Role of Endoscopy in the Diagnosis and Management of Esophageal Cancer
Abstract
1. Introduction
2. Epidemiology
2.1. Esophageal Squamous Cell Carcinoma
2.2. Esophageal Adenocarcinoma
2.3. Other Rare Esophageal Cancer Types
3. Screening and Surveillance
3.1. Barrett’s Esophagus
3.2. Diagnostic Modalities
3.2.1. White Light Endoscopy
3.2.2. Lugol Chromoendoscopy
3.2.3. Narrow-Band Imaging
3.2.4. Wide-Area Transepithelial Sampling
3.2.5. Multifunctional Ablative Gastrointestinal Imaging Capsule
3.2.6. Non-Endoscopic Cell-Collection Devices
4. Role of Endoscopy in Diagnosis and Staging
4.1. Diagnosis and Guidelines
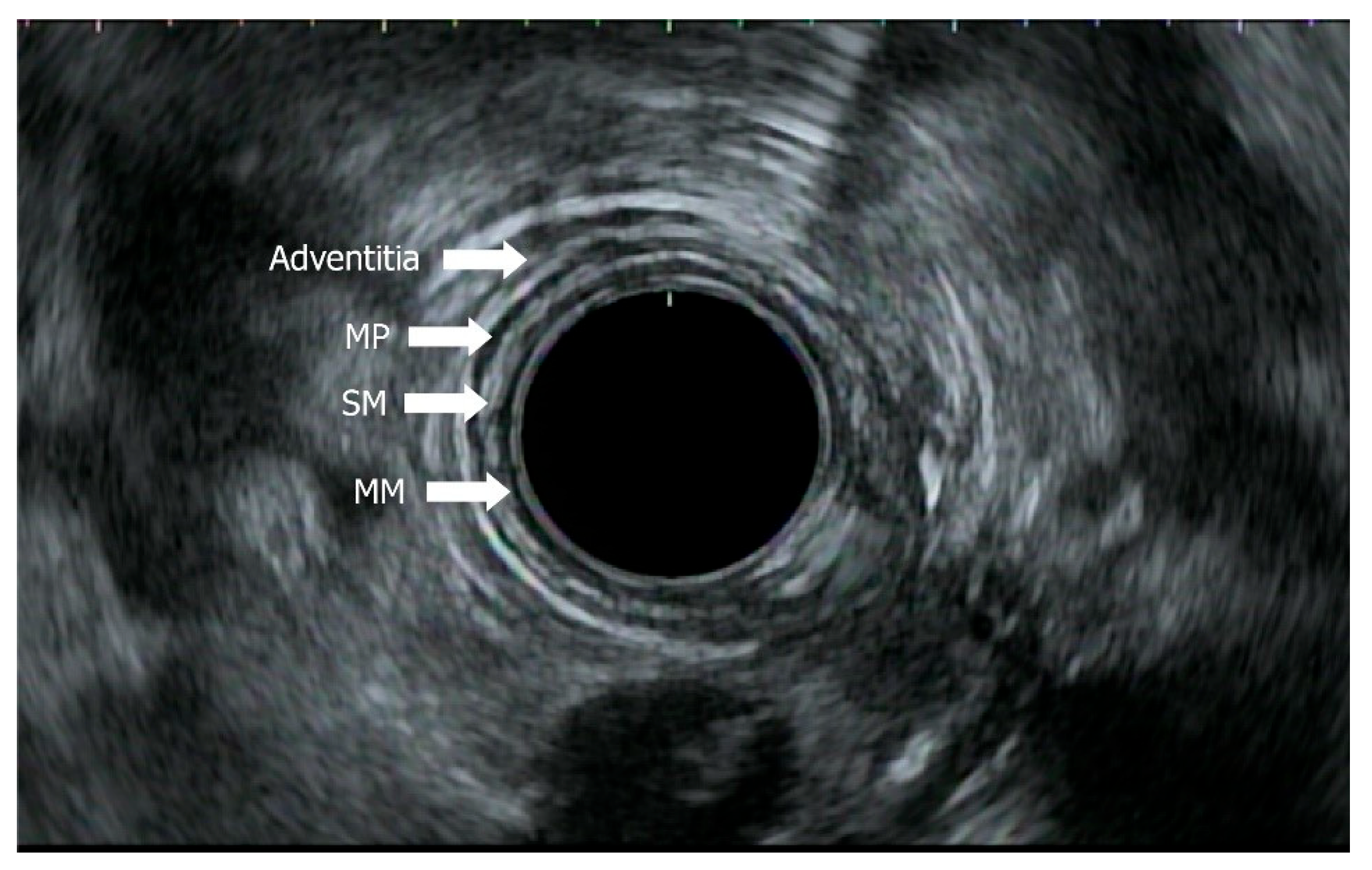
4.2. Role of EUS in Staging with EUS, Mini-EUS, Contrast EUS
5. Role of Endoscopy in Treatment
5.1. Resection
5.2. Ablative Techniques
5.2.1. Radiofrequency Ablation
5.2.2. Photodynamic Therapy
5.2.3. Argon Plasma Coagulation Therapy
5.2.4. Cryotherapy
6. Stents for the Management of Luminal Occlusion in Esophageal Cancer
7. Post-Treatment Surveillance
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Esophageal cancer |
| AGA | American Gastroenterological Association |
| AJCC | American Joint Committee on Cancer |
| APC | Argon plasma coagulation |
| ASGE | American Society of Gastrointestinal Endoscopy |
| ASIR | Age-standardized incident rates |
| ASMR | Age-standardized mortality rates |
| BE | Barrett’s esophagus |
| CE-IM | Complete eradication of intestinal metaplasia |
| CH-EUS | Contrast-enhanced endoscopic ultrasound |
| CLE | Confocal laser endoscopy |
| DESs | Drug-eluting stents |
| EAC | Esophageal adenocarcinoma |
| EET | Endoscopic eradication therapy |
| EMERALD | Oesophageal MicroRNAs of Barrett Adenocarcinoma and Dysplasia |
| EMR | Endoscopic mucosal resection |
| EOE | Eosinophilic esophagitis |
| ESD | Endoscopic submucosal dissection |
| GERD | Gastroesophageal reflux disease |
| EUS | Endoscopic ultrasound |
| EUS-MPs | Endoscopic ultrasound mini probes |
| FNA | Fine needle aspiration |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| GIST | Gastrointestinal stromal tumor |
| HGD | High-grade dysplasia |
| HRM | High-resolution microendoscopy |
| MAGIC | Multifunctional ablative gastrointestinal imaging capsule |
| NBI | Narrow band imaging |
| SEPSs | Self-expanding plastic stents |
| WATS | Wide-area transepithelial sampling |
Appendix A
| Category | Criteria |
|---|---|
| T category | |
| TX | Tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | High-grade dysplasia, malignant cells confined by basement membrane |
| T1 | Tumor invades lamina propria, muscularis mucosae, or submucosa |
| T1a | Tumor invades lamina propria or muscularis mucosae |
| T1b | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades adventitia |
| T4 | Tumor invades adjacent structures |
| T4a | Tumor invades the pleura, pericardium, azygos vein, diaphragm, or peritoneum |
| T4b | Tumor invades other adjacent structures, such as aorta, vertebral body, or trachea |
| N category | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1–2 regional lymph nodes |
