Oropharyngeal Interventions in Intubated Patients for Preventing Ventilator Associated Pneumonia: A Systematic Review and Multi-Variate Network Meta-Analysis Evaluating Pharmacological Agents
Abstract
1. Introduction
2. Methods
2.1. Search Methods
2.2. Eligibility Criteria
- Population: Children and adult patients receiving invasive mechanical ventilation.
- Interventions: Any topical oral intervention aimed at preventing VAP such as Chlorhexidine (various concentrations: 0.12%, 0.2%, 1%, 2%), Povidone-iodine, Probiotics (such as Lactobacillus), Antimicrobial drugs (such as polymyxin, tobramycin), Iseganan, Silver nanoparticles, Hydrogen peroxide, Sodium bicarbonate, Toothbrush, Potassium permanganate, Ozonated water, Nanosil, Miswak, Triclosan, Listerine, Nitrofurazone, Biotene, Amphotericin B, Chinese herbal formulation, Persica, Matrica, Achillea millefolium, Mentha spicata, Chamomile.
- Comparators: Standard of care, placebo, water, saline or any of the above interventions.
- Outcomes: The primary outcome was the incidence of VAP. We considered any definition used by the original study authors, such as clinical pulmonary infection score (CPIS), Centers for Disease Control and Prevention (CDC), American Thoracic Society/Infectious Disease Society of America, Chinese Society for Respiratory Disease or bacteriologic confirmation. The secondary outcomes were all-cause ICU mortality, duration of mechanical ventilation and ICU stay.
2.3. Study Selection and Data Extraction
2.4. Data Synthesis and Statistical Analysis
2.5. Supplementary Propensity Score Analysis
3. Results
3.1. Search Results
3.2. Network Meta-Analysis
3.3. Ranking of Treatments by SUCRA Plots
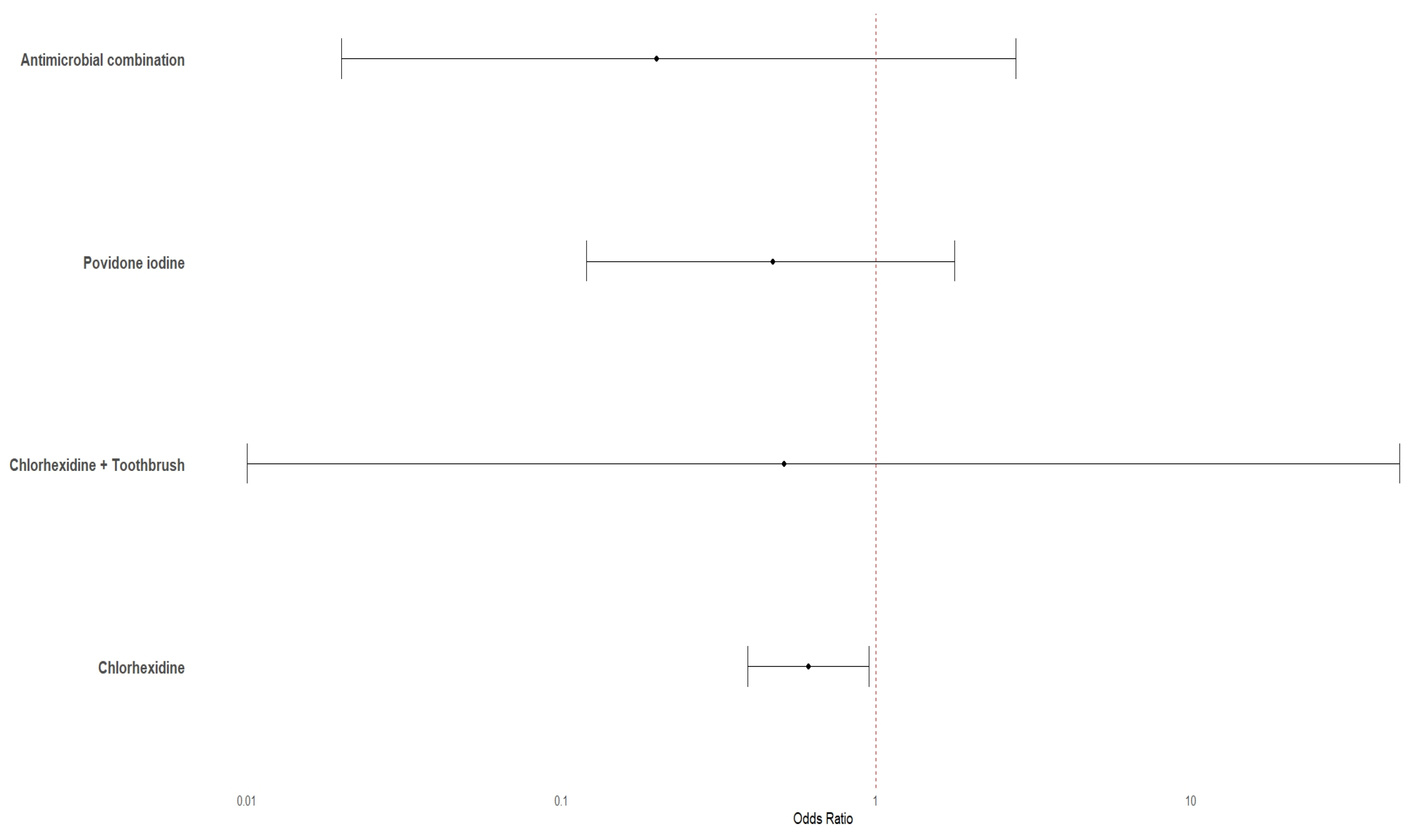
3.4. Pairwise Meta-Analysis with Reference Intervention
3.5. Bootstrap Analyses
3.6. Sub-Group Analyses
3.7. Multivariate Meta-Regression Analysis
3.8. Publication Bias, Risk of Bias and Leave-One-Out Sensitivity Analysis
3.9. Cumulative Meta-Analysis and Trial Sequential Analysis
3.10. Propensity Scores Matching Analysis
3.11. Grading the Strength of Evidence
4. Discussion
4.1. Key Findings
4.2. Comparison with Existing Literature
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Randolph, A.G. A practical approach to evidence-based medicine: Lessons learned from developing ventilator management protocols. Crit. Care Clin. 2003, 19, 515–527. [Google Scholar] [CrossRef]
- Charles, M.P. Ventilator-associated pneumonia. Australas. Med. J. 2014, 7, 334–344. [Google Scholar] [CrossRef]
- Timsit, J.F.; Esaied, W.; Neuville, M.; Bouadma, L.; Mourvillier, B. Update on ventilator-associated pneumonia. F1000Research 2017, 6, 2061. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-associated pneumonia in adults: A narrative review. Intensiv. Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Semet, C. The ongoing challenge of ventilator-associated pneumonia: Epidemiology, prevention, and risk factors for mortality in a secondary care hospital intensive care unit. Infect. Prev. Pract. 2023, 5, 100320. [Google Scholar] [CrossRef]
- Munro, N.; Ruggiero, M. Ventilator-Associated Pneumonia Bundle: Reconstruction for Best Care. AACN Adv. Crit. Care 2014, 25, 163–175. [Google Scholar] [CrossRef]
- Mastrogianni, M.; Katsoulas, T.; Galanis, P.; Korompeli, A.; Myrianthefs, P. The Impact of Care Bundles on Ventilator-Associated Pneumonia (VAP) Prevention in Adult ICUs: A Systematic Review. Antibiotics 2023, 12, 227. [Google Scholar] [CrossRef]
- Subbarao, K.C.; Nattuthurai, G.S.; Sundararajan, S.K.; Sujith, I.; Joseph, J.; Syedshah, Y.P. Gingival Crevicular Fluid: An Overview. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. S2), S135–S139. [Google Scholar] [CrossRef]
- Masur, H. Critically Ill Immunosuppressed Host. In Critical Care Medicine; Parrillo, J.E., Dellinger, R.P., Eds.; Mosby: St. Louis, MO, USA, 2008; pp. 1111–1132. [Google Scholar] [CrossRef]
- Hurley, J.C. Ventilator-associated pneumonia prevention methods using topical antibiotics: Herd protection or herd peril? Chest 2014, 146, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Winning, L.; Lundy, F.T.; Blackwood, B.; McAuley, D.F.; El Karim, I. Oral health care for the critically ill: A narrative review. Crit. Care 2021, 25, 353. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Memish, Z.A.; Bearman, G. Preventing ventilator-associated pneumonia: A position paper of the International Society for Infectious Diseases, 2024 update. Int. J. Infect. Dis. 2025, 151, 107305. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, X.; Zhang, Q.; Li, C.; Worthington, H.V.; Hua, F. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2020, 12, CD008367. [Google Scholar] [CrossRef]
- da Silva Pinto, A.C.; da Silva, B.M.; Santiago-Junior, J.F.; de Carvalho Sales-Peres, S.H. Efficiency of different protocols for oral hygiene combined with the use of chlorhexidine in the prevention of ventilator-associated pneumonia. J. Bras. Pneumol. 2021, 47, e20190286. [Google Scholar] [CrossRef] [PubMed]
- Kocaçal Güler, E.; Türk, G. Oral chlorhexidine against ventilator-associated pneumonia and microbial colonization in intensive care patients. West. J. Nurs. Res. 2019, 41, 901–919. [Google Scholar] [CrossRef]
- Silva, P.U.J.; Paranhos, L.R.; Meneses-Santos, D.; Blumenberg, C.; Macedo, D.R.; Cardoso, S.V. Combination of toothbrushing and chlorhexidine compared with exclusive use of chlorhexidine to reduce the risk of ventilator-associated pneumonia: A systematic review with meta-analysis. Clinics 2021, 76, e2659. [Google Scholar] [CrossRef]
- White, I.R. Network meta-analysis. Stata J. 2015, 15, 951–985. [Google Scholar] [CrossRef]
- Topical Oral Interventions for Preventing Ventilator-Associated Pneumonia: A Systematic Review and Network Meta-Analysis. Available online: https://doi.org/10.17605/OSF.IO/DUCWG (accessed on 18 October 2025).
