Cognitive Reserve in Mild Cognitive Impairment: A Systematic Review. J. Clin. Med. 2023, 12, 1759. [Google Scholar] [CrossRef]
- Kartschmit, N.; Mikolajczyk, R.; Schubert, T.; Lacruz, M.E. Measuring Cognitive Reserve (CR)–A Systematic Review of Measurement Properties of CR Questionnaires for the Adult Population. PLoS ONE 2019, 14, e0219851. [Google Scholar] [CrossRef]
- Perani, D.; Abutalebi, J. Bilingualism, Dementia, Cognitive and Neural Reserve. Curr. Opin. Neurol. 2015, 28, 618–625. [Google Scholar] [CrossRef]
- Berkes, M.; Bialystok, E. Bilingualism as a Contributor to Cognitive Reserve: What It Can Do and What It Cannot Do. Am. J. Alzheimers Dis. Other Demen. 2022, 37. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Aslan, D.H.; Sayre, M.K.; Bharadwaj, P.K.; Ally, M.; Maltagliati, S.; Lai, M.H.C.; Wilcox, R.R.; Klimentidis, Y.C.; Alexander, G.E. Sedentary Behavior and Incident Dementia Among Older Adults. JAMA 2023, 330, 934. [Google Scholar] [CrossRef] [PubMed]
- Gogniat, M.A.; Won, J.; Cruz, C.; Aranda, A.; Verma, A.; Gujral, S.; Weinstein, A.M.; Zaheed, A.B.; Cole, K.R.; Full, K.M.; et al. Sedentary Behavior, Cognition, and Brain Health in Older Adults: A Systematic Review. Front. Aging Neurosci. 2025, 17, 1622049. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Herold, F.; Cheval, B.; Wheeler, M.J.; Pindus, D.M.; Erickson, K.I.; Raichlen, D.A.; Alexander, G.E.; Müller, N.G.; Dunstan, D.W.; et al. Sedentary Behavior and Lifespan Brain Health. Trends Cogn. Sci. 2024, 28, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Wingood, M.; Gell, N.M.; Rosenberg, D.E.; Stoddard, G.J.; Bouldin, E.D. Associations of Cognitively Active Versus Passive Sedentary Behaviors and Cognition in Older Adults. J. Phys. Act. Health 2024, 21, 928–938. [Google Scholar] [CrossRef]
- Rizvi, N.; Lin, H.; Beiser, A.S.; Spartano, N.L. How Do Occupational Sedentary Behavior and Occupational Cognitive Complexity Relate to Cognitive Function? A Cross-Sectional Study. Health Sci. Rep. 2025, 8, e70949. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Lucey, B.P.; McCullough, A.; Landsness, E.C.; Toedebusch, C.D.; McLeland, J.S.; Zaza, A.M.; Fagan, A.M.; McCue, L.; Xiong, C.; Morris, J.C.; et al. Reduced Non–Rapid Eye Movement Sleep Is Associated with Tau Pathology in Early Alzheimer’s Disease. Sci. Transl. Med. 2019, 11, 474. [Google Scholar] [CrossRef]
- Altuna, M.; García-Sebastián, M.; Cipriani, R.; Capetillo-Zarate, E.; Alberdi, E.; Estanga, A.; Ecay-Torres, M.; Iriondo, A.; Saldias, J.; Cañada, M.; et al. Stepwise Approach to Alzheimer’s Disease Diagnosis in Primary Care Using Cognitive Screening, Risk Factors, Neuroimaging and Plasma Biomarkers. Sci. Rep. 2025, 15, 31526. [Google Scholar] [CrossRef]
- Tainta, M.; Ecay-Torres, M.; Barandiaran, M.; Estanga, A.; López, C.; Altuna, M.; Iriondo, A.; Saldias, J.; Garcia-Sebastian, M.; Cañada, M.; et al. CITA GO-ON Study. A Community Based Multidomain Lifestyle Intervention to Prevent Cognitive Decline. Protocol Design and Recruitment Process. Front. Aging Neurosci. 2025, 17, 1539711. [Google Scholar] [CrossRef]



| CUA-V- (n = 140) | CUA+ (n = 23) | CUV+ (n = 14) | p-Value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 55 (52–60) | 61 (55–66) | 62 (58.25–66.25) | 0.001 |
| Sex, female, n (%) | 79 (55.6%) | 11 (47.8%) | 6 (42.9%) | 0.08 |
| Years of education, median (IQR) | 14 (12–16) | 14 (10–16) | 13 (11–18.25) | 0.82 |
| APOE ε4 carrier, n (%) | 29 (20.7%) | 11 (47.8%) | 3 (21.4%) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estanga, A.; Tellaetxe-Elorriaga, I.; Ecay-Torres, M.; García Condado, J.; García-Sebastián, M.; Arriba, M.; López, C.; Ros, N.; Iriondo, A.; Reparaz-Escudero, I.; et al. Cognitive Age Delta as a Marker of Healthy and Pathological Cognitive Aging: The Role of Lifestyle, Cognitive Reserve, and Vascular Risk. J. Clin. Med. 2025, 14, 8176. https://doi.org/10.3390/jcm14228176
Estanga A, Tellaetxe-Elorriaga I, Ecay-Torres M, García Condado J, García-Sebastián M, Arriba M, López C, Ros N, Iriondo A, Reparaz-Escudero I, et al. Cognitive Age Delta as a Marker of Healthy and Pathological Cognitive Aging: The Role of Lifestyle, Cognitive Reserve, and Vascular Risk. Journal of Clinical Medicine. 2025; 14(22):8176. https://doi.org/10.3390/jcm14228176
Chicago/Turabian StyleEstanga, Ainara, Iñigo Tellaetxe-Elorriaga, Mirian Ecay-Torres, Jorge García Condado, Maite García-Sebastián, Maria Arriba, Carolina López, Naia Ros, Ane Iriondo, Imanol Reparaz-Escudero, and et al. 2025. "Cognitive Age Delta as a Marker of Healthy and Pathological Cognitive Aging: The Role of Lifestyle, Cognitive Reserve, and Vascular Risk" Journal of Clinical Medicine 14, no. 22: 8176. https://doi.org/10.3390/jcm14228176
APA StyleEstanga, A., Tellaetxe-Elorriaga, I., Ecay-Torres, M., García Condado, J., García-Sebastián, M., Arriba, M., López, C., Ros, N., Iriondo, A., Reparaz-Escudero, I., Erramuzpe, A., Martínez-Lage, P., & Altuna, M. (2025). Cognitive Age Delta as a Marker of Healthy and Pathological Cognitive Aging: The Role of Lifestyle, Cognitive Reserve, and Vascular Risk. Journal of Clinical Medicine, 14(22), 8176. https://doi.org/10.3390/jcm14228176







