Exoscopic Visualization for Transorbital Surgery: Preliminary Anatomical and Clinical Validation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Stepwise Dissection
2.2. Dissection Protocol
- Group A: the endoscope was used as the sole visualization tool.
- Group B: ETOA was performed using exoscopic visualization for the skin phase and endoscopic visualization for the orbital and intracranial phases.
- Group C: exoscopic visualization was used for the skin and orbital phases, while the endoscope was used for the intracranial phase.
- Group D: ETOA was performed entirely under exoscopic visualization.
2.3. Statistical Analysis
2.4. Skin Phase
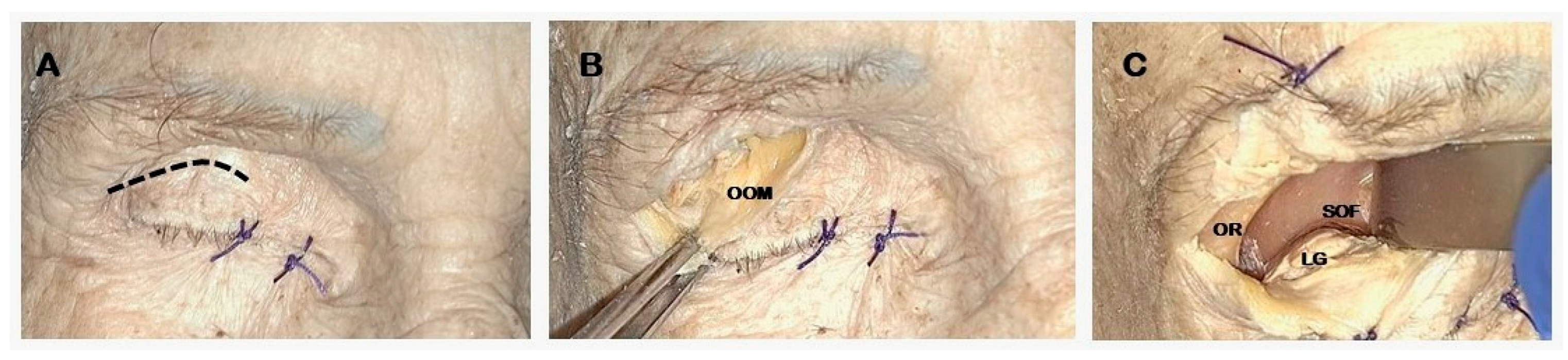
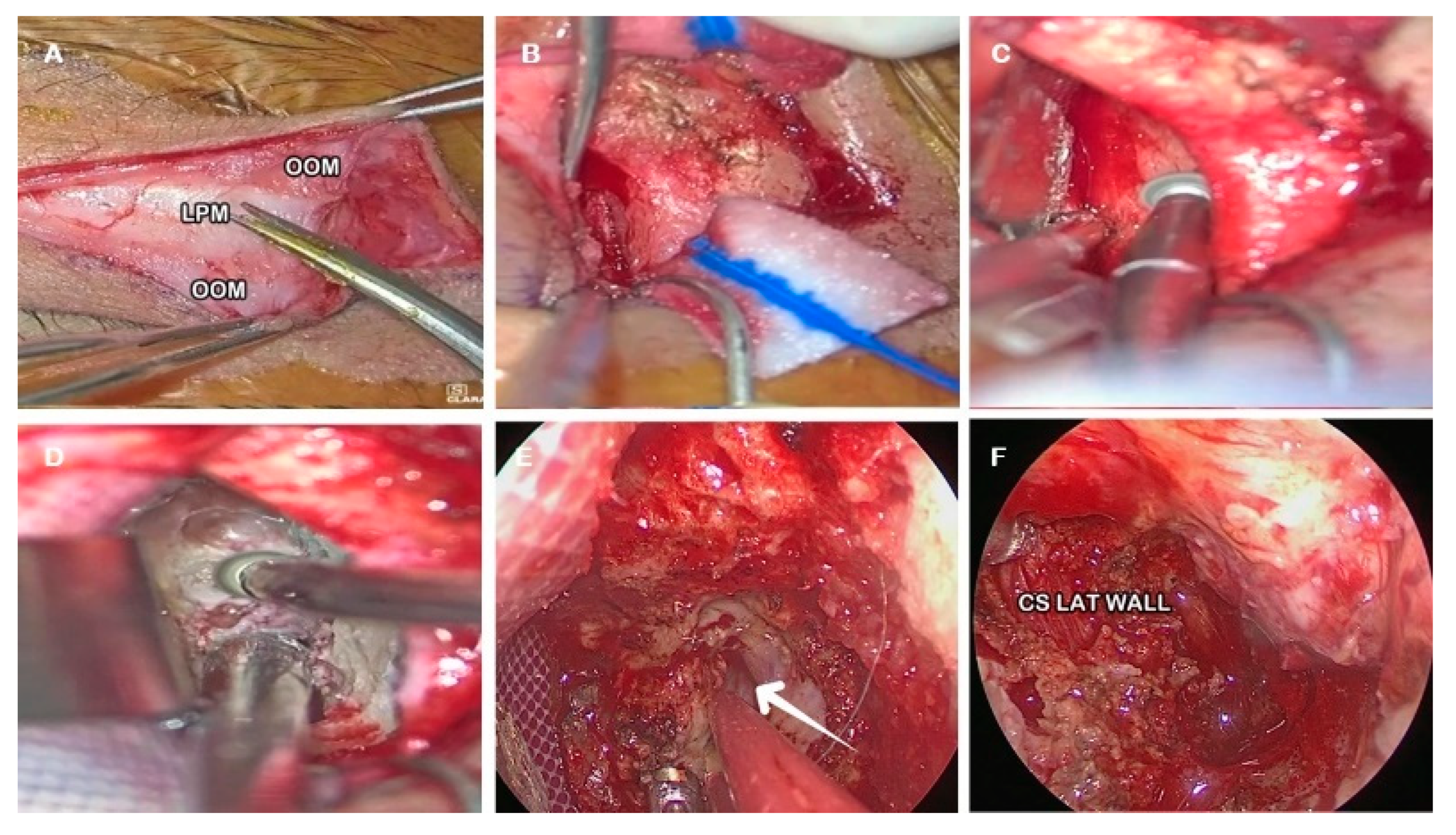
- Skin incision along the superior eyelid crease (Figure 1A).