| N2 | Metastasis in 3–6 regional lymph nodes |
| N3 | Metastasis in 7+ regional lymph nodes |
| M category | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
References
- Qi, L.; Sun, M.; Liu, W.; Zhang, X.; Yu, Y.; Tian, Z.; Ni, Z.; Zheng, R.; Li, Y. Global esophageal cancer epidemiology in 2022 and predictions for 2050: A comprehensive analysis and projections based on GLOBOCAN data. Chin. Med. J. 2024, 137, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Then, E.O.; Lopez, M.; Saleem, S.; Gayam, V.; Sunkara, T.; Culliford, A.; Gaduputi, V. Esophageal cancer: An updated surveillance epidemiology and end results database analysis. World J. Oncol. 2020, 11, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Maradey-Romero, C.; Fass, R. The relationship between eosinophilic esophagitis and esophageal cancer. Dis. Esophagus 2017, 30, 1–5. [Google Scholar] [CrossRef]
- Wadhwa, V.; Patel, N.; Grover, D.; Ali, F.S.; Thosani, N. Interventional gastroenterology in oncology. CA Cancer J. Clin. 2023, 73, 286–319. [Google Scholar] [CrossRef]
- Sheikh, M.; Roshandel, G.; McCormack, V.; Malekzadeh, R. Current status and future prospects for esophageal cancer. Cancers 2023, 15, 765. [Google Scholar] [CrossRef]
- Xie, S.; Rabbani, S.; Petrick, J.L.; Cook, M.B.; Lagergren, J. Racial and ethnic disparities in the incidence of esophageal cancer in the United States, 1992–2013. Am. J. Epidemiol. 2017, 186, 1341–1351. [Google Scholar] [CrossRef]
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.; Thrift, A.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, C.; Wang, N.; Pan, D.; Wang, S.; Sun, G. Hot tea drinking and the risk of esophageal cancer: A systematic review and meta-analysis. Nutr. Cancer 2022, 74, 2384–2391. [Google Scholar] [CrossRef]
- Lander, S.; Lander, E.; Gibson, M.K. Esophageal cancer: Overview, risk factors, and reasons for the rise. Curr. Gastroenterol. Rep. 2023, 25, 275. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Esophageal cancer: Genomic and molecular characterization, stem cell compartment and clonal evolution. Medicines 2017, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Mansour, N.M.; Groth, S.S.; Anandasabapathy, S. Esophageal adenocarcinoma: Screening, surveillance, and management. Annu. Rev. Med. 2017, 68, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Raja, S.; Kamath, S.; Jang, S.; Allende, D.; McNamara, M.; Videtic, G.; Murthy, S.; Bhatt, A. Esophageal adenocarcinoma: A dire need for early detection and treatment. Cleve Clin. J. Med. 2022, 89, 269–279. [Google Scholar] [CrossRef]
- Saddoughi, S.A.; Taswell, J.; Harmsen, W.S.; Inra, M.; Blackmon, S.; Nichols, F.; Cassivi, S.; Wigle, D.; Shen, K.R.; Allen, M. Surgical resection of rare esophageal cancers. Ann. Thorac. Surg. 2016, 101, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, D.B.; Zhao, Q.; Chen, G.; Liu, X.M.; Wang, Y.N.; Su, H.; Qin, Y.R.; He, Y.F.; Zou, Q.F. The genomic landscape of small cell carcinoma of the esophagus. Cell Res. 2018, 28, 771–774. [Google Scholar] [CrossRef]
