Abstract
A competent embryo and a receptive endometrium are essential for adequate embryo–maternal cross-talk and successful implantation. However, the majority of women undergoing IVF do not achieve pregnancy after the first embryo transfer, incriminating potential implantation issues. According to statistics, recurrent implantation failure (RIF), for which different definitions have been proposed, is estimated to affect about 10–25% of women undergoing in vitro fertilization (IVF). RIF is a complex condition with overlapping causes. The primary objective of this review is to explore factors such as gamete and embryo quality, chromosomal abnormalities, uterine environment, endometrial receptivity, immune cell biomarkers, and microbiota dysregulation to better understand and overcome RIF challenges. It also highlights the significance of comprehensive evaluations of novel therapies, such as activated autologous platelet-rich plasma (PRP) or peripheral blood mononuclear cell (PBMC) insemination, on pregnancy outcomes in patients with RIF.
1. Introduction
Different definitions have been proposed concerning the term of recurrent implantation failure (RIF). For the ASRM (American Society of Reproductive Medicine), RIF is a condition that occurs when embryos fail to implant after multiple in vitro fertilization (IVF) attempts, while the ESHRE (European Society of Human Reproduction and Embryology) reported that RIF can be considered after failure to achieve a clinical pregnancy after the transfer of at least three good-quality embryos or two transfers of euploid embryos, when uterine and endometrial factors have been ruled out. Consequently, different IVF centers may use different definitions. However, there is no single universally accepted definition; according to Coughlan et al., 2014, RIF is defined as failure to achieve a clinical pregnancy after transfer of ≥4 good-quality embryos in a minimum of three fresh or frozen IVF cycles in women under 40 years of age [1].
For some, the term RIF stands for conditions in which clinical pregnancy is not achieved after three consecutive IVF attempts, in which one to two embryos of high-grade quality are transferred in each cycle [2]. However, no standardized criteria exist to specify the number of failed cycles or the total number of embryos transferred during these attempts [3]. Different causes of RIF, including dysregulation of molecular mechanisms governing embryo–endometrium cross-talk, the two key players in the process of implantation, were reviewed recently along with available treatment options [4].
Implantation is a process driven by a dynamic interaction between the embryo and the endometrium [5]. This “cross-talk” is a highly intricate crucial process for implantation and the success of normal placentation [6]. This interaction involves a coordinated exchange of molecular signals between a competent blastocyst and a receptive endometrium, facilitating the attachment, which is a stable adhesion of the embryo to the endometrial cells mediated by cell adhesion molecules like integrins, selectins, and cadherins [7]. The invasion, whereby trophoblast cells invade the maternal tissue to establish a connection with maternal blood vessels ensuring nutrient and oxygen supply [8], is a step forward. Dysregulation in this cross-talk can lead to implantation failure and/or complications of pregnancy.
Multifactorial RIF may result from maternal factors, including increased body mass index (BMI), anatomical disorders, smoking, advanced age that affects oocyte quality, embryo competency and endometrial receptivity [9,10], and paternal factors, including sperm maturation, competency, as well as genetic and epigenetic disorders [11].
Multiple modifiable lifestyle and environmental factors influence implantation success and ART outcomes. Strong evidence links obesity and metabolic dysfunction with impaired endometrial receptivity and reduced implantation rates [12,13]. Smoking has a well-established negative impact on uterine blood flow and gamete quality, while vaping poses emerging but likely similar risks, and its cessation should be strongly encouraged [14,15]. A dose–response meta-analysis indicated a linear association, showing that increased alcohol exposure was progressively associated with decreased fecundability; excessive intake is consistently detrimental to reproductive outcomes [16].
Moreover, evidence regarding environmental endocrine disruptors (e.g., BPA, phthalates, PFAS) is still emerging but remains biologically plausible, considering their potential effects on the female reproductive system and embryogenesis, with particular attention to associated reproductive pathologies [17].
Additionally, inadequate patient investigation, deficient management strategy, suboptimal laboratory performance, and adverse embryo transfer conditions, including embryologist and clinician skills, can all contribute to RIF [18].