- Subcutaneous dissection of the orbicularis oculi muscle (OOM) to expose the orbital septum underneath (Figure 1B).
- Lateral orbital rim exposure: the periosteum was cut acceding the intraorbital space, proceeding with the dissection in a sub-periosteal plane separating the periorbita from the lateral orbital wall and exposing the ventral surface of the greater sphenoid wing and the lateral part of the orbital roof. A malleable retractor was then inserted to displace the orbital content medially (Figure 1C).
2.5. Endo-Orbital Phase
- Lateral orbital wall exposure and orbital retraction: dissection is led in a subperiosteal plane detaching periorbita until the lateral edges of the superior and inferior orbital fissures are encountered.
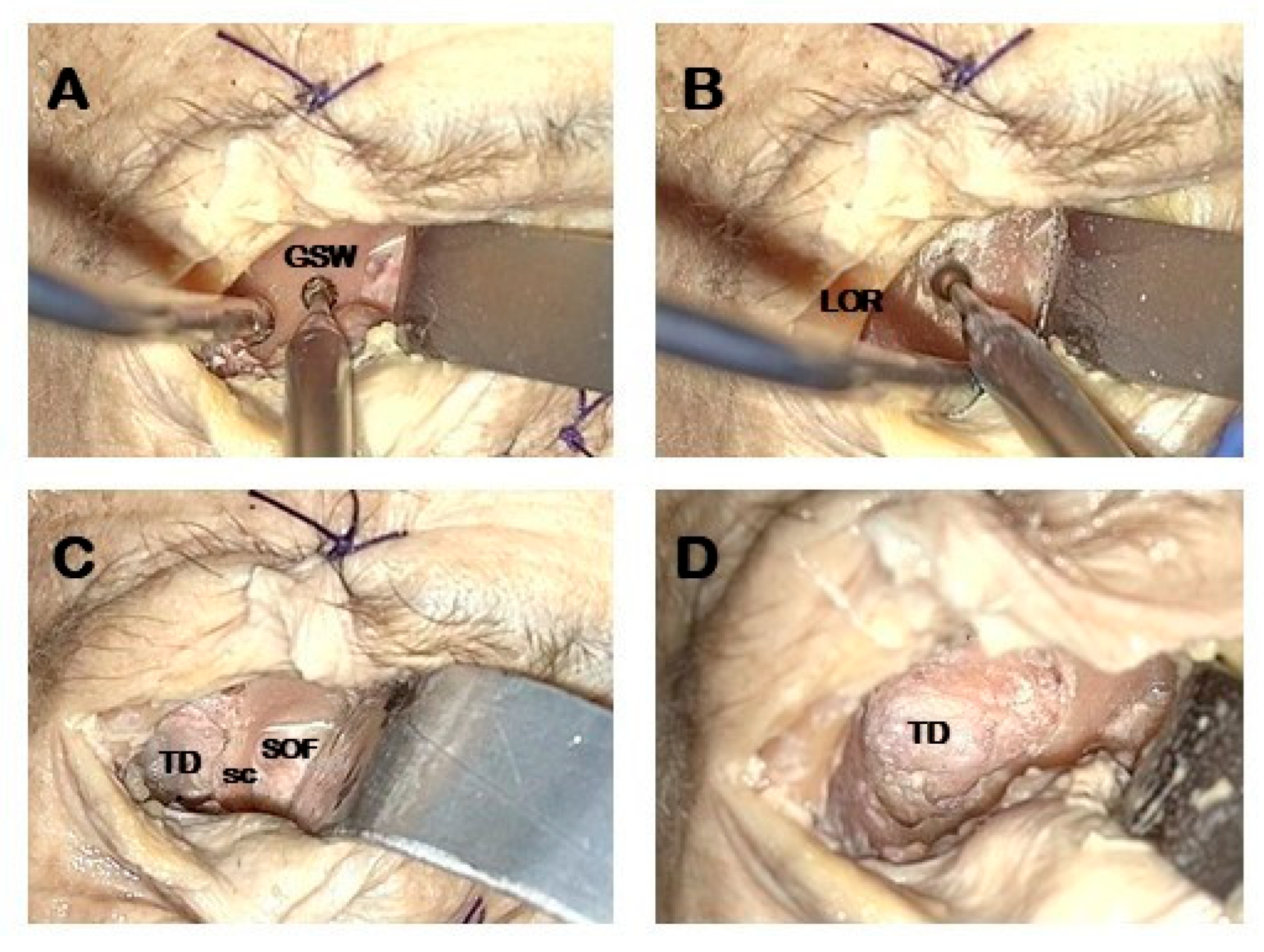
- Temporal fossa access: proximal portion of the lateral orbital wall of the greater sphenoid wing (GSW) is drilled acceding to the temporal fossa (Figure 2A).
- GSW drilling: distal drilling of the lateral orbital wall along the GSW exposes the dura mater of the temporal pole (Figure 2B).
- Sagittal crest (SC) resection: SC is a triangular bony ridge remnant of the GSW drilling dividing the medial temporal dura from the postero-lateral periorbital, that has to be removed to expose the entry point to perform interdural dissection [21] (Figure 2C). This maneuver allows the wide exposure of the dura mater of the temporal pole (Figure 2D).
2.6. Endocranial Phase and Meckel’s Cave Exposure
- Middle fossa floor flattening: High speed drilling of the GSW is pursued inferiorly to flatten the middle temporal fossa floor (Figure 3A).