- Cavalaris, C.P.; Okon, S.O.; Pena, L.R.; Friedman, M.S. Primary malignant melanoma of the esophagus. Dig. Dis. Sci. 2023, 68, 3203. [Google Scholar] [CrossRef]
- Lott, S.; Schmieder, M.; Mayer, B.; Henne-Bruns, D.; Knippschild, U.; Agaimy, A.; Schwab, M.; Kramer, K. Gastrointestinal stromal tumors of the esophagus: Evaluation of a pooled case series regarding clinicopathological features and clinical outcome. Am. J. Cancer Res. 2015, 5, 333. Available online: https://pubmed.ncbi.nlm.nih.gov/25628942/ (accessed on 24 June 2025).
- Chen, S.; Shi, Y.; Lu, Z.; Wang, M.; Cong, L.; Yang, B.; Chen, X.; Cai, J.; Yang, X. Esophageal Carcinosarcoma: Analysis of Clinical Features and Prognosis of 24 Cases and a Literature Review. Cancer Control 2021, 28, 10732748211004886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madan, A.K.; Long, A.E.; Weldon, C.B.; Jaffe, B.M. Esophageal carcinosarcoma. J. Gastrointest. Surg. 2001, 5, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.S.; Kim, W.T.; Ko, B.S.; Kim, E.H.; Kim, J.O.; Park, K.; Lee, S.W. A case of rapidly progressing leiomyosarcoma combined with squamous cell carcinoma in the esophagus. World J. Gastroenterol. 2013, 19, 5385–5388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pramesh, C.S.; Pantvaidya, G.H.; Moonim, M.T.; Jambhekar, N.A.; Sharma, S.; Deshpande, R.K. Leiomyosarcoma of the esophagus. Dis. Esophagus 2003, 16, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.; Fishman, D.; Gurudu, S.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359.e2. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Souza, R.F.; Yadlapati, R.H.; Sauer, B.; Wani, S. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am. J. Gastroenterol. 2022, 117, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Paiji, C.; Sedarat, A. Endoscopic management of esophageal cancer. Cancers 2022, 14, 3583. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hikichi, T.; Nakamura, J.; Hashimoto, M.; Kobashi, R.; Yanagita, T.; Takagi, T.; Suzuki, R.; Sugimoto, M.; Asama, H.; et al. Visibility of esophageal squamous cell carcinoma under iodine staining on texture and color enhancement imaging. DEN Open 2024, 5, e370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, W.; Kong, R.; Wang, N.; Bao, W.; Mao, X.; Lu, J. Confocal laser endomicroscopy for detection of early upper gastrointestinal cancer. Cancers 2023, 15, 776. [Google Scholar] [CrossRef]
- Ono, S.; Dobashi, A.; Furuhashi, H.; Koizumi, A.; Matsui, H.; Hara, Y.; Sumiyama, K. Characteristics of superficial esophageal squamous cell carcinomas undetectable with narrow-band imaging endoscopy. Gastroenterol. Rep. 2021, 9, 402–407. [Google Scholar] [CrossRef]
- Ishihara, R. Endoscopic Diagnosis and Treatment of Superficial Esophageal Squamous Cell Cancer: Present Status and Future Perspectives. Curr. Oncol. 2022, 29, 534–543. [Google Scholar] [CrossRef]
- Chiam, K.H.; Shin, S.H.; Choi, K.C.; Leiria, F.; Militz, M.; Singh, R. Current Status of Mucosal Imaging with Narrow-Band Imaging in the Esophagus. Gut Liver 2021, 15, 492–499. [Google Scholar] [CrossRef]