- Cumpston, M.; Li, T.; Page, M.; Chandler, J.; Welch, V.; Higgins, J.P.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; Volume 10, p. ED000142. [Google Scholar]
- Barker, T.H.; Hasanoff, S.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Habibi, N.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The revised JBI critical appraisal tool for the assessment of risk of bias for cohort studies. JBI Evid. Synth. 2025, 23, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Abele-Horn, M.; Dauber, A.; Bauernfeind, A.; Russwurm, W.; Seyfarth-Metzger, I.; Gleich, P.; Ruckdeschel, G. Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination (SOD). Intensive Care Med. 1997, 23, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo-Rodrigues, W.T.; Menegueti, M.G.; Gaspar, G.G.; Nicolini, E.A.; Auxiliadora-Martins, M.; Basile-Filho, A.; Martinez, R.; Bellissimo-Rodrigues, F. Effectiveness of a dental care intervention in the prevention of lower respiratory tract nosocomial infections among intensive care patients: A randomized clinical trial. Infect. Control Hosp. Epidemiol. 2014, 35, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, D.C.; Bonten, M.J.; Gaillard, C.A.; Paling, J.C.; van der Geest, S.I.; van Tiel, F.H.; Beysens, A.J.; de Leeuw, P.W.; Stobberingh, E.E. Prevention of ventilator-associated pneumonia by oral decontamination: A prospective, randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2001, 164, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Davidson, P.; Masters, J.; Rolls, K.; Ollerton, R. Effects of three approaches to standardized oral hygiene to reduce bacterial colonization and ventilator associated pneumonia in mechanically ventilated patients: A randomised control trial. Int. J. Nurs. Stud. 2011, 48, 681–688. [Google Scholar] [CrossRef]
- Berry, A. A comparison of Listerine® and sodium bicarbonate oral cleansing solutions on dental plaque colonisation and incidence of ventilator associated pneumonia in mechanically ventilated patients: A randomised control trial. Intensiv. Crit. Care Nurs. 2013, 29, 275–281. [Google Scholar] [CrossRef]
- Chacko, R.; Rajan, A.; Lionel, P.; Thilagavathi, M.; Yadav, B.; Premkumar, J. Oral decontamination techniques and ventilator-associated pneumonia. Br. J. Nurs. 2017, 26, 594–599. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, E.-Q.; Yang, Y.-J.; Zhao, S.-Y.; Zhu, C.; Wang, X.-F.; Jing, F.; Sheng, H.-Q.; Yang, Z.-T.; Chen, E.-Z. Prospective observational study to compare oral topical metronidazole versus 0.2% chlorhexidine gluconate to prevent nosocomial pneumonia. Am. J. Infect. Control 2016, 44, 1116–1122. [Google Scholar] [CrossRef]
- Chua, J.V.; Dominguez, E.A.; Sison, C.M.; Berba, R.P. The efficacy of povidone-iodine oral rinse in preventing ventilator-associated pneumonia: A randomized, double-blind, placebo-controlled (VAPOR) trial: Preliminary report. Philipp. J. Microbiol. Infect. Dis. 2004, 33, e61. [Google Scholar]
- Dahiya, U. Decontamination with chlorhexidine gluconate reduces the incidence of ventilator associated pneumonia. Nurs. J. India 2012, 103, 89. [Google Scholar] [CrossRef]
- Darbanian, N.; Nobahar, M.; Ghorbani, R. Effect of propolis mouthwash on the incidence of ventilator-associated pneumonia in intensive care unit patients: A comparative randomized triple-blind clinical trial. BMC Oral. Health 2024, 24, 636. [Google Scholar] [CrossRef] [PubMed]
- de Lacerda Vidal, C.F.; Vidal, A.K.; Monteiro, J.G., Jr.; Cavalcanti, A.; Henriques, A.P.; Oliveira, M.; Godoy, M.; Coutinho, M.; Sobral, P.D.; Vilela, C.Â.; et al. Impact of oral hygiene involving toothbrushing versus chlorhexidine in the prevention of ventilator-associated pneumonia: A randomized study. BMC Infect. Dis. 2017, 17, 112. [Google Scholar] [CrossRef]
- De Riso, I.I.A.J.; Ladowski, J.S.; Dillon, T.A.; Justice, J.W.; Peterson, A.C. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest 1996, 109, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Deschepper, M.; Waegeman, W.; Eeckloo, K.; Vogelaers, D.; Blot, S. Effects of chlorhexidine gluconate oral care on hospital mortality: A hospital-wide, observational cohort study. Intensiv. Care Med. 2018, 44, 1017–1026. [Google Scholar] [CrossRef]
- Dobakhti, F.; Zargar, A.; Naghibi, T. The Impact of Chlorhexidine Mucoadhesive Gel in the Prevention of Ventilator-Associated Pneumonia: A Randomized Clinical Trial. Bull. Emerg. Trauma. 2023, 11, 26–31. [Google Scholar] [CrossRef]
- Dobakhti, F.; Eskandari, M.; Tavakolizadeh, M.; Forouzideh, N.; Dobakhti, P.; Jamshidi, M.; Naghibi, T. Impact of Rose Water Mouthwash on Prevention of Ventilator-Associated Pneumonia in Intensive Care Unit: A Randomized Controlled Trial. Tanaffos 2023, 22, 112–119. [Google Scholar]
- Gerlach, A.T.; Enwere, E.N.; Elofson, K.; Forbes, R.C. Impact of chlorhexidine mouthwash prophylaxis on probable ventilator-associated pneumonia in a surgical intensive care unit. Int. J. Crit. Illn. Inj. Sci. 2016, 6, 3–8. [Google Scholar] [CrossRef]
- Feng, S.; Sun, X.; Chen, Y. Application of different mouthwashes in oral nursing for patients with orotracheal intubation. China Med. Pharm. 2012, 8, 100. [Google Scholar]
- Fourrier, F.; Cau-Pottier, E.; Boutigny, H.; Roussel-Delvallez, M.; Jourdain, M.; Chopin, C. Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensiv. Care Med. 2000, 26, 1239–1247. [Google Scholar] [CrossRef]
- Fourrier, F.; Dubois, D.; Pronnier, P.; Herbecq, P.; Leroy, O.; Desmettre, T.; Pottier-Cau, E.; Boutigny, H.; Di Pompéo, C.; Durocher, A.; et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: A double-blind placebo-controlled multicenter study. Crit. Care Med. 2005, 33, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Franata, D.; Muchtar, F.; Palinrungi, A.S.; Rum, M.; Adil, A. The effectiveness of 0.12% chlorhexidine compared to fluoride toothpaste as an oral hygiene agent in reducing the incidence of VAP and BAL microorganisms in patients with MV in the ICU of Dr. Wahidin Sudirohusodo Hospital. Crit. Care Shock 2025, 28, 5. [Google Scholar]
- Galhardo, L.F.; Ruivo, G.F.; Santos, F.; Ferreira, T.T.; Santos, J.; Leao, M.V.; Pallos, D. Impact of oral care and antisepsis on the prevalence of ventilator-associated pneumonia. Oral Health Prev. Dent. 2020, 18, a44443. [Google Scholar] [CrossRef]
- Genuit, T.; Bochicchio, G.; Napolitano, L.M.; McCarter, R.J.; Roghman, M.-C. Prophylactic chlorhexidine oral rinse decreases ventilator-associated pneumonia in surgical ICU patients. Surg. Infect. 2001, 2, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Gharebaghi, N.; Hasanloei, M.A.V.; Mosarrezaii, A.; Rad, S.M. The effect of combined mouth wash Gentamycine, Colistin and Vancomycine in prevention of Ventilator Associated Pneumonia in mechanical ventilatory patients admitted to intensive care unit: A randomized Clinical Trial. Sci. J. Kurd. Univ. Med. Sci. 2020, 25, 105–116. [Google Scholar] [CrossRef]
- Gholami, Z.; Dianati, M.; Maghami, M.; Afazel, M.R.; Azizi-Fini, I. The Effect of Zataria multiflora Boiss. Mouthwash on the Oral Microbial Load in Patients under Mechanical Ventilation: A Randomized Controlled Trial. Tradit. Integr. Med. 2022, 7, 187–194. [Google Scholar] [CrossRef]
- Haghighat, S.; Mahjobipoor, H.; Gavarti, S.G. Comparative study of the effect of three oral care protocols on ventilator-associated pneumonia in critically ill patients: A clinical trial. Iran. J. Nurs. Midwifery Res. 2022, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hanifi, N.; Masoumi, M.; Jamshidi, M.R.; Faghihzadeh, S. The effect of ozonated water and chlorhexidine gluconate on prevention of ventilator-associated pneumonia: A double-blind, randomized, clinical trial. Iran. Red Crescent Med. J. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Hashemi, S.T.; Alikiaii, B.; Fallah-Medvari, M.A.; Karimi, F.; Fallah-Medvari, A. Comparison of Effects of Chlorhexidine Mouthwash versus Stop-Snoring Mouthwash in Prevention of Ventilator-Associated Pneumonia. J. Isfahan Med. Sch. 2018, 36, 227–232. [Google Scholar]