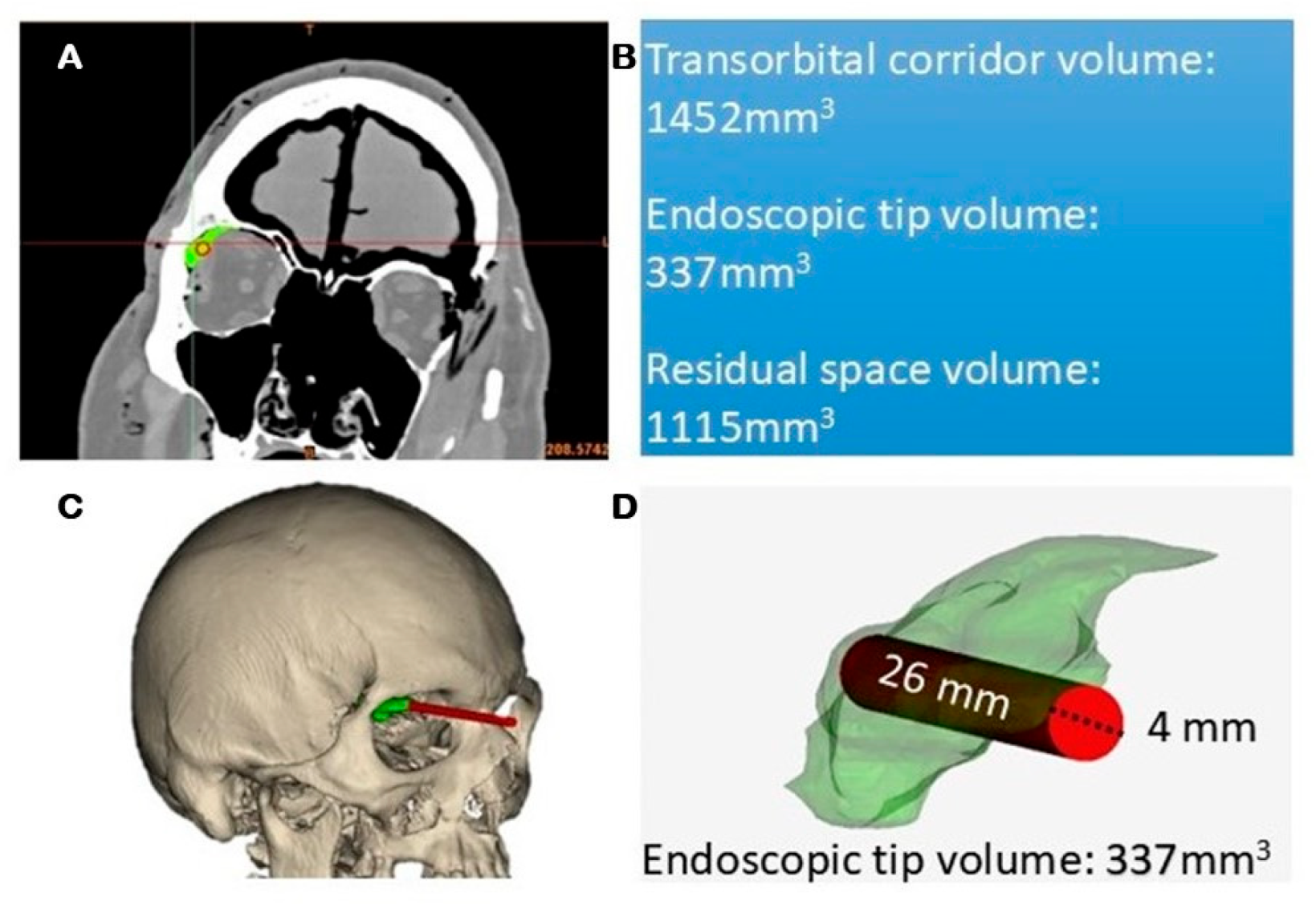
2.7. Quantitative Radiological Analysis
3. Results
3.1. Skin Phase
3.2. Orbital Phase
3.3. Intracranial Phase
3.4. Timing
Ergonomics
3.5. Quantitative CT-Based Analysis
3.6. Illustrative Cases
4. Discussion
4.1. Skin Phase
4.2. Orbital Phase
4.3. Intracranial Phase
4.4. Considerations
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montemurro, N.; Scerrati, A.; Ricciardi, L.; Trevisi, G. The Exoscope in Neurosurgery: An Overview of the Current Literature of Intraoperative Use in Brain and Spine Surgery. J. Clin. Med. 2021, 11, 223. [Google Scholar] [CrossRef]
- Siller, S.; Zoellner, C.; Fuetsch, M.; Trabold, R.; Tonn, J.-C.; Zausinger, S. A High-Definition 3D Exoscope as an Alternative to the Operating Microscope in Spinal Microsurgery. J. Neurosurg. Spine 2020, 33, 705–714. [Google Scholar] [CrossRef]
- Ricciardi, L.; Chaichana, K.L.; Cardia, A.; Stifano, V.; Rossini, Z.; Olivi, A.; Sturiale, C.L. The Exoscope in Neurosurgery: An Innovative “Point of View”. A Systematic Review of the Technical, Surgical, and Educational Aspects. World Neurosurg. 2019, 124, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Andaluz, N.; Cavallo, L.M.; de Notaris, M.; Dallan, I.; Solari, D.; Zimmer, L.A.; Keller, J.T.; Zuccarello, M.; Prats-Galino, A.; et al. Endoscopic Transorbital Superior Eyelid Approach: Anatomical Study from a Neurosurgical Perspective. J. Neurosurg. 2018, 129, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.S.; Bergeron, C.M.; Ellenbogen, R.G. Transorbital Neuroendoscopic Surgery. Neurosurgery 2010, 67, ons16–ons28. [Google Scholar] [CrossRef] [PubMed]
- de Notaris, M.; Sacco, M.; Corrivetti, F.; Grasso, M.; Corvino, S.; Piazza, A.; Kong, D.-S.; Iaconetta, G. The Transorbital Approach, A Game-Changer in Neurosurgery: A Guide to Safe and Reliable Surgery Based on Anatomical Principles. J. Clin. Med. 2023, 12, 6484. [Google Scholar] [CrossRef]
- Bly, R.A.; Ramakrishna, R.; Ferreira, M.; Moe, K.S. Lateral Transorbital Neuroendoscopic Approach to the Lateral Cavernous Sinus. J. Neurol. Surg. Part B Skull Base 2014, 75, 11–17. [Google Scholar] [CrossRef]
- Dallan, I.; Sellari-Franceschini, S.; Turri-Zanoni, M.; de Notaris, M.; Fiacchini, G.; Fiorini, F.R.; Battaglia, P.; Locatelli, D.; Castelnuovo, P. Endoscopic Transorbital Superior Eyelid Approach for the Management of Selected Spheno-Orbital Meningiomas: Preliminary Experience. Oper. Neurosurg. 2018, 14, 243–251. [Google Scholar] [CrossRef]
- Kong, D.-S.; Lee, W.J.; Kim, G.J.; Hong, C.-K. The Feasibility and Clinical Outcome of Endoscopic Transorbital Transcavernous Approaches with or without Petrosectomy for Petroclival Lesions. J. Neurosurg. 2024, 142, 1058–1065. [Google Scholar] [CrossRef]
- Kong, D.-S.; Kim, Y.H.; Hong, C.-K. Optimal Indications and Limitations of Endoscopic Transorbital Superior Eyelid Surgery for Spheno-Orbital Meningiomas. J. Neurosurg. 2020, 134, 1472–1479. [Google Scholar] [CrossRef]