- Suresh Kumar, V.C.; Harne, P.; Patthipati, V.S.; Subedi, A.; Masood, U.; Sharma, A.; Goyal, F.; Aggarwal, N.; Sapkota, B. Wide-area transepithelial sampling in adjunct to forceps biopsy increases the absolute detection rates of Barrett’s oesophagus and oesophageal dysplasia: A meta-analysis and systematic review. BMJ Open Gastroenterol. 2020, 7, e000494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, H.; Li, D.; Liang, R.; Adrales, G.; Li, X. Multifunctional ablative gastrointestinal imaging capsule (MAGIC) for esophagus surveillance and interventions. BME Front. 2024, 5, 0041. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.C.; di Pietro, M.; O’Donovan, M.; Maroni, R.; Muldrew, B.; Debiram-Beecham, I.; Gehrung, M.; Offman, J.; Tripathi, M.; Smith, S.G.; et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: A multicentre, pragmatic, randomised controlled trial. Lancet 2020, 396, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.R.; Wani, S.; Gyawali, C.P.; Komanduri, S. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett’s Esophagus: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 2696–2706.e1. [Google Scholar] [CrossRef]
- Iyer, P.; Taylor, W.; Slettedahl, S.; Lansing, R.L.; Hemminger, L.L.; Cayer, F.K.; Mahoney, D.W.; Giakoumopoulos, M.; Allawi, H.; Wu, T.; et al. Validation of a methylated DNA marker panel for the nonendoscopic detection of Barrett’s esophagus in a multisite case-control study. Gastrointest. Endosc. 2021, 94, 498–505. [Google Scholar] [CrossRef]
- Moinova, H.R.; LaFramboise, T.; Lutterbaugh, J.D.; Chandar, A.K.; Dumot, J.; Faulx, A.; Brock, W.; Cabrera, O.D.l.C.; Guda, K.; Barnholtz-Sloan, J.S.; et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 2018, 10, eaao5848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Gierken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef]
- Rice, T.W.; Chen, L.; Hofstetter, W.L.; Smithers, B.M.; Rusch, V.W.; Wijnhoven, B.P.L.; Chen, K.L.; Davies, A.R.; D’Journo, X.B.; Kesler, K.A.; et al. Worldwide esophageal cancer collaboration: Pathologic staging data. Dis. Esophagus 2016, 29, 724–733. [Google Scholar] [CrossRef]
- Thosani, N.; Singh, H.; Kapadia, A.; Ochi, N.; Lee, J.H.; Ajani, J.; Swisher, S.G.; Hofstetter, W.L.; Guha, S.; Bhutani, M. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: A systematic review and meta-analysis. Gastrointest. Endosc. 2012, 75, 242–253. [Google Scholar] [CrossRef]
- DaVee, T.; Ajani, J.A.; Lee, J.H. Is endoscopic ultrasound examination necessary in the management of esophageal cancer? World J. Gastroenterol. 2017, 23, 751–762. [Google Scholar] [CrossRef]
- Radlinski, M.; Shami, V.M. Role of endoscopic ultrasound in esophageal cancer. World J. Gastrointest. Endosc. 2022, 14, 205–214. [Google Scholar] [CrossRef]
- Thakkar, S.; Kaul, V. Endoscopic ultrasound staging of esophageal cancer. Gastroenterol. Hepatol. 2020, 16, 14–20. Available online: https://pubmed.ncbi.nlm.nih.gov/33867884/ (accessed on 29 May 2025).