- Hu, X.; Chen, X. Application of improved oral nursing method to orotracheal intubation. Chin. J. Misdiagnostics 2009, 9, 4058–4059. [Google Scholar]
- Long, Y.; Mou, G.; Zuo, Y.; Lv, F.; Feng, Q.; Du, J. Effect of modified oral nursing method on the patients with orotracheal intubation. J. Nurses Train. 2012, 27, 2290–2293. [Google Scholar]
- Mo, Z.D.; Li, X.L.; Ke, J.Y.; Wu, J.P.; Chen, X.W. Analysis of risk factors in ventilator-associated pneumonia and preventive effect of oral care. Chin. J. Nosocomiology 2016, 26, 698–699. [Google Scholar]
- Xu, J.; Feng, B.; He, L.; Shen, H.; Chen, X.Y. Influence of different oral nursing methods on ventilator-associated pneumonia and oral infection in the patients undergoing mechanical ventilation. J. Nurs. Sci. 2007, 7, 56–57. [Google Scholar]
- Xu, H.L. Application of improved oral nursing method to the prevention of ventilator-associated pneumonia. J. Qilu Nurs. 2008, 14, 15–16. [Google Scholar]
- Zhao, Y. Research on application of Yikou gargle in prevention of ventilation associated pneumonia. Chin. J. Nosocomiology 2012, 23, 5232–5233. [Google Scholar]
- Irani, H.; Sargazi, G.; Dahmardeh, A.R.; Mofrad, Z.P. The effect of oral care with miswak versus chlorhexidine on the incidence of ventilator-associated pneumonia: A clinical trial study. Med. Surg. Nurs. J. 2019, 8, e100387. [Google Scholar] [CrossRef]
- Izadi, M.; Bagheri, M.; Far, A.B.; Bagheri-Baghdasht, M.S.; Ghasemzadeh, G.; Sureda, A.; Soodmand, M. Reduce the risk of ventilator-associated pneumonia in ICU patients by Ozonated water mouthwash: A double-blind randomized clinical trial. Am. J. Infect. Control 2022, 51, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Jácomo, A.D.N.; Carmona, F.; Matsuno, A.K.; Manso, P.H.; Carlotti, A.P.C.P. Effect of oral hygiene with 0.12% chlorhexidine gluconate on the incidence of nosocomial pneumonia in children undergoing cardiac surgery. Infect. Control Hosp. Epidemiol. 2011, 32, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Jahanshir, M.; Nobahar, M.; Ghorbani, R.; Malek, F. Effect of clove mouthwash on the incidence of ventilator-associated pneumonia in intensive care unit patients: A comparative randomized triple-blind clinical trial. Clin. Oral. Investig. 2023, 27, 3589–3600. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.; Samany, F.Q.; Farhood, G.G.; Qodrati, S.; Falakaflaki, B. Evaluating the effect of chlorhexidine and tooth brushing in preventing the ventilator associated pneumonia. J. Adv. Med. Biomed. 2016, 24, 9–17. [Google Scholar]
- Karakaya, Z.; Duyu, M.; Yersel, M.N. Oral mucosal mouthwash with chlorhexidine does not reduce the incidence of ventilator-associated pneumonia in critically ill children: A randomised controlled trial. Aust. Crit. Care 2022, 35, 336–344. [Google Scholar] [CrossRef]
- Kawyannejad, R.; AminiSaman, J.; Mohammadi, S.; Amini, S.; Mirzaei, M.; Karimpour, H. Comparing the effects of orthodentol and chlorhexidine mouthwash on prevention of ventilator-associated pneumonia in patients of intensive care unit: A randomized controlled clinical trial. Sci. J. Kurd. Univ. Med. Sci. 2020, 25, 93–104. [Google Scholar] [CrossRef]
- Khaky, B.; Yazdannik, A.; Mahjoubipour, H. Evaluating the efficacy of nanosil mouthwash on the preventing pulmonary infection in intensive care unit: A randomized clinical trial. Med. Arch. 2018, 72, 206–209. [Google Scholar] [CrossRef]
- Kiabi, F.H.; Baradari, A.G.; Kiasari, A.Z.; Shahheidari, M. The difference in mouthwash side effects of persica and chlorhexidine for preventing ventilator-induced pneumonia among patients admitted to the intensive care unit. Open Public Health J. 2023, 16, e187494452305085. [Google Scholar] [CrossRef]
- Klarin, B.; Adolfsson, A.; Torstensson, A.; Larsson, A. Can probiotics be an alternative to chlorhexidine for oral care in the mechanically ventilated patient? A multicentre, prospective, randomised controlled open trial. Crit. Care 2018, 22, 272. [Google Scholar] [CrossRef] [PubMed]
- Koeman, M.; van der Ven, A.J.A.M.; Hak, E.; Joore, H.C.A.; Kaasjager, K.; de Smet, A.G.A.; Ramsay, G.; Dormans, T.P.J.; Aarts, L.P.H.J.; de Bel, E.E.; et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2006, 173, 1348–1355. [Google Scholar] [CrossRef]
- Kollef, M.; Pittet, D.; García, M.S.; Chastre, J.; Fagon, J.-Y.; Bonten, M.; Hyzy, R.; Fleming, T.R.; Fuchs, H.; Bellm, L.; et al. A randomized double-blind trial of iseganan in prevention of ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2006, 173, 91–97. [Google Scholar] [CrossRef]
- Kusahara, D.M.; Peterlini, M.A.S.; Pedreira, M.L.G. Oral care with 0.12% chlorhexidine for the prevention of ventilator-associated pneumonia in critically ill children: Randomised, controlled and double blind trial. Int. J. Nurs. Stud. 2012, 49, 1354–1363. [Google Scholar] [CrossRef]
- Lev, A.; Aied, A.S.; Arshed, S. The effect of different oral hygiene treatments on the occurrence of ventilator associated pneumonia (VAP) in ventilated patients. J. Infect. Prev. 2015, 16, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Leyderman, I.N.; Marichev, A.O.; Kasherininov, I.U.; Lesteva, N.A.; Ponomareva, A.D.; Sivcov, A.O.; Ryabova, D.V.; Nosenko, M.M.; Ablesimov, G.A. The Main Effects of the Original Oral Care Protocol Implementation in Patients on Invasive Mechanical Ventilation. Obs. Reanimatol.—Gen. Reanimatol. 2024, 20, 39–47. [Google Scholar] [CrossRef]
- Li, D.-F.; Shi, C.-X.; Zhao, L.; Shi, F.-Z.; Jiang, M.-L.; Kang, W.-Q. Prevention of neonatal ventilator-associated pneumonia through oral care with the combined use of colostrum and sodium bicarbonate. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.-Y.; Chang, C.-K.; Maa, S.-H.; Wang, C.; Chen, C.C.-H. Brushing teeth with purified water to reduce ventilator-associated pneumonia. J. Nurs. Res. 2011, 19, 289–297. [Google Scholar] [CrossRef]
- Lorente, L.; Lecuona, M.; Jiménez, A.; Palmero, S.; Pastor, E.; Lafuente, N.; Ramos, M.J.; Mora, M.L.; Sierra, A. Ventilator-associated pneumonia with or without toothbrushing: A randomized controlled trial. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2621–2629. [Google Scholar] [CrossRef]
- Maarefvand, A.; Heidari, M.R.; Ebadi, A.; Kazemnejad, A. Comparing the effects of matrica and chlorhexidine on the prevention of ventilator-associated pneumonia. Mod. Care J. 2015, 12, 114–118. [Google Scholar]
- Tavakoli, H.; Meidani, M.; Khorvash, F.; Abbasi, S.; Cheshmavar, M. Oropharyngeal irrigation to prevent ventilator-associated-pneumonia: Comparing potassium permangenate with chlorhexidine. Int. J. Prev. Med. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Meinberg, M.C.; Cheade, M.D.; Miranda, A.L.; Fachini, M.M.; Lobo, S.M. The use of 2% chlorhexidine gel and toothbrushing for oral hygiene of patients receiving mechanical ventilation: Effects on ventilator-associated pneumonia. Rev. Bras. Ter. Intensiv. 2012, 24, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, R.S.; Rahnama, M.; Abdollahimohammad, A.; Naderifar, M. Comparison of rinsing with chlorhexidine and matrica mouthwashes on the ventilator-associated pneumonia. Rom. J. Mil. Med. 2022, 125, 213–219. [Google Scholar] [CrossRef]
- Mohseni, P.; Sediqi, L.; Shiri, H.; Kamali, A. Comparative Study of the Effect of Botanical Extract Mouthwashes of Tea Tree Oil/Aloe Vera and Chlorhexidine on Prevention of Ventilator-associated Pneumonia in Admitted Patients in Intensive Care Units of Arak Hospitals, 2022. J. Maz. Univ. Med. Sci. 2024, 33, 125–137. [Google Scholar]
- Mori, H.; Hirasawa, H.; Oda, S.; Shiga, H.; Matsuda, K.; Nakamura, M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med. 2006, 32, 230–236. [Google Scholar] [CrossRef]
- Nasiriani, K.; Torki, F.; Jarahzadeh, M.H.; Maybodi, F.R. The effect of brushing with a soft toothbrush and distilled water on the incidence of ventilator-associated pneumonia in the intensive care unit. Tanaffos 2016, 15, 101–107. [Google Scholar] [PubMed]