- Kong, D.-S.; Kim, Y.H.; Lee, W.-J.; Kim, Y.-H.; Hong, C.-K. Indications and Outcomes of Endoscopic Transorbital Surgery for Trigeminal Schwannoma Based on Tumor Classification: A Multicenter Study with 50 Cases. J. Neurosurg. 2022, 138, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, J.A.; Rosen, K.U.; Chae, J.K.; Pandey, A.; Bander, E.D.; Godfrey, K.; Schwartz, T.H. The Endoscopic Lateral Transorbital Approach for the Removal of Select Sphenoid Wing and Middle Fossa Meningiomas. Surgical Technique and Short-Term Outcomes. Oper. Neurosurg. 2023, 26, 165–172. [Google Scholar] [CrossRef]
- Bander, E.D.; Carnevale, J.A.; Tosi, U.; Godfrey, K.J.; Schwartz, T.H. Lateral Transorbital Endoscope-Assisted Approach to the Cavernous Sinus. Oper. Neurosurg. 2023, 25, 359–364. [Google Scholar] [CrossRef]
- Jeon, C.; Hong, C.-K.; Woo, K.I.; Hong, S.D.; Nam, D.-H.; Lee, J.-I.; Choi, J.W.; Seol, H.J.; Kong, D.-S. Endoscopic Transorbital Surgery for Meckel’s Cave and Middle Cranial Fossa Tumors: Surgical Technique and Early Results. J. Neurosurg. 2018, 131, 1126–1135. [Google Scholar] [CrossRef]
- Almeida, J.P.; Omay, S.B.; Shetty, S.R.; Chen, Y.-N.; Ruiz-Treviño, A.S.; Liang, B.; Anand, V.K.; Levine, B.; Schwartz, T.H. Transorbital Endoscopic Eyelid Approach for Resection of Sphenoorbital Meningiomas with Predominant Hyperostosis: Report of 2 Cases. J. Neurosurg. 2018, 128, 1885–1895. [Google Scholar] [CrossRef]
- Jeon, C.; Hong, C.-K.; Chong, K.; Lee, W.J.; Kim, G.J.; Lee, J.-I.; Nam, D.-H.; Seol, H.J.; Choi, J.W.; Shin, H.J.; et al. Endoscopic Transorbital Approach for Resection of Mediobasal Temporal Lesions Using an Optic Radiation-Sparing Strategy. J. Neurosurg. 2025, 142, 819–828. [Google Scholar] [CrossRef]
- Ricciuti, V.; Peppucci, E.; Montalbetti, A.; Piras, G.; Spena, G.; Giussani, C.G.; Zoia, C. Endoscopic Transorbital Approach for Recurrent Spheno-Orbital Meningiomas: Single Center Case Series. Neurosurg. Rev. 2024, 47, 706. [Google Scholar] [CrossRef]
- Corrivetti, F.; de Notaris, M.; Seneca, V.; Di Nuzzo, G.; Catapano, G. Is It Time for a Paradigm Shift in the Surgical Management of Trigeminal Schwannomas? Evaluating the Role of the Transorbital Approach: An Anatomo-Clinical Study and Systematic Literature Review. World Neurosurg. 2024, 190, e1025–e1037. [Google Scholar] [CrossRef]
- Iwami, K.; Fujii, M.; Watanabe, T.; Osuka, K. Exo- and Endoscopic Lateral Orbital Wall Approach for the Medial Temporal Lobe Glioma: How I Do It. Acta Neurochir. 2024, 166, 110. [Google Scholar] [CrossRef] [PubMed]