- Iglesias-Garcia, J.; de la Iglesia-Garcia, D.; Lariño-Noia, J.; Dominguez-Muñoz, J.E. Endoscopic ultrasound (EUS) guided elastography. Diagnostics 2023, 13, 1686. [Google Scholar] [CrossRef]
- Iglesias-García, J.; Lariño-Noia, J.; Domínguez-Muñoz, J.E. New imaging techniques: Endoscopic ultrasound-guided elastography. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 551–567. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Jenssen, C.; Arcidiacono, P.G.; Cui, X.; Giovannini, M.; Hocke, M.; Iglesias-Garcia, J.; Saftoiu, A.; Sun, S.; Chiorean, L. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc. Ultrasound 2015, 4, 176. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Machado, P.; Eisenbrey, J.R.; Gummadi, S.; Forsberg, F.; Wessner, C.; Kumar, A.R.; Chiang, A.; Infantolino, A.; Schlachterman, A.; et al. Identification of sentinel lymph nodes in esophageal cancer patients using contrast-enhanced EUS with peritumoral injections. Endosc. Ultrasound 2023, 12, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yamashita, Y.; Kamata, K.; Ang, T.L.; Imazu, H.; Ohno, E.; Hirooka, Y.; Fusaroli, P.; Seo, D.; Napoleon, B.; et al. The Asian federation of societies for ultrasound in medicine and biology (AFSUMB) guidelines for contrast-enhanced endoscopic ultrasound. Ultrasound Med. Biol. 2021, 47, 1433–1447. [Google Scholar] [CrossRef]
- Fusaroli, P.; Kypraios, D.; Eloubeidi, M.A.; Caletti, G. Levels of evidence in endoscopic ultrasonography: A systematic review. Dig. Dis. Sci. 2012, 57, 602–609. [Google Scholar] [CrossRef]
- Seifert, H.; Fusaroli, P.; Arcidiacono, P.G.; Braden, B.; Herth, F.; Hocke, M.; Larghi, A.; Napoleon, B.; Rimbas, M.; Ungureanu, B.S.; et al. Controversies in EUS: Do we need miniprobes? Endosc. Ultrasound 2021, 10, 246–269. [Google Scholar] [CrossRef]
- Ishihara, R.; Mizusawa, J.; Kushima, R.; Matsuura, N.; Yano, T.; Kataoka, T.; Fukuda, H.; Hanaoka, N.; Yoshio, T.; Abe, S.; et al. Assessment of the Diagnostic Performance of Endoscopic Ultrasonography After Conventional Endoscopy for the Evaluation of Esophageal Squamous Cell Carcinoma Invasion Depth. JAMA Netw. Open 2021, 4, e2125317. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Y.; Wang, T.; Gao, D.; Hu, B. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: A meta-analysis. Gastrointest. Endosc. 2014, 79, 233–241.e2. [Google Scholar] [CrossRef] [PubMed]
- Komanduri, S.; Kahrilas, P.J.; Krishnan, K.; McGorisk, T.; Bidari, K.; Grande, D.; Keefer, L.; Pandolfino, J. Recurrence of Barrett’s esophagus is rare following endoscopic eradication therapy coupled with effective reflux control. Off. J. Am. Coll. Gastroenterol. ACG 2017, 112, 556. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Singh, S.; Ragunathan, K.; Katzka, D.A.; Wang, K.K.; Iyer, P.G. Risk of recurrence of Barrett’s esophagus after successful endoscopic therapy. Gastrointest. Endosc. 2016, 83, 1090–1106.e3. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Qumseya, B.; Sultan, S.; Agrawal, D.; Chandrasekhara, V.; Harnke, B.; Kothari, S.; McCarter, M.; Shaukat, A.; Wang, A.; et al. Endoscopic eradication therapy for patients with Barrett’s esophagus-associated dysplasia and intramucosal cancer. Gastrointest. Endosc. 2018, 87, 907–931.e9. [Google Scholar] [CrossRef]
- Calpin, G.G.; Davey, M.G.; Donlon, N.E. Management of early oesophageal cancer: An overview. World J. Gastrointest. Surg. 2024, 16, 1255–1258. [Google Scholar] [CrossRef]
- Forbes, N.; Elhanafi, S.; Al-Haddad, M.A.; Thosani, N.; Draganov, P.V.; Othman, M.O.; Ceppa, E.P.; Kaul, V.; Feely, M.M.; Sahin, I.; et al. American Society for Gastrointestinal Endoscopy guideline on endoscopic submucosal dissection for the management of early esophageal and gastric cancers: Summary and recommendations. Gastrointest. Endosc. 2023, 98, 271–284. [Google Scholar] [CrossRef]
- Naveed, M.; Kubiliun, N. Endoscopic treatment of early-stage esophageal cancer. Curr. Oncol. Rep. 2018, 20, 71. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Xiang, J.; Zhang, Y.; Kong, Y.; Garfield, D.; Li, H. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2013, 146, 1198–1203. [Google Scholar] [CrossRef]
- Shaheen, N.J. Endoscopic treatment of esophageal neoplasia: A decade of evolution. Trans. Am. Clin. Clim. Assoc. 2020, 131, 297–314. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7358467/ (accessed on 31 May 2025).