- Nicolosi, L.N.; Rubio, M.d.C.; Martinez, C.D.; González, N.N.; Cruz, M.E. Effect of oral hygiene and 0.12% chlorhexidine gluconate oral rinse in preventing ventilator-associated pneumonia after cardiovascular surgery. Respir. Care 2014, 59, 504–509. [Google Scholar] [CrossRef]
- Nobahar, M.; Razavi, M.R.; Malek, F.; Ghorbani, R. Effects of hydrogen peroxide mouthwash on preventing ventilator-associated pneumonia in patients admitted to the intensive care unit. Braz. J. Infect. Dis. 2016, 20, 444–450. [Google Scholar] [CrossRef]
- Ory, J.; Raybaud, E.; Chabanne, R.; Cosserant, B.; Faure, J.S.; Guérin, R.; Calvet, L.; Pereira, B.; Mourgues, C.; Guelon, D.; et al. Comparative study of 2 oral care protocols in intensive care units. Am. J. Infect. Control 2017, 45, 245–250. [Google Scholar] [CrossRef]
- Özçaka, Ö.; Başoğlu, O.K.; Buduneli, N.; Taşbakan, M.S.; Bacakoğlu, F.; Kinane, D.F. Chlorhexidine decreases the risk of ventilator--associated pneumonia in intensive care unit patients: A randomized clinical trial. J. Periodontal Res. 2012, 47, 584–592. [Google Scholar] [CrossRef]
- Panchabhai, T.S.; Dangayach, N.S.; Krishnan, A.; Kothari, V.M.; Karnad, D.R. Oropharyngeal cleansing with 0.2% chlorhexidine for prevention of nosocomial pneumonia in critically ill patients: An open-label randomized trial with 0.01% potassium permanganate as control. Chest 2009, 135, 1150–1156. [Google Scholar] [CrossRef]
- Pedreira, M.L.; Kusahara, D.M.; de Carvalho, W.B.; Núñez, S.C.; Peterlini, M.A.S. Oral care interventions and oropharyngeal colonization in children receiving mechanical ventilation. Am. J. Crit. Care 2009, 18, 319–328. [Google Scholar] [CrossRef]
- Pobo, A.; Lisboa, T.; Rodriguez, A.; Sole, R.; Magret, M.; Trefler, S.; Gómez, F.; Rello, J.; RASPALL Study Investigators. A randomized trial of dental brushing for preventing ventilator-associated pneumonia. Chest 2009, 136, 433–439. [Google Scholar] [CrossRef]
- Pugin, J.; Auckenthaler, R.; Lew, D.P.; Suter, P.M. Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia: A randomized, placebo-controlled, double-blind clinical trial. JAMA 1992, 265, 2704–2710. [Google Scholar] [CrossRef]
- Rezvani, M.; Alikiaii, B.; Kiani, P. Comparison of the effect of chlorhexidine mouthwash with Matrika mouthwash drop on probable ventilator-associated pneumonia in intensive care unit. Arch. Anesthesiol. Crit. Care 2018, 4, 492–496. [Google Scholar]
- Rodriguez-Roldán, J.; Altuna-Cuesta, A.; López, A.; Carrillo, A.; Garcia, J.; León, J.; Martínez-Pellus, A.J. Prevention of nosocomial lung infection in ventilated patients: Use of an antimicrobial pharyngeal nonabsorbable paste. Crit. Care Med. 1990, 18, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Kes, D.; Yildirim, T.A.; Kuru, C.; Pazarlıoglu, F.; Ciftci, T.; Ozdemir, M. Effect of 0.12% chlorhexidine use for oral care on ventilator-associated respiratory infections: A randomized controlled trial. J. Trauma Nurs. JTN 2021, 28, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Houston, S.; Hougland, P.; Anderson, J.J.; LaRocco, M.; Kennedy, V.; Gentry, L.O. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am. J. Crit. Care 2002, 11, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Thao, P.T.N.; Ishikane, M.; Xuan, P.T.; Kutsuna, S.; Dai, H.Q.; Ohtsu, H.; Kimura, T.; Kiyohara, H.; Shimada, Y.; et al. Physical oral care prevents ventilator-associated pneumonia in Vietnam: A prospective interventional study. J. Infect. Chemother. 2022, 28, 1632–1638. [Google Scholar] [CrossRef]
- Scannapieco, A.F.; Yu, J.; Raghavendran, K.; Vacanti, A.; Owens, S.I.; Wood, K.; Mylotte, J.M. A randomized trial of chlorhexidine gluconate on oral bacterial pathogens in mechanically ventilated patients. Crit. Care 2009, 13, R117. [Google Scholar] [CrossRef]
- Sebastian, M.R.; Lodha, R.; Kapil, A.; Kabra, S.K. Oral mucosal decontamination with chlorhexidine for the prevention of ventilator-associated pneumonia in children—A randomized, controlled trial. Pediatr. Crit. Care Med. 2012, 13, e305–e310. [Google Scholar] [CrossRef] [PubMed]
- Seguin, P.; Tanguy, M.; Laviolle, B.; Tirel, O.; Mallédant, Y. Effect of oropharyngeal decontamination by povidone-iodine on ventilator-associated pneumonia in patients with head trauma. Crit. Care Med. 2006, 34, 1514–1519. [Google Scholar] [CrossRef]
- Seguin, P.; Laviolle, B.; Dahyot-Fizelier, C.; Dumont, R.; Veber, B.; Gergaud, S.; Asehnoune, K.; Mimoz, O.; Donnio, P.-Y.; Bellissant, E.; et al. Effect of oropharyngeal povidone-iodine preventive oral care on ventilator-associated pneumonia in severely brain-injured or cerebral hemorrhage patients: A multicenter, randomized controlled trial. Crit. Care Med. 2014, 42, 1–8. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kaur, J. Randomized control trial on efficacy of chlorhexidine mouth care in prevention of ventilator associated pneumonia (VAP). Nurs. Midwifery Res. J. 2012, 8, 169–178. [Google Scholar] [CrossRef]
- Shorofi, S.A.; Golchin-Mehr, S.; Mousavinasab, S.N.; Arbon, P.; Saeedi, M.; Ebrahimzadeh, M.A. Effects of Zataria multiflora mouthwash and chlorhexidine compared to chlorhexidine alone on the incidence of ventilator-associated pneumonia in patients admitted to intensive care units. Complement. Ther. Clin. Pract. 2025, 60, 101966. [Google Scholar] [CrossRef]
- Singh, P.; Arshad, Z.; Srivastava, V.K.; Singh, G.P.; Gangwar, R.S. Efficacy of oral care protocols in the prevention of ventilator-associated pneumonia in mechanically ventilated patients. Cureus 2022, 14, e23750. [Google Scholar] [CrossRef]
- Siriyanyongwong, P.; Teanpaisan, R.; Pahumunto, N.; Uppanisakorn, S.; Vattanavanit, V. Efficacy of Moraceae with chlorhexidine mouthwash on the microbial flora of critically ill intubated patients: A randomized controlled pilot study. Sci. Rep. 2022, 12, 17261. [Google Scholar] [CrossRef]
- Stefanescu, B.M.; Hétu, C.; Slaughter, J.C.; O’sHea, T.M.; Shetty, A.K. A pilot study of Biotene OralBalance® gel for oral care in mechanically ventilated preterm neonates. Contemp. Clin. Trials 2013, 35, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Takeyasu, Y.; Yamane, G.-Y.; Tonogi, M.; Watanabe, Y.; Nishikubo, S.; Serita, R.; Imura, K. Ventilator-associated pneumonia risk decreased by use of oral moisture gel in oral health care. Bull. Tokyo Dent. Coll. 2014, 55, 95–102. [Google Scholar] [CrossRef]
- Tantipong, H.; Morkchareonpong, C.; Jaiyindee, S.; Thamlikitkul, V. Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infect. Control Hosp. Epidemiol. 2008, 29, 131–136. [Google Scholar] [CrossRef]
- Tuon, F.F.; Gavrilko, O.; de Almeida, S.; Sumi, E.R.; Alberto, T.; Rocha, J.L.; Rosa, E.A. Prospective, randomised, controlled study evaluating early modification of oral microbiota following admission to the intensive care unit and oral hygiene with chlorhexidine. J. Glob. Antimicrob. Resist. 2017, 8, 159–163. [Google Scholar] [CrossRef]
- Vyas, N.; Mathur, P.; Jhawar, S.; Prabhune, A.; Vimal, P. Effectiveness of Oral hygiene with chlorhexidine mouthwash with 0.12% and 0.2% concentration on incidence of ventilator associated pneumonia (VAP) in intubated patients–A parallel arm double blind randomized controlled trial. Ann. Int. Med. Dent. Res. 2021, 7, 6. [Google Scholar]
- Yadav, M.S.; Singh, V.K.; Singh, D. Efficacy of Gentamicin versus Chlorhexidine as a Sole Prophylactic Oral Decontaminant in Reducing the Incidence of Ventilator Associated Pneumonia: A Randomised Clinical Study. J. Clin. Diagn. Res. 2022, 16, 40–43. [Google Scholar] [CrossRef]