- Dallan, I.; Di Somma, A.; Prats-Galino, A.; Solari, D.; Alobid, I.; Turri-Zanoni, M.; Fiacchini, G.; Castelnuovo, P.; Catapano, G.; de Notaris, M. Endoscopic Transorbital Route to the Cavernous Sinus through the Meningo-Orbital Band: A Descriptive Anatomical Study. J. Neurosurg. 2017, 127, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Corrivetti, F.; de Notaris, M.; Di Somma, A.; Dallan, I.; Enseñat, J.; Topczewski, T.; Solari, D.; Cavallo, L.M.; Cappabianca, P.; Prats-Galino, A. “Sagittal Crest”: Definition, Stepwise Dissection, and Clinical Implications from a Transorbital Perspective. Oper. Neurosurg. 2022, 22, e206–e212. [Google Scholar] [CrossRef] [PubMed]
- Froelich, S.C.; Aziz, K.M.A.; Levine, N.B.; Theodosopoulos, P.V.; van Loveren, H.R.; Keller, J.T. Refinement of the Extradural Anterior Clinoidectomy: Surgical Anatomy of the Orbitotemporal Periosteal Fold. Neurosurgery 2007, 61, 179–185, discussion 185–186. [Google Scholar] [CrossRef]
- Corrivetti, F.; Guizzardi, G.; Bove, I.; Enseñat, J.; Prats-Galino, A.; Solari, D.; Cavallo, L.M.; Iaconetta, G.; Di Somma, A.; de Notaris, M. Transorbital Exposure of the Internal Carotid Artery: A Detailed Anatomic and Quantitative Roadmap for Safe Successful Surgery. Oper. Neurosurg. 2023, 26, 314–322. [Google Scholar] [CrossRef]
- de Divitiis, E.; Cavallo, L.M.; Cappabianca, P.; Esposito, F. Extended Endoscopic Endonasal Transsphenoidal Approach for the Removal of Suprasellar Tumors: Part 2. Neurosurgery 2007, 60, 46–58, discussion 58–59. [Google Scholar] [CrossRef]
- Garneau, J.C.; Laitman, B.M.; Cosetti, M.K.; Hadjipanayis, C.; Wanna, G. The Use of the Exoscope in Lateral Skull Base Surgery: Advantages and Limitations. Otol. Neurotol. 2019, 40, 236–240. [Google Scholar] [CrossRef]
- Hafez, A.; Haeren, R.H.L.; Dillmann, J.; Laakso, A.; Niemelä, M.; Lehecka, M. Comparison of Operating Microscope and Exoscope in a Highly Challenging Experimental Setting. World Neurosurg. 2021, 147, e468–e475. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, L.M.; Frank, G.; Cappabianca, P.; Solari, D.; Mazzatenta, D.; Villa, A.; Zoli, M.; D’Enza, A.I.; Esposito, F.; Pasquini, E. The Endoscopic Endonasal Approach for the Management of Craniopharyngiomas: A Series of 103 Patients. J. Neurosurg. 2014, 121, 100–113. [Google Scholar] [CrossRef]
- Kassam, A.B.; Prevedello, D.M.; Carrau, R.L.; Snyderman, C.H.; Thomas, A.; Gardner, P.; Zanation, A.; Duz, B.; Stefko, S.T.; Byers, K.; et al. Endoscopic Endonasal Skull Base Surgery: Analysis of Complications in the Authors’ Initial 800 Patients. J. Neurosurg. 2011, 114, 1544–1568. [Google Scholar] [CrossRef] [PubMed]
- Maghalashvili, E.; Corrivetti, F.; Shalamberidze, B.; Corvino, S.; Chkhikvishvili, T.; de Notaris, M. Endoscopic Transorbital Resection of Temporal Pole Cavernoma: 2-Dimensional Operative Video. Oper. Neurosurg. 2024, 28, 442. [Google Scholar] [CrossRef]
- Gerges, M.M.; Godil, S.S.; Younus, I.; Rezk, M.; Schwartz, T.H. Endoscopic Transorbital Approach to the Infratemporal Fossa and Parapharyngeal Space: A Cadaveric Study. J. Neurosurg. 2019, 133, 1948–1959. [Google Scholar] [CrossRef]