- Han, C.; Sun, Y. Efficacy and safety of endoscopic submucosal dissection versus endoscopic mucosal resection for superficial esophageal carcinoma: A systematic review and meta-analysis. Dis. Esophagus 2021, 34, doaa081. [Google Scholar] [CrossRef]
- Shi, Q.; Ju, H.; Yao, L.Q.; Zhou, P.H.; Xu, M.D.; Chen, T.; Zhou, J.M.; Chen, T.Y.; Zhong, Y.S. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy 2014, 46, 640–644. [Google Scholar] [CrossRef]
- Hanaoka, N.; Ishihara, R.; Takeuchi, Y.; Uedo, N.; Higashino, K.; Ohta, T.; Kanzaki, H.; Hanafusa, M.; Nagai, K.; Matsui, F.; et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: A controlled prospective study. Endoscopy 2012, 44, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Kobayashi, M.; Takeuchi, M.; Sato, Y.; Narisawa, R.; Aoyagi, Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest. Endosc. 2011, 74, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Arima, M.; Iizuka, T.; Oyama, T.; Katada, C.; Kato, M.; Goda, K.; Goto, O.; Tanaka, K.; Yano, T.; et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig. Endosc. 2020, 32, 452–493. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Hirai, Y.; Uozumi, T.; Makiguchi, M.E.; Nonaka, S.; Suzuki, H.; Yoshinaga, S.; Oda, I.; Saito, Y. Endoscopic resection of esophageal squamous cell carcinoma: Current indications and treatment outcomes. DEN Open 2021, 2, e45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toyonaga, T.; Man-I, M.; East, J.E.; Nishino, E.; Ono, W.; Hirooka, T.; Ueda, C.; Iwata, Y.; Sugiyama, T.; Dozaiku, T.; et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: Complication rates and long-term outcomes. Surg. Endosc. 2013, 27, 1000–1008. [Google Scholar] [CrossRef]
- Iwai, N.; Dohi, O.; Yamada, S.; Harusato, A.; Horie, R.; Yasuda, T.; Yamada, N.; Horii, Y.; Majima, A.; Zen, K.; et al. Prognostic risk factors associated with esophageal squamous cell carcinoma patients undergoing endoscopic submucosal dissection: A multi-center cohort study. Surg Endosc. 2021, 36, 2279–2289. [Google Scholar] [CrossRef]
- Nagami, Y.; Ominami, M.; Shiba, M.; Minamino, H.; Fukunaga, S.; Kameda, N.; Sugimori, S.; Machida, H.; Tanigawa, T.; Yamagami, H.; et al. The five-year survival rate after endoscopic submucosal dissection for superficial esophageal squamous cell neoplasia. Dig. Liver Dis. 2017, 49, 427–433. [Google Scholar] [CrossRef]
- Qi, Z.P.; Chen, T.; Li, B.; Ren, Z.; Yao, L.Q.; Shi, Q.; Cai, S.L.; Zhong, Y.S.; Zhou, P.H. Endoscopic submucosal dissection for early esophageal cancer in elderly patients with relative indications for endoscopic treatment. Endoscopy 2018, 50, 839–845. [Google Scholar] [CrossRef]
- Yamashina, T.; Ishihara, R.; Nagai, K.; Matsuura, N.; Matsui, F.; Ito, T.; Fujii, M.; Yamamoto, S.; Hanaoka, N.; Takeuci, Y.; et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am. J. Gastroenterol. 2013, 108, 544–551. [Google Scholar] [CrossRef]