- Yu, X.-R.; Xu, N.; Huang, S.-T.; Zhang, Q.-L.; Wang, Z.-C.; Cao, H.; Chen, Q. Effects of different oral care strategies on postoperative pneumonia in infants with mechanical ventilation after cardiac surgery: A prospective randomized controlled study. Transl. Pediatr. 2021, 10, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-R.; Huang, S.-T.; Xu, N.; Dai, W.-S.; Wang, Z.-C.; Cao, H.; Chen, Q. Comparison of the effect of breast milk and sodium bicarbonate solution for oral care in infants with tracheal intubation after cardiothoracic surgery. Breastfeed. Med. 2020, 16, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Zand, F.; Zahed, L.; Mansouri, P.; Dehghanrad, F.; Bahrani, M.; Ghorbani, M. The effects of oral rinse with 0.2% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated pneumonia in adults’ intensive care units. J. Crit. Care 2017, 40, 318–322. [Google Scholar] [CrossRef]
- Zarinfar, N.; Ghaznavi-Rad, E.; Mahmoodiyeh, B.; Reyhani, A. Comparison of three interventional approaches to prevent ventilator-associated pneumonia in intensive care units (ICUs): A clinical trial study. Qatar Med. J. 2021, 2021, 21. [Google Scholar] [CrossRef]
- Bellissimo-Rodrigues, F.; Bellissimo-Rodrigues, W.T.; Viana, J.M.; Teixeira, G.C.A.; Nicolini, E.; Auxiliadora-Martins, M.; Passos, A.D.C.; Martinez, E.Z.; Basile-Filho, A.; Martinez, R. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infect. Control Hosp. Epidemiol. 2009, 30, 952–958. [Google Scholar] [CrossRef]
- Bopp, M.; Darby, M.; Loftin, K.C.; Broscious, S. Effects of daily oral care with 0.12% chlorhexidine gluconate and a standard oral care protocol on the development of nosocomial pneumonia in intubated patients: A pilot study. Am. Dent. Hyg. Assoc. 2006, 80, 9. [Google Scholar]
- Ćabov, T.; Macan, D.; Husedžinović, I.; Škrlin-Šubić, J.; Bošnjak, D.; Šestan-Crnek, S.; Perić, B.; Kovač, Z.; Golubović, V. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive-care patients: A randomized placebo-controlled study. Wien. Klin. Wochenschr. 2010, 122, 397–404. [Google Scholar] [CrossRef]
- de Smet, A.; Kluytmans, J.; Cooper, B.; Mascini, E.; Benus, R.; van der Werf, T.; van der Hoeven, J.; Pickkers, P.; Bogaers-Hofman, D.; van der Meer, N.; et al. Decontamination of the digestive tract and oropharynx in ICU patients. New Engl. J. Med. 2009, 360, 20–31. [Google Scholar] [CrossRef]
- Loha, S.; Kumar, S.; Reena; Yadav, G.; Jayanthi, A.; Rath, A.; Banerjee, T.; Yadav, R.S. The effect of alkalinization of oral cavity by sodium bicarbonate mouth wash to decrease ventilator-associated pneumonia in traumatic brain injury patients: A prospective randomized controlled study. Trends Anaesth. Crit. Care 2022, 46, 2–7. [Google Scholar] [CrossRef]
- Munro, C.L.; Grap, M.J.; Jones, D.J.; McClish, D.K.; Sessler, C.N. Chlorhexidine, toothbrushing, and preventing ventilator-associated pneumonia in critically ill adults. Am. J. Crit. Care 2009, 18, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, T.B.S.; Vivas, X.S.G.; Santos, C.B.; Mestre, V.d.F.; Maddela, N.R.; Santana, L.E.G.; de Almeida, R.S.C. Evaluation of brushing efficiency in reducing oral microbiota in mechanically ventilated patients admitted to an intensive care unit. Infect. Prev. Pract. 2024, 6, 100346. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lighvan, N.L.; McCredie, V.; Pechlivanoglou, P.; Krahn, M.; Quiñonez, C.; Azarpazhooh, A. Chlorhexidine-related mortality rate in critically ill subjects in intensive care units: A systematic review and meta-analysis. Respir. Care 2019, 64, 337–349. [Google Scholar] [CrossRef]
- Park, H.M.; Ryu, S.; Jo, E.; Yoo, S.K.; Kim, Y.W. A Study on the Biofilm Removal Efficacy of a Bioelectric Toothbrush. Bioengineering 2023, 10, 1184. [Google Scholar] [CrossRef]
- Ohsumi, T.; Takenaka, S.; Sakaue, Y.; Suzuki, Y.; Nagata, R.; Hasegawa, T.; Ohshima, H.; Terao, Y.; Noiri, Y. Adjunct use of mouth rinses with a sonic toothbrush accelerates the detachment of a Streptococcus mutans biofilm: An in vitro study. BMC Oral Health 2020, 20, 161. [Google Scholar] [CrossRef]
- Ramirez, D.M.; Schweizer, F. Development of polymyxin- and aminoglycoside-based outer membrane permeabilizers: A review. Front. Microbiol. 2025, 16, 1625300. [Google Scholar] [CrossRef] [PubMed]
- Maihoub, S.; Krasznai, M.; Molnár, A. A comparison of locally acting antiseptics, increasingly recommended as an alternative to antibiotics. Orvosi Hetil. 2024, 165, 1621–1627. [Google Scholar] [CrossRef]
- Sridharan, K.; Hasan, H.; Al Jufairi, M.; Al Daylami, A.; Pasha, S.A.A.; Al Ansari, E. Drug utilisation in adult, paediatric and neonatal intensive care units, with an emphasis on systemic antimicrobials. Anaesthesiol. Intensiv. Ther. 2021, 53, 18–24. [Google Scholar] [CrossRef] [PubMed]


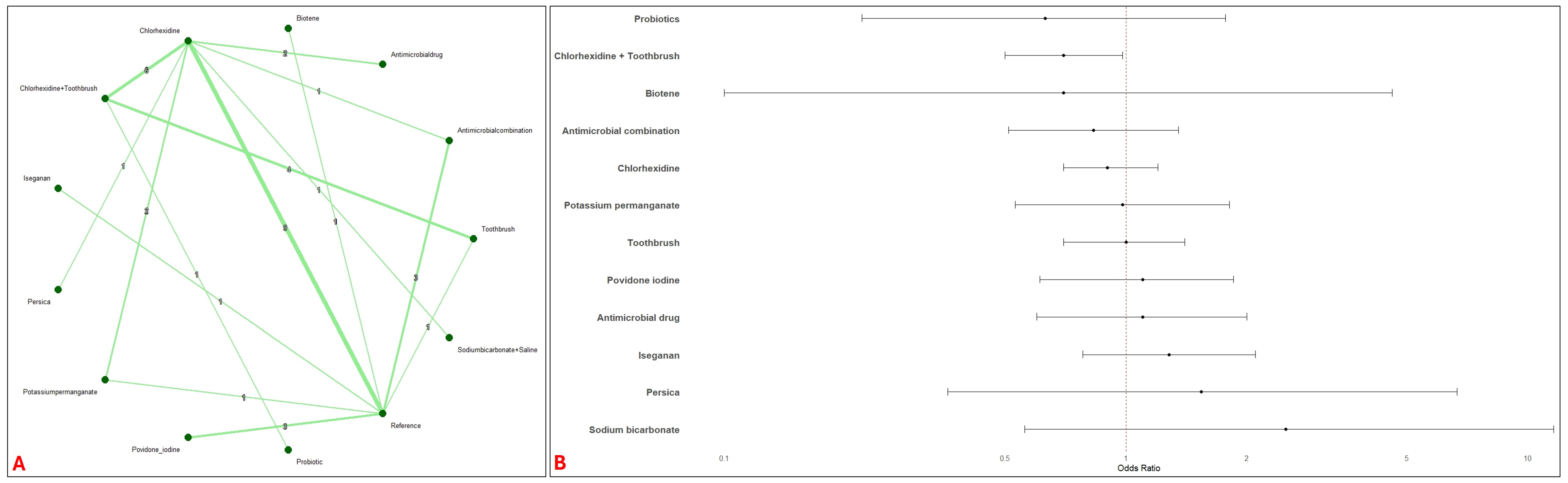

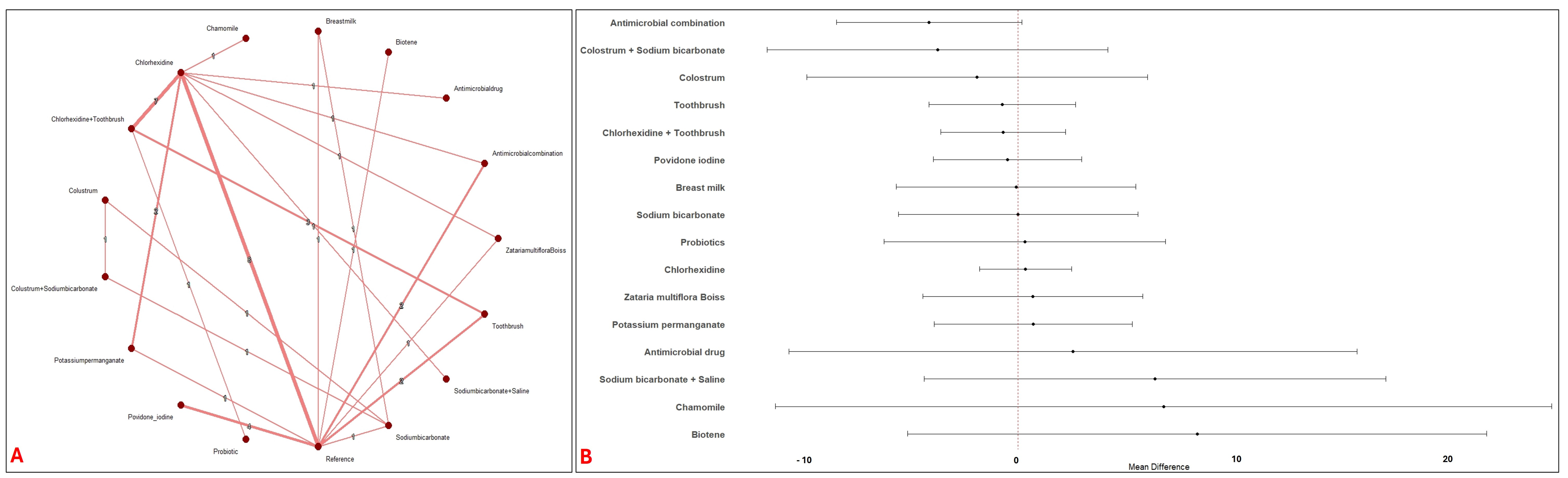
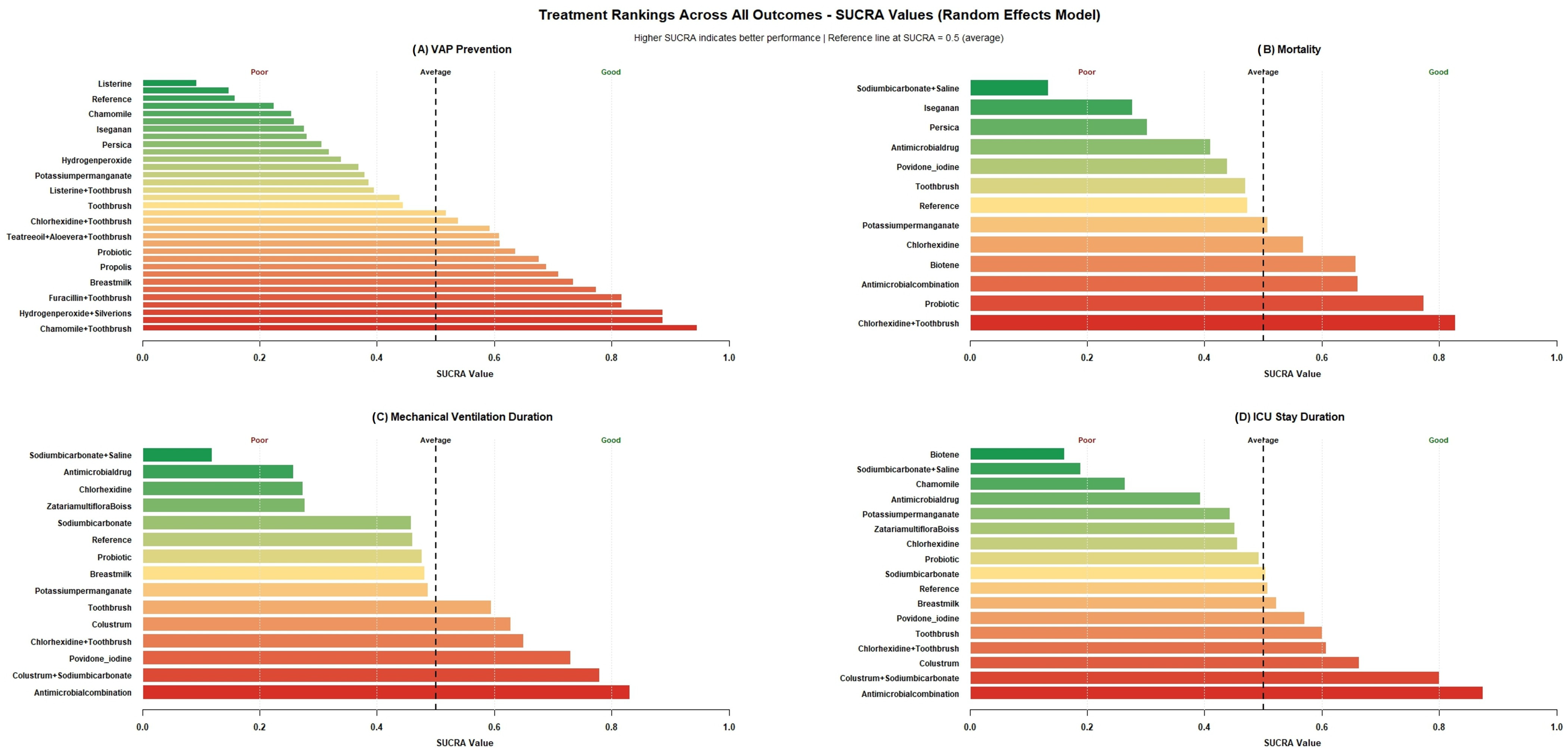
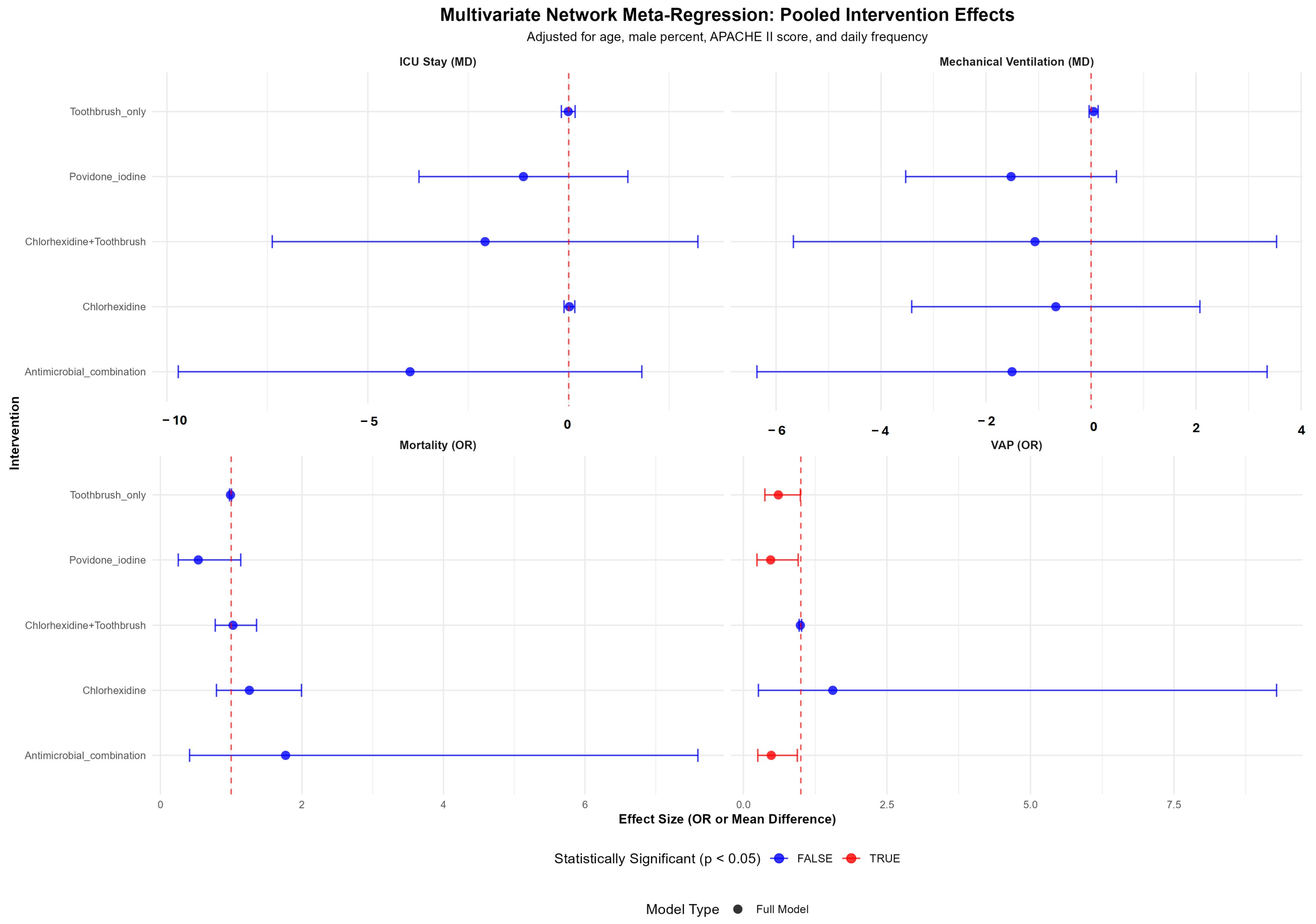

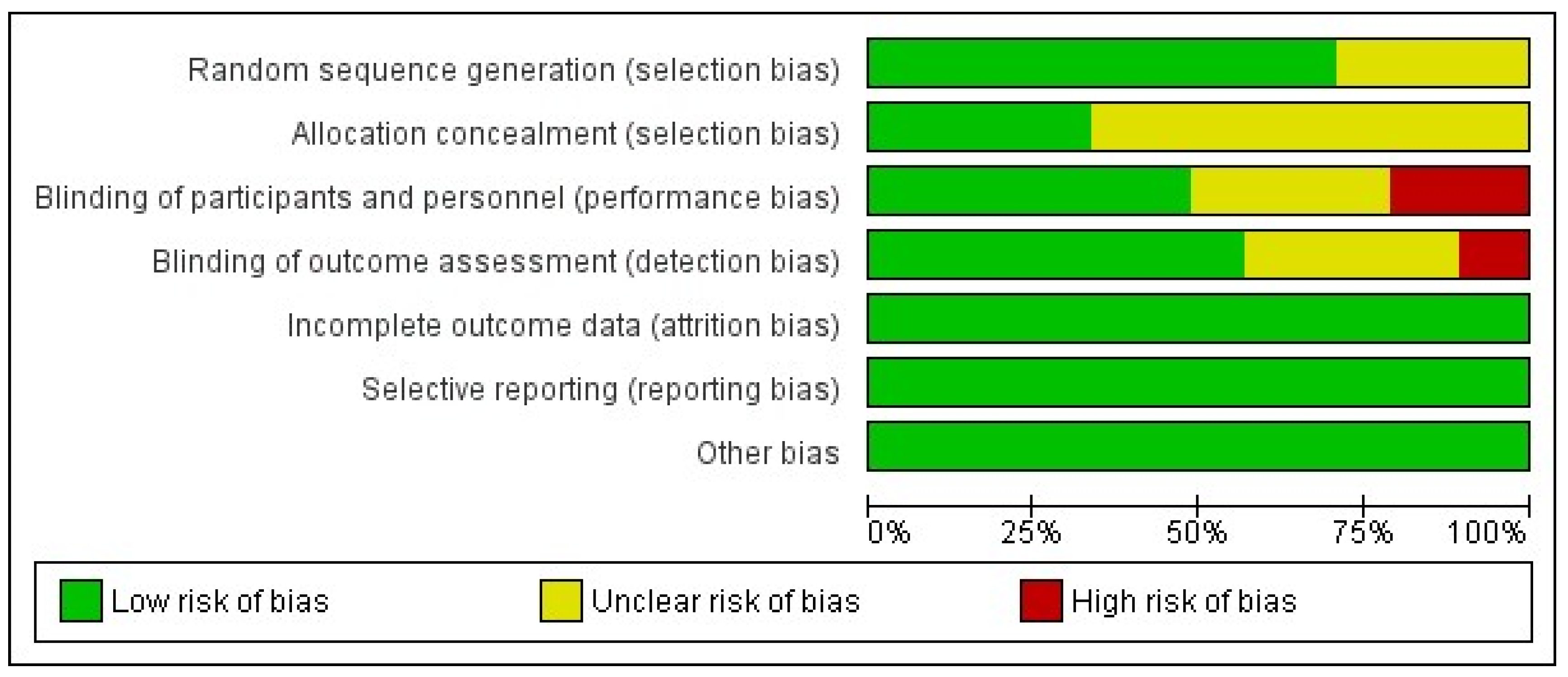
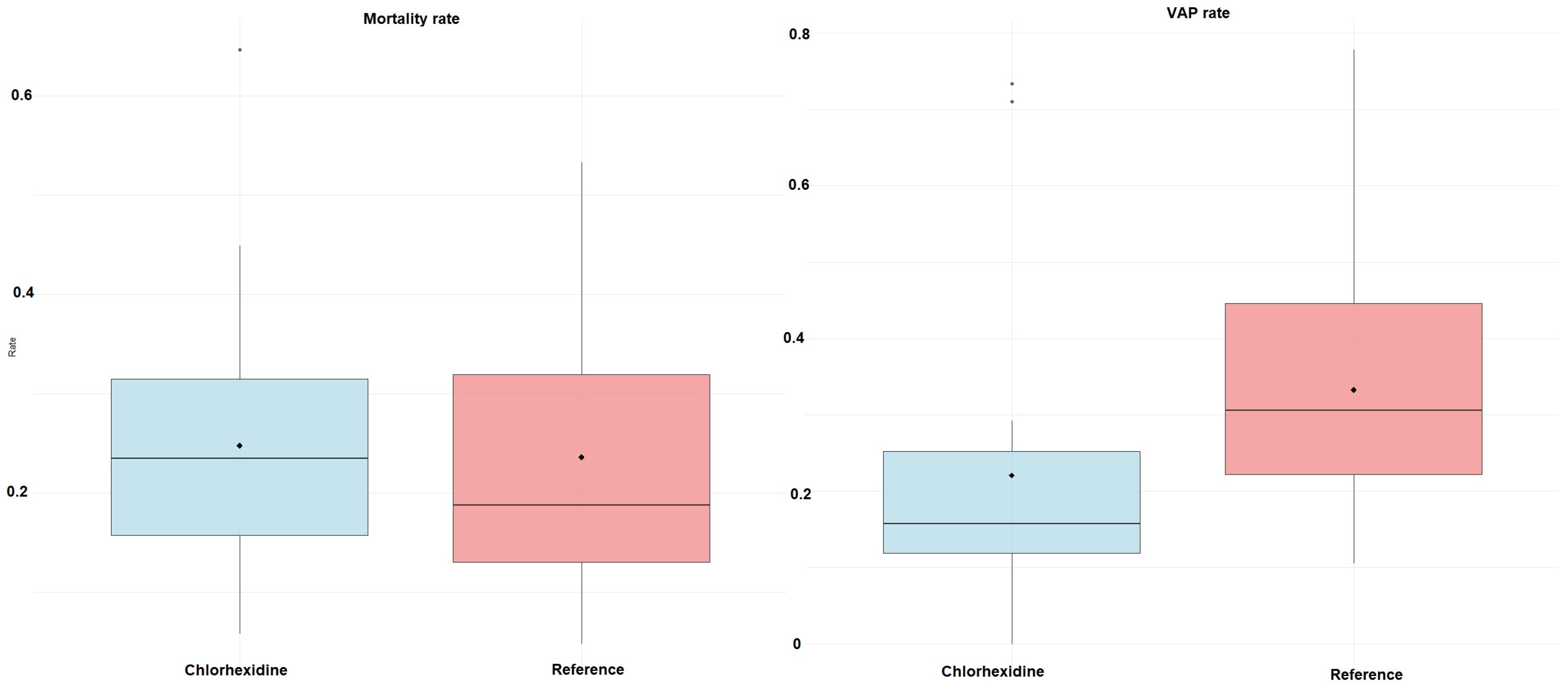
| Study Id | Year | Country | ICU Setting | Study Design | VAP Bundle Adequacy | Interventions | Toothbrush Co-Intervention | Concentration (%) | Daily Frequency | Total Numbers | Mean Age (Years) | Male (%) | Severity of Illness Scale | Severity Score | VAP Definition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abele-Horn [22] | 1997 | Germany | Anesthesiology | RCT | 1 | Antimicrobial combination | No | 2 | 4 | 58 | 39.9 | 50 | APACHE II | 16 | CPIS |
| Standard of care | No | NA | Not mentioned | 30 | 44.7 | 80 | APACHE II | 18 | CPIS | ||||||
| Bellissimo-Rodrigues [23] | 2014 | Brazil | Mixed | RCT | 0 | Chlorhexidine + Toothbrush | Yes | 2 or 0.12 | Not mentioned | NA | NA | NA | Not provided | NA | NA |
| Chlorhexidine | No | 2 or 0.12 | Not mentioned | NA | NA | NA | Not provided | NA | NA | ||||||
| Bergmans [24] | 2001 | Netherlands | Mixed | RCT | 2 | Antimicrobial combination | No | 2 | 4 | 87 | 56.6 | 68 | APACHE II | 21 | CDC |
| Water | No | NA | 4 | 61 | 58.7 | 77 | APACHE II | 21.2 | CDC | ||||||
| Berry [25] | 2011 | Australia | Mixed | RCT | 1 | Water + Toothbrush | Yes | NA | 12 | 78 | 59.1 | 56.4 | APACHE II | 21.64 | Pugin’s |
| Sodium bicarbonate + Toothbrush | Yes | NA | 12 | 76 | 60.4 | 55.3 | APACHE II | 22 | Pugin’s | ||||||
| Chlorhexidine + Toothbrush | Yes | 0.2 | 12 | 71 | 58.2 | 49.3 | APACHE II | 22.8 | Pugin’s | ||||||
| Berry [26] | 2013 | Australia | Mixed | RCT | 4 | Listerine + Toothbrush | Yes | NA | 2 | 127 | 59.96 | 57.5 | APACHE II | 21.21 | Pugin’s |
| Sodium bicarbonate + Toothbrush | Yes | NA | 12 | 133 | 54.93 | 59.4 | APACHE II | 21.38 | Pugin’s | ||||||
| Water + Toothbrush | Yes | NA | 12 | 138 | 58.82 | 60.9 | APACHE II | 20.86 | Pugin’s | ||||||
| Chacko [27] | 2017 | India | Medical | RCT | 2 | Chlorhexidine | No | 0.2 | 8 | 104 | 45.91 | 67.3 | Not provided | NA | CDC |
| Chlorhexidine + Toothbrush | Yes | 0.2 | 8 | 102 | 41.02 | 44.1 | Not provided | NA | CDC | ||||||
| Chen [28] | 2016 | China | Emergency | RCT | 2 | Antimicrobial drug | No | 0.08 | 2 | 40 | 64.7 | 60 | APACHE II | 16.1 | CPIS |
| Chlorhexidine | No | 0.2 | 2 | 155 | 62.6 | 99 | APACHE II | 16.1 | CPIS | ||||||
| Chua [29] | 2004 | Philippines | Mixed | RCT | 2 | Povidone iodine | No | 1 | 3 | 22 | 51.4 | 31.8 | APACHE II | 15.9 | CDC |
| Water | No | NA | 3 | 20 | 55.2 | 50 | APACHE II | 17 | CDC | ||||||
| Dahiya [30] | 2012 | India | Medical | RCT | 0 | Chlorhexidine | No | 0.2 | 2 | 35 | NA | NA | Not provided | NA | Not specified |
| Hydrogen peroxide | No | NA | 2 | 35 | NA | NA | Not provided | NA | Not specified | ||||||
| Darbanian [31] | 2024 | Iran | Medical | RCT | 2 | Propolis | No | 0.06 | 2 | 55 | NA | 49.1 | Not provided | NA | CPIS |
| Chlorhexidine | No | 0.2 | 2 | 55 | NA | 49.1 | Not provided | NA | CPIS | ||||||
| de Lacerda Vidal [32] | 2017 | Brazil | Mixed | RCT | 3 | Chlorhexidine + Toothbrush | Yes | 0.12 | 2 | 105 | 59.4 | 48.6 | APACHE II | 21.9 | ATS/IDSA |
| Chlorhexidine | No | 0.12 | 2 | 108 | 63.2 | 50 | APACHE II | 22.2 | ATS/IDSA | ||||||
| De Riso [33] | 1996 | USA | Cardiothoracic | RCT | 1 | Chlorhexidine | No | 0.12 | 2 | 173 | 64.1 | 68.8 | Not provided | NA | NA |