- Almeida, J.P.; Ruiz-Treviño, A.S.; Shetty, S.R.; Omay, S.B.; Anand, V.K.; Schwartz, T.H. Transorbital Endoscopic Approach for Exposure of the Sylvian Fissure, Middle Cerebral Artery and Crural Cistern: An Anatomical Study. Acta Neurochir. 2017, 159, 1893–1907. [Google Scholar] [CrossRef]
- Veldeman, M.; Rossmann, T.; Huhtakangas, J.; Nurminen, V.; Eisenring, C.; Sinkkonen, S.T.; Niemela, M.; Lehecka, M. Three-Dimensional Exoscopic Versus Microscopic Resection of Vestibular Schwannomas: A Comparative Series. Oper. Neurosurg. 2023, 24, 507–513. [Google Scholar] [CrossRef]
- Sistiaga, I.L.; Catalán-Uribarrena, G.; Valcárcel, F.; Garcia-Orozco, Y.; Altamirano-Cruz, J.; Oñate, M.; Pomposo, I. Endoscopic Transorbital Approach for Repair of Spontaneous CSF Leak and Frontal Sinus Encephalocele. Neurosurg. Focus Video 2025, 12, V9. [Google Scholar] [CrossRef] [PubMed]
- Chibbaro, S.; Ganau, M.; Scibilia, A.; Todeschi, J.; Zaed, I.; Bozzi, M.T.; Ollivier, I.; Cebula, H.; Santin, M.D.N.; Djennaoui, I.; et al. Endoscopic Transorbital Approaches to Anterior and Middle Cranial Fossa: Exploring the Potentialities of a Modified Lateral Retrocanthal Approach. World Neurosurg. 2021, 150, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Guizzardi, G.; Mosteiro, A.; Hoyos, J.; Ferres, A.; Topczewski, T.; Reyes, L.; Alobid, I.; Matas, J.; Cavallo, L.M.; Cappabianca, P.; et al. Endoscopic Transorbital Approach to the Middle Fossa: Qualitative and Quantitative Anatomic Study. Oper. Neurosurg. 2022, 23, e267–e275. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A.; Mosteiro, A.; Guizzardi, G.; Roldán, P.; Torales, J.; Matas Fassi, J.; Cavallo, L.M.; Solari, D.; Prats-Galino, A.; Di Somma, A.; et al. Endoscopic Transorbital Resection of the Temporal Lobe: Anatomic Qualitative and Quantitative Study. Front. Neuroanat. 2023, 17, 1282226. [Google Scholar] [CrossRef]
- Carnevale, J.A.; Ramirez-Loera, C.; Goldberg, J.L.; Godfrey, K.J.; Schwartz, T.H. Transorbital Endoscopic Approach for Middle Fossa Floor/Lateral Cavernous Sinus Meningioma: 2-Dimensional Operative Video. Oper. Neurosurg. 2023, 24, e201–e202. [Google Scholar] [CrossRef]
- Di Somma, A.; Guizzardi, G.; Sanchez España, J.C.; Matas Fassi, J.; Topczewski, T.E.; Ferres, A.; Mosteiro, A.; Reyes, L.; Tercero, J.; Lopez, M.; et al. Complications of the Superior Eyelid Endoscopic Transorbital Approach to the Skull Base: Preliminary Experience with Specific Focus on Orbital Outcome. J. Neuro-Ophthalmol. 2024, 44, 92–100. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Paolini, F.; Meccio, F.; Giovannini, E.A.; Provenzano, A.; Bonosi, L.; Brunasso, L.; Costanzo, R.; Gerardi, R.M.; Di Bonaventura, R.; et al. Assessing the Training in Neurosurgery with the Implementation of VITOM-3D Exoscope: Learning Curve on Experimental Model in Neurosurgical Practice. Brain Sci. 2023, 13, 1409. [Google Scholar] [CrossRef]