- Katada, C.; Muto, M.; Momma, K.; Arima, M.; Tajiri, H.; Kanamaru, C.; Ooyanagi, H.; Endo, H.; Michida, T.; Hasuike, N.; et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae-a multicenter retrospective cohort study. Endoscopy 2007, 39, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hashimoto, S.; Mizuno, K.I.; Kobayashi, T.; Tominaga, K.; Sato, H.; Kohisa, J.; Ikarashi, S.; Hayashi, K.; Takeuchi, M.; et al. Management decision based on lymphovascular involvement leads to favorable outcomes after endoscopic treatment of esophageal squamous cell carcinoma. Endoscopy 2018, 50, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Manner, H.; Rabenstein, T.; Pech, O.; Braun, K.; May, A.; Pohl, J.; Behrens, A.; Vieth, M.; Ell, C. Ablation of residual Barrett’s epithelium after endoscopic resection: A randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014, 46, 6–12. [Google Scholar] [CrossRef]
- van Vilsteren, F.G.I.; Pouw, R.E.; Seewald, S.; Herrero, L.A.; Sondermeijer, C.M.T.; Visser, M.; ten Kate, F.J.W.; Teng, K.C.Y.K.; Soehendra, N.; Rosch, T.; et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: A multicentre randomised trial. Gut 2011, 60, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Brown, N.J.; Davis, M.F.; Stephenson, T.J.; Stoddard, C.J.; Reed, M.W.R. Aminolevulinic acid-induced photodynamic therapy: Safe and effective ablation of dysplasia in Barrett’s esophagus. Dis. Esophagus 2000, 13, 18–22. [Google Scholar] [CrossRef]
- Overholt, B.F.; Wang, K.K.; Burdick, J.S.; Lightdale, C.J.; Kimmey, M.; Nava, H.R.; Sivak, M.V., Jr.; Nishioka, N.; Barr, H.; Marcon, N.; et al. Five-year efficacy and safety of photodynamic therapy with photofrin in Barrett’s high-grade dysplasia. Gastrointest. Endosc. 2007, 66, 460–468. [Google Scholar] [CrossRef]
- Shah, S.N.; Chehade, N.E.H.; Tavangar, A.; Choi, A.; Monachese, M.; Chang, K.; Samarasena, J.B. Hybrid argon plasma coagulation in Barrett’s esophagus: A systematic review and meta-analysis. Clin. Endosc. 2023, 56, 38–49. [Google Scholar] [CrossRef]
- Chandan, S.; Bapaye, J.; Khan, S.R.; Deliwala, S.; Mohan, B.P.; Ramai, D.; Dhindsa, B.S.; Goyal, H.; Kassab, L.L.; Aziz, M.; et al. Safety and efficacy of liquid nitrogen spray cryotherapy in Barrett’s neoplasia—A comprehensive review and meta-analysis. Endosc. Int. Open 2022, 10, E1462–E1473. [Google Scholar] [CrossRef]
- Lal, P.; Thota, P.N. Cryotherapy in the management of premalignant and malignant conditions of the esophagus. World J. Gastroenterol. 2018, 24, 4862–4869. [Google Scholar] [CrossRef]
- Tsai, F.C.; Ghorbani, S.; Greenwald, B.D.; Jang, S.; Dumot, J.A.; McKinley, M.J.; Shaheen, N.J.; Habra, D.; Wolfsen, H.C.; Abrams, J.A.; et al. Safety and efficacy of endoscopic spray cryotherapy for esophageal cancer. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Kachaamy, T.; Prakash, R.; Kundranda, M.; Batish, R.; Weber, J.; Hendrickson, S.; Yoder, L.; Do, H.; Magat, T.; Nayar, R.; et al. Liquid nitrogen spray cryotherapy for dysphagia palliation in patients with inoperable esophageal cancer. Gastrointest. Endosc. 2018, 88, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Cash, B.D.; Johnston, L.R.; Johnston, M.H. Cryospray ablation (CSA) in the palliative treatment of squamous cell carcinoma of the esophagus. World J. Surg. Oncol. 2007, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y. A review of self-expanding esophageal stents for the palliation therapy of inoperable esophageal malignancies. Biomed. Res. Int. 2019, 2019, 9265017. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, T.; Morales, J.P.; Salter, R.; Adam, A. Esophageal cancer: Self-expanding metallic stents. Abdom. Imaging 2005, 30, 456–464. [Google Scholar] [CrossRef]