| Water | No | NA | 2 | 180 | 63.5 | 68.3 | Not provided | NA | NA | ||||||
| Deschepper [34] | 2018 | Belgium | Mixed | Cohort | 0 | Chlorhexidine | No | 0.05 or 0.12 | 2 or 3 | NA | NA | NA | Not provided | NA | NA |
| No Chlorhexidine | No | NA | 2 or 3 | NA | NA | NA | Not provided | NA | NA | ||||||
| Dobakhti [35] | 2023 | Iran | Surgical | RCT | 0 | Chlorhexidine | No | 0.2 | 3 | 32 | NA | 62.5 | APACHE II | 18.12 | CPIS |
| Chlorhexidine | No | 0.2 | 3 | 32 | NA | 56.2 | APACHE II | 18.9 | CPIS | ||||||
| Dobakhti [36] | 2023 | Iran | Surgical | RCT | 0 | Rose water + Chlorhexidine | No | 0.12 | 3 | 40 | 45.75 | 60 | APACHE II | 18.25 | CPIS |
| Chlorhexidine | No | 0.12 | 3 | 40 | 45.99 | 62.5 | APACHE II | 19.37 | CPIS | ||||||
| Enwere [37] | 2016 | USA | Surgical | Cohort | 2 | Chlorhexidine + Toothbrush | Yes | 0.12 | 2 | 64 | 57 | 67.2 | Not provided | NA | CDC |
| Toothbrush | Yes | NA | 2 | 94 | 59 | 71.3 | Not provided | NA | CDC | ||||||
| Feng [38] | 2012 | China | Medical | RCT | 0 | Povidone iodine + Toothbrush | Yes | 0.05 | 4 | 71 | 43.7 | NA | Not provided | NA | CSRD |
| Nitrofurazone + Toothbrush | Yes | NA | 4 | 65 | 38.5 | NA | Not provided | NA | CSRD | ||||||
| Saline + Toothbrush | Yes | NA | 4 | 68 | 40.3 | NA | Not provided | NA | CSRD | ||||||
| Fourrier [39] | 2000 | France | Medical | RCT | 0 | Chlorhexidine | No | 0.2 | 3 | 30 | 51.2 | 63.3 | APACHE II | 37 | NA |
| Sodium bicarbonate + Saline | No | NA | 4 | 30 | 50.4 | 63.3 | APACHE II | 33 | NA | ||||||
| Fourrier [40] | 2005 | France | Medical | RCT | 0 | Chlorhexidine | No | 0.2 | 3 | 114 | 61 | 72.8 | Not provided | NA | Clinical + Radiological + Microbiological |
| Placebo | No | NA | 3 | 114 | 61.1 | 64 | Not provided | NA | Clinical + Radiological + Microbiological | ||||||
| Franata [41] | 2025 | Indonesia | Unclear | RCT | 0 | Chlorhexidine | No | 0.12 | 2 | 10 | 39.2 | NA | Not provided | NA | CPIS |
| Fluoride toothpaste + Toothbrush | Yes | NA | 2 | 10 | 48.5 | NA | Not provided | NA | CPIS | ||||||
| Galhardo [42] | 2020 | Brazil | Unclear | Cohort | 0 | Chlorhexidine + Toothbrush | Yes | 0.12 | Not mentioned | 329 | NA | NA | Not provided | NA | Clinical |
| No Chlorhexidine | No | NA | Not mentioned | 229 | NA | NA | Not provided | NA | Clinical | ||||||
| Genuit [43] | 2000 | USA | Surgical | Non-randomized | 0 | Chlorhexidine | No | 0.12 | 2 | 56 | 68.8 | 100 | Not provided | NA | NA |
| No Chlorhexidine | No | NA | Not mentioned | 39 | 69.2 | 98 | Not provided | NA | NA | ||||||
| Gharebaghi [44] | 2020 | Iran | Medical | RCT | 0 | Antimicrobial combination | No | 2 | 4 | 52 | NA | NA | Not provided | NA | Clinical + Radiological + Microbiological |
| Chlorhexidine | No | 0.2 | 4 | 53 | NA | NA | Not provided | NA | Clinical + Radiological + Microbiological | ||||||
| Gholami [45] | 2021 | Iran | Unclear | RCT | 0 | Zataria multiflora Boiss. | No | 10 | 3 | 30 | NA | 73.3 | Not provided | NA | NA |
| Chlorhexidine | No | 0.2 | 3 | 30 | NA | 66.7 | Not provided | NA | NA | ||||||
| Saline | No | NA | 3 | 30 | NA | 46.7 | Not provided | NA | NA | ||||||
| Haghighat [46] | 2022 | Iran | Mixed | RCT | 0 | Chlorhexidine | No | 0.2 | 2 | 20 | NA | 70 | Not provided | NA | CPIS |
| Chlorhexidine + Toothbrush | Yes | 0.2 | 2 | 40 | NA | 62.5 | Not provided | NA | CPIS | ||||||
| Hanifi [47] | 2017 | Iran | Surgical | RCT | 0 | Ozonated water | No | NA | 3 | 39 | 44.42 | 64.1 | Not provided | NA | CPIS |
| Chlorhexidine | No | 0.2 | 3 | 30 | 44.61 | 71.4 | Not provided | NA | CPIS | ||||||
| Hashemi [48] | 2018 | Iran | Unclear | RCT | 0 | Chlorhexidine | No | 0.2 | 4 | 43 | 48.44 | 60.46 | Not provided | NA | CDC |
| Stop-snoring herbal mouthwash | No | NA | 4 | 43 | 54.06 | 60 | Not provided | NA | CDC | ||||||
| Hu [49] | 2009 | China | Unclear | RCT | 0 | Saline | No | NA | 2 | 25 | NA | 64 | Not provided | NA | Clinical + Radiological + Microbiological |
| Saline | No | NA | 2 | 22 | NA | 59.1 | Not provided | NA | Clinical + Radiological + Microbiological | ||||||
| Long [50] | 2012 | China | Unclear | RCT | 0 | Povidone iodine + Toothbrush | Yes | NA | 3 | 31 | 60.06 | 64.5 | APACHE II | 17.94 | Clinical + Radiological + Microbiological |
| Povidone iodine | No | NA | 3 | 30 | 63.67 | 60 | APACHE II | 18.23 | Clinical + Radiological + Microbiological | ||||||
| Mo [51] | 2016 | China | Cardiothoracic | RCT | 0 | Saline | No | NA | 4 | 105 | 59.14 | 57.1 | Not provided | NA | CSRD |
| Saline | No | NA | 4 | 105 | 56.71 | 64.8 | Not provided | NA | CSRD | ||||||
| Xu [52] | 2007 | China | Unclear | RCT | 0 | Saline | No | NA | 2 | 44 | NA | NA | Not provided | NA | CSRD |
| Saline | No | NA | 2 | 58 | NA | NA | Not provided | NA | CSRD | ||||||
| Saline | No | NA | 2 | 62 | NA | NA | Not provided | NA | CSRD | ||||||
| Xu [53] | 2008 | China | Unclear | RCT | 0 | Saline | No | NA | 2 | 64 | NA | NA | Not provided | NA | CSRD |
| Saline | No | NA | 2 | 52 | NA | NA | Not provided | NA | CSRD | ||||||
| Zhao [54] | 2012 | China | Unclear | RCT | 0 | Triclosan | No | NA | 4 | 162 | NA | NA | Not provided | NA | Microbiological |
| Saline | No | NA | 4 | 162 | NA | NA | Not provided | NA | Microbiological | ||||||
| Irani [55] | 2019 | Iran | Surgical | RCT | 1 | Miswak + Toothbrush | Yes | NA | 2 | 35 | 33.65 | 82.9 | Not provided | NA | CPIS |
| Chlorhexidine | No | 0.2 | 2 | 35 | 34.83 | 74.3 | Not provided | NA | CPIS | ||||||
| Izadi [56] | 2023 | Iran | Unclear | RCT | 0 | Chlorhexidine | No | 0.2 | 3 | 37 | 63.7 | 34.2 | Not provided | NA | CPIS |
| Ozonated water | No | NA | 3 | 36 | 60.3 | 58.3 | Not provided | NA | CPIS | ||||||
| Jacomo [57] | 2011 | Brazil | Cardiothoracic | RCT | 0 | Chlorhexidine | No | 0.12 | 2 | 87 | 1.02 | 48 | PRISM | 3 | CDC |
| Water | No | NA | 2 | 73 | 0.9 | 48 | PRISM | 3 | CDC | ||||||
| Jahanshir [58] | 2023 | Iran | Unclear | RCT | 0 | Clove | No | 6.66 | 2 | 84 | 55.21 | 61.9 | Not provided | NA | CPIS |
| Chlorhexidine | No | 0.12 | 2 | 84 | 59.7 | 67.9 | Not provided | NA | CPIS | ||||||
| Jamshidi [59] | 2015 | Iran | Unclear | RCT | 0 | Chlorhexidine | No | 0.2 | 3 | 60 | 41.3 | 53.9 | Not provided | NA | CPIS |
| Toothbrush | Yes | NA | 3 | 61 | 41.3 | 53.9 | Not provided | NA | CPIS | ||||||
| Chlorhexidine + Toothbrush | Yes | 0.2 | 3 | 59 | 41.3 | 53.9 | Not provided | NA | CPIS | ||||||
| Karakaya [60] | 2022 | Turkey | PICU | RCT | 3 | Chlorhexidine | No | 0.12 | 6 | 88 | 4.3 | 47.7 | PRISM | 18 | CDC |
| Saline | No | 0.9 | 6 | 86 | 3.2 | 30.2 | PRISM | 18 | CDC | ||||||
| Kawyannejad [61] | 2020 | Iran | Unclear | RCT | 3 | Satureja plant | No | NA | 3 | 40 | 42.22 | 57.5 | Not provided | NA | CPIS |
| Chlorhexidine | No | 0.2 | 3 | 40 | 44.76 | 60 | Not provided | NA | CPIS | ||||||
| Khaky [62] | 2018 | Iran | Unclear | RCT | 0 | Hydrogen peroxide + Silver ions | No | NA | 3 | 37 | 41.6 | 72.5 | SOFA | 7.5 | CPIS |
| Chlorhexidine | No | 2 | 3 | 38 | 44.1 | 67.5 | SOFA | 7.3 | CPIS | ||||||
| Kiabi [63] | 2023 | Iran | Unclear | RCT | 2 | Persica | No | NA | 2 | 25 | NA | 45.04 | SOFA | 8.8 | CPIS |
| Chlorhexidine | No | 0.2 | 2 | 25 | NA | 45.04 | SOFA | 7.72 | CPIS | ||||||
| Klarin [64] | 2018 | Sweden | Medical | RCT | 0 | Probiotic | No | NA | 2 | 69 | 66 | 58 | APACHE II | 22 | Clinical + Radiological + Microbiological |
| Chlorhexidine + Toothbrush | Yes | 0.1 | 2 | 68 | 65.5 | 52.9 | APACHE II | 24 | Clinical + Radiological + Microbiological | ||||||
| Koeman [65] | 2006 | Netherlands | Mixed | RCT | 2 | Chlorhexidine | No | 2 | 4 | 127 | 60.9 | 52 | APACHE II | 22.2 | Clinical + Radiological + Microbiological |
| Chlorhexidine + Colistin | No | 2 | 4 | 128 | 62.4 | 56 | APACHE II | 23.7 | Clinical + Radiological + Microbiological | ||||||
| Saline | No | 0.9 | 4 | 130 | 62.1 | 72 | APACHE II | 21.8 | Clinical + Radiological + Microbiological | ||||||
| Kollef [66] | 2006 | Multinational | Mixed | RCT | 0 | Iseganan | No | NA | 6 | 362 | 60.5 | 62.9 | APACHE II | 19.6 | Clinical + Radiological + Microbiological |