- Bradaschia, L.; Vincitorio, F.; Titolo, P.; Battiston, B.; Garbossa, D. The Role of Exoscope in the Peripheral Nerve Surgery: Proof of Concept: 2-Dimensional Operative Video. Oper. Neurosurg. 2025. [Google Scholar] [CrossRef]
- Calloni, T.; Carone, G.; Cavaliere, M.; de Laurentis, C.; Bussa, M.; Antolini, L.; Nicolosi, F.; Carrabba, G.G.; Fontanella, M.M.; Cenzato, M.; et al. Comparison of Operative Microscope and Exoscope for Execution of Microanastomoses on an Artificial Model. Front. Surg. 2025, 12, 1573333. [Google Scholar] [CrossRef] [PubMed]


| Group | Visualization Technique | Surgical Comfort | Maneuverability | Image Quality |
|---|---|---|---|---|
| A | Endoscopic only | 3.2 | 3.5 | 4.6 |
| B | Exoscopic (skin) + Endoscopic | 4.1 | 4.3 | 4.4 |
| C | Exoscopic (skin + orbit) + Endoscopic | 4.3 | 4.5 | 4.2 |
| D | Exoscopic only | 3.6 | 3.8 | 3.9 |
| EXOSCOPE | ENDOSCOPE | ||
|---|---|---|---|
| Advantages | Limitations | Advantages | Limitations |
| Distance from the field allows improved instrument maneuverability Adjustable magnification Improved working space Avoid lens tarnishing during drilling | Requires constant adjustment to maintain proper angle No freehand control Poor visualization around corners Limited view in depth | Excellent visualization within orbital corridor High magnification and excellent deep-field visualization | Low resolution outside cavities Not designed for superficial layers Instrument crowding inside narrow corridor Lens tarnishing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrivetti, F.; de Notaris, M.; Corvino, S.; Piazza, A.; Porto, E.; Leo, S.; Cavaliere, C.; De Simone, M.; Iaconetta, G.; Kong, D.-S. Exoscopic Visualization for Transorbital Surgery: Preliminary Anatomical and Clinical Validation Study. J. Clin. Med. 2025, 14, 8165. https://doi.org/10.3390/jcm14228165
Corrivetti F, de Notaris M, Corvino S, Piazza A, Porto E, Leo S, Cavaliere C, De Simone M, Iaconetta G, Kong D-S. Exoscopic Visualization for Transorbital Surgery: Preliminary Anatomical and Clinical Validation Study. Journal of Clinical Medicine. 2025; 14(22):8165. https://doi.org/10.3390/jcm14228165
Chicago/Turabian StyleCorrivetti, Francesco, Matteo de Notaris, Sergio Corvino, Amedeo Piazza, Edoardo Porto, Stefano Leo, Carlo Cavaliere, Matteo De Simone, Giorgio Iaconetta, and Doo-Sik Kong. 2025. "Exoscopic Visualization for Transorbital Surgery: Preliminary Anatomical and Clinical Validation Study" Journal of Clinical Medicine 14, no. 22: 8165. https://doi.org/10.3390/jcm14228165
APA StyleCorrivetti, F., de Notaris, M., Corvino, S., Piazza, A., Porto, E., Leo, S., Cavaliere, C., De Simone, M., Iaconetta, G., & Kong, D.-S. (2025). Exoscopic Visualization for Transorbital Surgery: Preliminary Anatomical and Clinical Validation Study. Journal of Clinical Medicine, 14(22), 8165. https://doi.org/10.3390/jcm14228165