In turn, the immune tolerance system can also play a critical role in implantation and pregnancy maintenance [10]. Successful implantation requires balanced uterine immunity. A one-to-one matched cohort study involving 193 patients between 2012 and 2014 revealed that uterine immune profile balance is a key factor to the success of implantation and pregnancy maintenance [19]. In addition, imbalance between the pro-inflammatory (IL-1, TNF-α) and anti-inflammatory (IL-10, TGF-β) cytokines can also be a causative factor in RIF [20]. In this context, the autoimmune factor can cause pregnancy loss, and research shows that autoantibodies targeting phospholipids can hinder implantation by causing micro thrombosis or inflammation in uterine vessels [21,22].
Abnormal uterine environment caused by different pathologies, such as anatomical abnormalities of the Mullerian tract or myomas, intrauterine adhesions, polyps, and thin endometrium, can impair embryo implantation and cause RIF [11]. These anomalies can distort the uterine cavity and increase the risk of miscarriage [18]. Among the various uterine disorders, chronic endometritis (CE), defined as a persistent inflammation of the endometrium [23], often results from intrauterine bacterial infections (such as Mycoplasma and Ureaplasma, Chlamydia, Escherichia coli, Enterococcus faecalis, Streptococcus) and can be involved in RIF [24]. On other hand, it was reported that vaginal Lactobacillus was significantly lower in patients with RIF compared with healthy women [25]. A systematic review and meta-analysis, involving a population of 1038 women treated between 1990 and 2024, revealed that CE seems to be linked to infertility and recurrent pregnancy loss [26].
RIF is still a complex and poorly understood area. The present review aims to find out and summarize the etiology of RIF involving gametes and embryo factors, uterine cavity environment pathophysiology, and immune tolerance balance system.
2. Looking at Gametes and Embryos
Oocyte quality is determined by several factors, including cytoplasmic maturation, which ensures the presence of healthy mitochondria and other resources to support early embryonic growth [27]. Nuclear maturation is equally essential for proper chromosomal alignment and segregation to minimize chromosome abnormalities and genome decay risks. Additionally, the zona pellucida must remain intact to facilitate sperm binding and protect the embryo during its initial stages. With advancing maternal age, ovaries are increasingly exposed to oxidative stress because of mitochondrial dysfunction decreasing inherent antioxidant defense (Figure 1), which significantly impacts oocyte quality and leads to an increased risk of chromosome disorders and implantation failure [28].
Similarly, sperm quality is critical to reproductive success. Sperm genome and epigenome decays are key factors in IVF outcomes, as high levels of DNA fragmentation and/or chromatin decondensation can compromise embryo quality and implantation potential (Figure 2).
Furthermore, sperm motility and morphology influence the ability of sperm to reach and fertilize the oocyte [29]. Lastly, chromatin compaction, which ensures proper DNA packaging, is essential for normal embryonic development [30]. Together, these factors determine the overall reproductive potential of the gametes and influence pregnancy outcomes. Molecular mechanisms and available treatments of both age-related and -unrelated ovarian issues were reviewed recently [31,32].
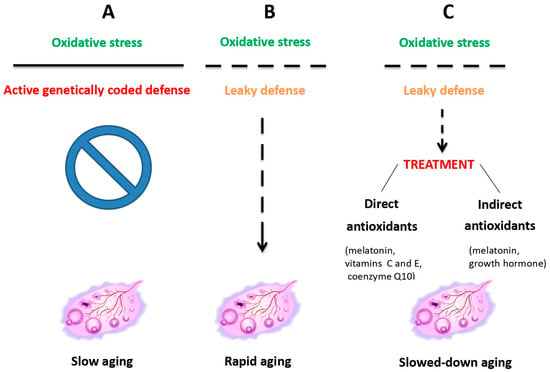
Figure 1.
Ovarian aging due to oxidative stress resulting from mitochondrial dysfunction is counteracted by inherent antioxidant defense, which, when leaky, can be treated with external antioxidant administration. Reprinted from Ref. [31].