| Placebo | No | NA | 6 | 347 | 57.5 | 57.5 | APACHE II | 19.3 | Clinical + Radiological + Microbiological | ||||||
| Kushara [67] | 2012 | Brazil | PICU | RCT | 2 | Chlorhexidine + Toothbrush | Yes | 0.12 | 2 | 46 | 1 | 60.9 | Not provided | NA | CPIS/CDC/NHSN |
| Toothbrush | Yes | NA | 2 | 50 | 2.9 | 64 | Not provided | NA | CPIS/CDC/NHSN | ||||||
| Lev [68] | 2015 | Israel | Mixed | RCT | 0 | Sodium bicarbonate + Hydrogen peroxide + Vitamin E + Toothbrush | Yes | NA | 3 | 45 | 68.7 | 55.5 | APACHE II | 19.1 | NHSN |
| Chlorhexidine | No | 0.2 | 3 | 45 | 71.8 | 53.3 | APACHE II | 18.2 | NHSN | ||||||
| Leyderman [69] | 2024 | Russia | Surgical | RCT | 3 | Chlorhexidine + Toothbrush | Yes | 0.05 | 3 | 25 | 65 | NA | APACHE II | 13.05 | CPIS |
| Chlorhexidine | No | 0.05 | 2 | 22 | 70 | NA | APACHE II | 13.1 | CPIS | ||||||
| Li [70] | 2021 | China | NICU | RCT | 0 | Colostrum + Sodium bicarbonate | No | 2.5 | 4 | 40 | NA | NA | Not provided | NA | Clinical + Radiological + Microbiological |
| Colostrum | No | NA | 4 | 40 | NA | NA | Not provided | NA | Clinical + Radiological + Microbiological | ||||||
| Sodium bicarbonate | No | 2.5 | 4 | 40 | NA | NA | Not provided | NA | Clinical + Radiological + Microbiological | ||||||
| Li-Yin [71] | 2011 | Taiwan | Surgical | RCT | 2 | Water + Toothbrush | Yes | NA | 2 | 28 | 60.7 | 60.7 | APACHE II | 19.6 | CPIS |
| Water | No | NA | 2 | 25 | 60.5 | 68 | APACHE II | 19.4 | CPIS | ||||||
| Lorente [72] | 2012 | Spain | Mixed | RCT | 3 | Chlorhexidine + Toothbrush | Yes | 0.12 | 3 | 217 | 61 | 67.3 | APACHE II | 17.88 | Clinical + Radiological + Microbiological |
| Chlorhexidine | No | 0.12 | 3 | 219 | 60.4 | 66.2 | APACHE II | 19.16 | Clinical + Radiological + Microbiological | ||||||
| Maarefvand [73] | 2015 | Iran | Unclear | RCT | 0 | Chamomile | No | NA | 2 | 30 | 45.93 | 46.7 | APACHE II | 17.7 | CPIS |
| Chlorhexidine | No | 0.12 | 2 | 60 | 51.63 | 50 | APACHE II | 17.63 | CPIS | ||||||
| Meidani [74] | 2018 | Iran | Unclear | RCT | 0 | Chlorhexidine | No | 0.2 | 3 | 50 | 50.6 | 74 | Not provided | NA | CDC |
| Potassium permanganate | No | 0.01 | 3 | 50 | 49.8 | 74 | Not provided | NA | CDC | ||||||
| Placebo | No | NA | 3 | 50 | 51.7 | 66 | Not provided | NA | CDC | ||||||
| Meinberg [75] | 2012 | Brazil | Surgical | RCT | 0 | Chlorhexidine + Toothbrush | Yes | 2 | 4 | 28 | 40.1 | NA | APACHE II | 17.9 | Clinical + Radiological + Microbiological |
| Toothbrush | Yes | 2 | 4 | 24 | 41 | NA | APACHE II | 16.7 | Clinical + Radiological + Microbiological | ||||||
| Moghaddam [76] | 2022 | Iran | Unclear | RCT | 2 | Chamomile + Toothbrush | Yes | 10 | 2 | 40 | 45.1 | NA | Not provided | NA | CPIS |
| Chlorhexidine + Toothbrush | Yes | 0.2 | 2 | 40 | 39.85 | NA | Not provided | NA | CPIS | ||||||
| Mohseni [77] | 2024 | Iran | Unclear | RCT | 2 | Tea tree oil + Aloe vera+ Toothbrush | Yes | NA | 2 | 31 | 45 | 64.5 | Not provided | NA | CPIS |
| Chlorhexidine + Toothbrush | Yes | 0.2 | 2 | 31 | 45 | 64.5 | Not provided | NA | CPIS | ||||||
| Mori [78] | 2006 | Japan | Mixed | Cohort | 0 | Povidone iodine | No | Not known | 8 | 1248 | 53 | 62 | APACHE II | 13.9 | Clinical + Radiological + Microbiological |
| No oral care | No | NA | NA | 414 | 53 | 66 | APACHE II | 13.4 | Clinical + Radiological + Microbiological | ||||||
| Nasiriani [79] | 2016 | Iran | Unclear | RCT | 0 | Chlorhexidine + Saline+ Toothbrush | Yes | Not known | 2 | 84 | 44.9 | 66.7 | Not provided | NA | CPIS |
| Chlorhexidine + Saline | No | Not known | 2 | 84 | 44.2 | 67.9 | Not provided | NA | CPIS | ||||||
| Nicolosi [80] | 2014 | Argentina | Cardiothoracic | Cohort | 0 | Chlorhexidine + Toothbrush | Yes | 0.12 | 2 | 150 | 62.3 | 81.3 | Not provided | NA | Clinical + Radiological |
| Standard of care | No | NA | 2 | 150 | 63.1 | 86 | Not provided | NA | Clinical + Radiological | ||||||
| Nobahar [81] | 2016 | Iran | Mixed | RCT | 1 | Hydrogen peroxide | No | 3 | 2 | 34 | 66 | 50 | Not provided | NA | CPIS |
| Saline | No | 0.9 | 2 | 34 | 63.4 | 47.1 | Not provided | NA | CPIS | ||||||
| Ory [82] | 2017 | France | Mixed | Cohort | 0 | Chlorhexidine | No | 0.5 | 3 | 932 | 64.6 | 69.3 | SAPS2 | 54 | Clinical + Radiological + Microbiological |
| Chlorhexidine + Toothbrush | Yes | 0.5 | 3 | 1151 | 63.6 | 67.5 | SAPS2 | 53.3 | Clinical + Radiological + Microbiological | ||||||
| Ozcaka [83] | 2012 | Turkey | Respiratory | RCT | 0 | Chlorhexidine | No | 0.2 | 4 | 29 | 60.5 | NA | APACHE II | 23.9 | Not specified |
| Saline | No | 0.9 | 4 | 32 | 56 | NA | APACHE II | 24.7 | Not specified | ||||||
| Panchabai [84] | 2009 | India | Medical | RCT | 3 | Chlorhexidine | No | 0.2 | 2 | 88 | NA | NA | Not provided | NA | Clinical + Radiological + Microbiological |
| Potassium permanganate | No | 0.01 | 2 | 83 | NA | NA | Not provided | NA | Clinical + Radiological + Microbiological | ||||||
| Pedreira [85] | 2009 | Brazil | PICU | RCT | 0 | Chlorhexidine + Toothbrush | Yes | 0.12 | 2 | 27 | NA | NA | Not provided | NA | NA |
| Toothbrush | No | NA | 2 | 29 | NA | NA | Not provided | NA | NA | ||||||
| Pobo [86] | 2009 | Spain | Mixed | RCT | 1 | Chlorhexidine | No | 0.12 | 3 | 73 | 52.6 | 63 | APACHE II | 18.7 | ATS/IDSA |
| Chlorhexidine + Toothbrush | Yes | 0.12 | 3 | 74 | 55.3 | 66.2 | APACHE II | 18.8 | ATS/IDSA | ||||||
| Pugin [87] | 1991 | Switzerland | Surgical | RCT | 1 | Antimicrobial combination | No | NA | 1 | 25 | 45 | 76 | APACHE II | 15.8 | CPIS |
| Placebo | No | NA | 1 | 27 | 46 | 74.1 | APACHE II | 14.7 | CPIS | ||||||
| Rezvani [88] | 2018 | Iran | Mixed | RCT | 0 | Chamomile | No | NA | 3 | 45 | 60.78 | 60 | Not provided | NA | Not specified |
| Chlorhexidine | No | 0.2 | 3 | 45 | 59.18 | 55 | Not provided | NA | Not specified | ||||||
| Rodriguez-Roldan [89] | 1990 | Spain | Mixed | RCT | 1 | Chlorhexidine + Antimicrobial combination | N0 | 0.1 | 4 | 13 | 54 | 53.8 | APACHE II | 16 | Clinical + Radiological + Microbiological |
| Chlorhexidine | No | 0.1 | 4 | 15 | 49 | 66.7 | APACHE II | 18 | Clinical + Radiological + Microbiological | ||||||
| Kes [90] | 2021 | Turkey | Surgical | RCT | 0 | Chlorhexidine | No | 0.12 | 3 | 29 | 72.79 | 62.1 | APACHE II | 16.1 | CPIS |
| Sodium bicarbonate | No | NA | 3 | 28 | 77.37 | 57.1 | APACHE II | 16.6 | CPIS | ||||||
| Houston [91] | 2002 | USA | Cardiothoracic | RCT | 0 | Chlorhexidine | No | 0.12 | 2 | 19 | NA | NA | Not provided | NA | CDC |
| Listerine | No | NA | 2 | 18 | NA | NA | Not provided | NA | CDC | ||||||
| Saito [92] | 2022 | Vietnam | Mixed | Cohort | 1 | Toothbrush | Yes | NA | Not mentioned | 300 | 57.5 | 56.3 | APACHE II | 20 | CPIS |
| Standard of care | No | NA | Not mentioned | 303 | 56 | 58.1 | APACHE II | 17 | CPIS | ||||||
| Scannapieco [93] | 2009 | USA | Unclear | RCT | 0 | Chlorhexidine | No | 0.12 | 1 | 47 | 44.8 | 91.5 | APACHE II | 18.5 | CPIS |
| Chlorhexidine | No | 0.12 | 2 | 50 | 47.6 | 88 | APACHE II | 19.7 | CPIS | ||||||
| Water | No | 0.12 | 1 | 49 | 50 | 73.5 | APACHE II | 19.1 | CPIS | ||||||
| Sebastian [94] | 2012 | India | PICU | RCT | 2 | Chlorhexidine | No | 1 | 3 | 41 | NA | 56.1 | PIM2 | 19 | CDC |
| Placebo | No | NA | 3 | 45 | NA | 60 | PIM2 | 23.5 | CDC | ||||||
| Seguin [95] | 2006 | France | Surgical | RCT | 2 | Povidone iodine | No | 10 | 6 | 36 | 38 | 78 | SAPS2 | 39 | BAL + Microbiological |
| Saline | No | 0.9 | 6 | 31 | 38 | 77 | SAPS2 | 41 | BAL + Microbiological | ||||||
| Standard of care | No | NA | 6 | 31 | 41 | 74 | SAPS2 | 40 | BAL + Microbiological | ||||||
| Seguin [96] | 2013 | France | Surgical | RCT | 2 | Povidone iodine | No | 10 | 6 | 85 | 48 | 71 | SAPS2 | 47 | Clinical + Radiological + Microbiological |
| Water | No | NA | 6 | 82 | 48 | 78 | SAPS2 | 46 | Clinical + Radiological + Microbiological | ||||||
| Sharma [97] | 2012 | India | Mixed | RCT | 0 | Chlorhexidine | No | 0.12 | 2 | 130 | NA | 74.6 | Not provided | NA | CPIS |