- So, H.; Ahn, J.Y.; Han, S.; Jung, K.; Na, H.K.; Lee, J.H.; Jeong, K.W.; Kim, D.H.; Choi, K.D.; Song, H.J.; et al. Efficacy and safety of fully covered self-expanding metal stents for malignant esophageal obstruction. Dig. Dis. Sci. 2018, 63, 234–241. [Google Scholar] [CrossRef]
- Martin, R.C.G.; Woodall, C.; Duvall, R.; Scoggins, C.R. The use of self-expanding silicone stents in esophagectomy strictures: Less cost and more efficiency. Ann. Thorac. Surg. 2008, 86, 436–440. [Google Scholar] [CrossRef]
- Holm, A.N.; de la Mora Levy, J.G.; Gostout, C.J.; Topazian, M.D.; Baron, T.H. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointest. Endosc. 2008, 67, 20–25. [Google Scholar] [CrossRef]
- Bhatt, A.; Kamath, S.; Murthy, S.C.; Raja, S. Multidisciplinary evaluation and management of early stage esophageal cancer. Surg. Oncol. Clin. N. Am. 2020, 29, 613–630. [Google Scholar] [CrossRef]
- Grady, W.M.; Yu, M.; Markowitz, S.D.; Chak, A. Barrett’s Esophagus and Esophageal Adenocarcinoma Biomarkers. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2486–2494. [Google Scholar] [CrossRef]
- Dong, J.; Buas, M.F.; Gharahkhani, P.; Kendall, B.J.; Onstad, L.; Zhao, S.; Anderson, L.A.; Wu, A.; Ye, W.; Bird, N.C.; et al. Determining Risk of Barrett’s Esophagus and Esophageal Adenocarcinoma Based on Epidemiologic Factors and Genetic Variants. Gastroenterology 2018, 154, 1273–1281.e3. [Google Scholar] [CrossRef]
- Miyoshi, J.; Mannucci, A.; Scarpa, M.; Gao, F.; Toden, S.; Whitsett, T.; Inge, L.J.; Bremner, R.M.; Takayama, T.; Cheng, Y.; et al. Liquid biopsy to identify Barrett’s oesophagus, dysplasia and oesophageal adenocarcinoma: The EMERALD multicentre study. Gut 2025, 74, 169–181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakao, E.; Yoshio, T.; Kato, Y.; Namikawa, K.; Tokai, Y.; Yoshimizu, S.; Horiuchi, Y.; Ishiyama, A.; Hirasawa, T.; Kurihara, N.; et al. Randomized controlled trial of an artificial intelligence diagnostic system for the detection of esophageal squamous cell carcinoma in clinical practice. Endoscopy 2025, 57, 210–217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Pan, S.; Mortan, R.; Ali, F.S.; Thosani, N.; Wadhwa, V. Role of Endoscopy in the Diagnosis and Management of Esophageal Cancer. J. Clin. Med. 2025, 14, 8169. https://doi.org/10.3390/jcm14228169
Ma J, Pan S, Mortan R, Ali FS, Thosani N, Wadhwa V. Role of Endoscopy in the Diagnosis and Management of Esophageal Cancer. Journal of Clinical Medicine. 2025; 14(22):8169. https://doi.org/10.3390/jcm14228169
Chicago/Turabian StyleMa, Jennifer, Sharon Pan, Rachel Mortan, Faisal Shaukat Ali, Nirav Thosani, and Vaibhav Wadhwa. 2025. "Role of Endoscopy in the Diagnosis and Management of Esophageal Cancer" Journal of Clinical Medicine 14, no. 22: 8169. https://doi.org/10.3390/jcm14228169
APA StyleMa, J., Pan, S., Mortan, R., Ali, F. S., Thosani, N., & Wadhwa, V. (2025). Role of Endoscopy in the Diagnosis and Management of Esophageal Cancer. Journal of Clinical Medicine, 14(22), 8169. https://doi.org/10.3390/jcm14228169