| Saline | No | 0.9 | 2 | 130 | NA | 72.3 | Not provided | NA | CPIS | ||||||
| Shorofi [98] | 2025 | Iran | Mixed | RCT | 0 | Zataria multiflora Boiss. + Chlorhexidine | No | 0.2 | 2 | 60 | 46.57 | 66.7 | Not provided | NA | CPIS |
| Chlorhexidine | No | 0.2 | 2 | 60 | 50.58 | 66.7 | Not provided | NA | CPIS | ||||||
| Singh [99] | 2022 | India | Unclear | RCT | 3 | Chlorhexidine + Toothbrush | Yes | 0.2 | 2 | 110 | 39.02 | 50 | Not provided | NA | Not specified |
| Chlorhexidine | No | 0.2 | 2 | 110 | 39.1 | 50.9 | Not provided | NA | Not specified | ||||||
| Siriyanyongwong [100] | 2022 | Thailand | Medical | RCT | 0 | Chlorhexidine + Moraceae + Toothbrush | Yes | 0.02 | 3 | 15 | 68 | 53.3 | SOFA | 9 | CDC |
| Chlorhexidine | No | 0.12 | 3 | 15 | 63 | 53.3 | SOFA | 8 | CDC | ||||||
| Stefanescu [101] | 2013 | USA | NICU | RCT | 1 | Biotene | No | NA | 6 | 20 | 0.5 | 35 | APGAR | 3 | CDC |
| Water | No | NA | 6 | 21 | 0.48 | 52 | APGAR | 4 | CDC | ||||||
| Takeyasu [102] | 2014 | Japan | Unclear | RCT | 2 | Povidone iodine + Toothbrush | Yes | 1 | 3 | 84 | 67.9 | 65.5 | Not provided | NA | Not specified |
| Toothbrush | Yes | NA | 3 | 58 | 67.9 | 65.5 | Not provided | NA | Not specified | ||||||
| Tantipong [103] | 2008 | Thailand | Mixed | RCT | 0 | Chlorhexidine + Toothbrush | Yes | 2 | 4 | 102 | 56.5 | 49.1 | APACHE II | 16.7 | Clinical + Radiological + Microbiological |
| Saline + Toothbrush | Yes | 0.9 | 4 | 105 | 60.3 | 48.6 | APACHE II | 18.2 | Clinical + Radiological + Microbiological | ||||||
| Tuon [104] | 2017 | Brazil | Mixed | RCT | 0 | Chlorhexidine | No | 2 | 2 | 8 | 53.1 | 62.5 | APACHE II | NA | CDC |
| Saline | No | 0.9 | 2 | 8 | 42.8 | 50 | Not provided | NA | CDC | ||||||
| Vyas [105] | 2020 | India | Unclear | RCT | 1 | Chlorhexidine | No | 0.12 | 3 | 70 | 47 | 78.57 | Not provided | NA | CPIS |
| Chlorhexidine | No | 0.2 | 3 | 70 | 48.5 | 68.57 | Not provided | NA | CPIS | ||||||
| Yadav [106] | 2022 | India | Unclear | RCT | 1 | Antimicrobial drug | No | 2 | 4 | 82 | 33.21 | 50 | APACHE II | 21.9 | CPIS |
| Chlorhexidine | No | 2 | 4 | 69 | 33.75 | 47.8 | APACHE II | 22.58 | CPIS | ||||||
| Yu [107] & [108] | 2021 | China | NICU | RCT | 0 | Breast milk | No | NA | 8 | 31 | 0.15 | NA | Not provided | NA | Clinical + Radiological + Microbiological |
| Saline | No | NA | 8 | 31 | 0.14 | NA | Not provided | NA | Clinical + Radiological + Microbiological | ||||||
| Sodium bicarbonate | No | NA | 8 | 31 | 0.15 | NA | Not provided | NA | Clinical + Radiological + Microbiological | ||||||
| Zand [109] | 2017 | Iran | Mixed | RCT | 1 | Chlorhexidine | No | 0.2 | 2 | 57 | 45.43 | 80.7 | APACHE IV | 61.33 | CPIS |
| Chlorhexidine | No | 2 | 2 | 57 | 44.45 | 80.7 | APACHE IV | 56.01 | CPIS | ||||||
| Zarinfar [110] | 2021 | Iran | Mixed | RCT | 4 | Standard of care | No | NA | Not mentioned | 43 | 60.4 | 48.8 | APACHE II | NA | CPIS |
| Chlorhexidine | No | 0.12 | 2 | 43 | 53.5 | 60.5 | APACHE II | NA | CPIS | ||||||
| Chlorhexidine | No | 0.12 | 2 | 43 | 50 | 44.2 | APACHE II | NA | CPIS | ||||||
| Bellissimo-Rodrigues [111] | 2009 | Brazil | Mixed | RCT | 2 | Chlorhexidine | No | 0.12 | 3 | 98 | 62.5 | 48 | APACHE II | 17 | CDC |
| Placebo | No | NA | 3 | 96 | 54 | 53 | APACHE II | 19 | CDC | ||||||
| Bopp [112] | 2006 | USA | Mixed | RCT | 0 | Chlorhexidine | No | 0.12 | 2 | 2 | 40 | 50 | Not provided | NA | Not specified |
| Hydrogen peroxide or Listerine | No | NA | 6 | 3 | 73.7 | 33 | Not provided | NA | Not specified | ||||||
| Cabov [113] | 2010 | Croatia | Surgical | RCT | 0 | Chlorhexidine | No | 0.2 | 3 | 30 | 57 | 63.3 | SAPS2 | 30 | Clinical + Radiological + Microbiological |
| Placebo | No | NA | 3 | 30 | 52 | 66.7 | SAPS2 | 28.2 | Clinical + Radiological + Microbiological | ||||||
| de Smet [114] | 2009 | Netherlands | Mixed | RCT | 0 | Antimicrobial combination | No | 2 | 4 | 1904 | 61.4 | 63.7 | APACHE II | 19.5 | Not specified |
| Standard of care | No | NA | Not mentioned | 1990 | 61.4 | 61.3 | APACHE II | 18.6 | Not specified | ||||||
| Loha [115] | 2022 | India | Unclear | RCT | 5 | Chlorhexidine | No | 2 | 3 | 44 | 56.86 | 50 | APACHE II | 21.21 | CPIS |
| Chlorhexidine + Sodium bicarbonate | No | 2 & 0.9 | 2 | 44 | 54.96 | 47.7 | APACHE II | 21.28 | CPIS | ||||||
| Munro [116] | 2009 | USA | Mixed | RCT | 0 | Toothbrush | Yes | NA | 3 | 49 | 47.1 | 57 | APACHE III | 76.4 | Not specified |
| Chlorhexidine | No | 0.12 | 2 | 44 | 46.1 | 59 | APACHE III | 80.4 | Not specified | ||||||
| Chlorhexidine + Toothbrush | Yes | 0.12 | 3 | 48 | 47.3 | 58 | APACHE III | 76.2 | Not specified | ||||||
| Standard of care | No | NA | Not mentioned | 51 | 46.8 | 73 | APACHE III | 76.2 | Not specified | ||||||
| Zambrano [117] | 2024 | Brazil | Unclear | RCT | 0 | Chlorhexidine | No | 0.12 | 2 | 45 | 63 | 55 | Not provided | NA | Not specified |
| Chlorhexidine + Toothbrush | Yes | 0.12 | 2 | 45 | 65 | 69 | Not provided | NA | Not specified |
| Comparisons with Reference Interventions for the Risk of VAP | Comparative Risks of VAP per 1000 Patients with Reference Interventions (95% Confidence Intervals) | Effect Estimates (ORs) and the Quality of Evidence | |
|---|---|---|---|
| Assumed Risk 1 | Corresponding Risk | ||
| Random effect estimates from network meta-analysis | |||
| Chlorhexidine | 311 | 199 (151 to 258) | 0.55 [0.39, 0.77]; Moderate 2 |
| Antimicrobial combination | 123 (63 to 224) | 0.31 [0.15, 0.64]; Very low 2,3 | |
| Povidone iodine | 150 (87 to 243) | 0.39 [0.21, 0.71]; Very low 2,3 | |
| Chlorhexidine + Toothbrush | 147 (102 to 205) | 0.38 [0.25, 0.57]; Very low 2,3 | |
| Toothbrush alone | 172 (112 to 258) | 0.46 [0.28, 0.77]; Very low 2,3 | |
| Miswak + Toothbrush | 18 (0 to 260) | 0.04 [0, 0.78]; Very low 2,3 | |
| Chamomile + Toothbrush | 18 (4 to 67) | 0.04 [0.01, 0.16]; Very low 2,3 | |
| Hydrogen peroxide + Silver ions | 22 (0 to 190) | 0.05 [0, 0.52]; Very low 2,3 | |
| Hyrogen peroxide+ Vitamin E + Toothbrush | 47 (9 to 193) | 0.11 [0.02, 0.53]; Very low 2,3 | |
| Nitrofurazone + Toothbrush | 51 (13 to 156) | 0.12 [0.03, 0.41]; Very low 2,3 | |
| Ozonated water | 63 (22 to 178) | 0.15 [0.05, 0.48]; Very low 2,3 | |
| Clove | 79 (26 to 235) | 0.19 [0.06, 0.68]; Very low 2,3 | |
| Propolis | 87 (26 to 260) | 0.21 [0.06, 0.78]; Very low 2,3 | |
| Satureja plant | 90 (22 to 280) | 0.22 [0.05, 0.86]; Very low 2,3 | |
| Random effect estimates from pairwise comparisons | |||
| Chlorhexidine | 300 | 208 (143 to 289) | 0.61 [0.39, 0.95]; Moderate 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridharan, K.; Sivaramakrishnan, G.; Alotaibi, G.A. Oropharyngeal Interventions in Intubated Patients for Preventing Ventilator Associated Pneumonia: A Systematic Review and Multi-Variate Network Meta-Analysis Evaluating Pharmacological Agents. J. Clin. Med. 2025, 14, 8174. https://doi.org/10.3390/jcm14228174
Sridharan K, Sivaramakrishnan G, Alotaibi GA. Oropharyngeal Interventions in Intubated Patients for Preventing Ventilator Associated Pneumonia: A Systematic Review and Multi-Variate Network Meta-Analysis Evaluating Pharmacological Agents. Journal of Clinical Medicine. 2025; 14(22):8174. https://doi.org/10.3390/jcm14228174
Chicago/Turabian StyleSridharan, Kannan, Gowri Sivaramakrishnan, and Ghazi Abdulrahman Alotaibi. 2025. "Oropharyngeal Interventions in Intubated Patients for Preventing Ventilator Associated Pneumonia: A Systematic Review and Multi-Variate Network Meta-Analysis Evaluating Pharmacological Agents" Journal of Clinical Medicine 14, no. 22: 8174. https://doi.org/10.3390/jcm14228174
APA StyleSridharan, K., Sivaramakrishnan, G., & Alotaibi, G. A. (2025). Oropharyngeal Interventions in Intubated Patients for Preventing Ventilator Associated Pneumonia: A Systematic Review and Multi-Variate Network Meta-Analysis Evaluating Pharmacological Agents. Journal of Clinical Medicine, 14(22), 8174. https://doi.org/10.3390/jcm14228174