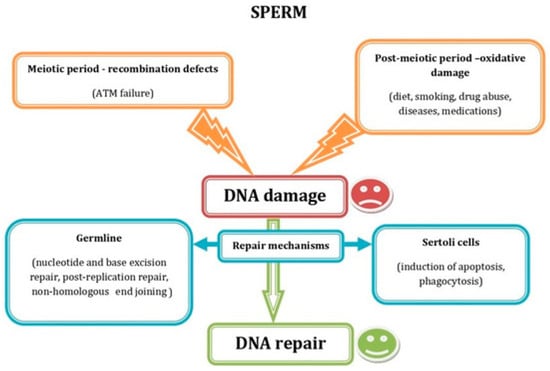
Figure 2.
Schematic representation showing the most common factors causing sperm DNA damage during the meiotic and the postmeiotic period, as well as the DNA repair mechanisms acting in germ and Sertoli cells. Reprinted from Ref. [4].
As for the male factor, most studies to date have focused on the correlation between paternal advanced age [33,34], environmental exposure [35], and the quality of spermatozoa including WHO Criteria’s and genome integrity [36]. A review of numerous IVF/ICSI cycles, including cases involving surgically retrieved sperm, showed that severe male factors can affect fertilization rates, embryo development, and clinical outcome.
Chromosomal abnormalities in embryos play a significant role in RIF by contributing to implantation failure, miscarriage, and chromosomes and/or genes syndromes in newborns, along with specific genetic defects that can independently result in early reproductive failure [37]. Aneuploid embryos are less able to progress to the blastocyst stage or implant successfully, although euploidy can be re-established (embryo self-correction) spontaneously in some of them. In fact, aneuploidy arising at different stages of oocyte development can be repaired through inherent mechanisms acting in the oocyte, zygote, and embryo (Figure 3).
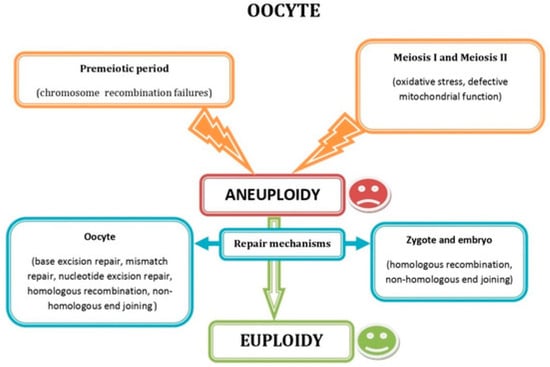
Figure 3.
Schematic representation showing the most common factors causing oocyte aneuploidy during the premeiotic and the meiotic period, as well as the DNA repair mechanisms acting in oocytes, zygotes, and embryos. Reprinted from Ref. [4].
Even if implantation occurs, aneuploid embryos are more likely to result in miscarriage due to their inability to support normal fetal development [38]. Preimplantation Genetic Testing for Aneuploidy (PGT-A), known as Preimplantation Genetic Screening (PGS), is a technique used in IVF to assess the chromosomal content of embryos before transfer to the uterus. Data from patients undergoing PGT-A, often due to factors such as advanced maternal age, recurrent pregnancy loss, or multiple failed IVF cycles, highlight a high prevalence of chromosomal abnormalities in embryos and oocytes. By identifying euploid embryos, PGT-A can enhance the chances of a successful implantation, ultimately improving IVF outcomes [38] and reducing time to pregnancy. However, PGT-A also has serious limitations related to low reliability and invasiveness. To overcome this issue, replacing the conventional PGT-A with noninvasive methods for genome investigation via analysis of free DNA from spent culture media, along with the use of other noninvasive biomarkers related to chromosomal ploidy status, is currently being considered [32].
Interleukins have been found to play a crucial role in embryo development. In women undergoing IVF, higher levels of IL-6 in follicular fluid (FF) were associated with a reduction in embryo fragmentation and an increased pregnancy rate compared to those with lower IL-6 levels [39].
There is a lack of consistency in the clinical definition of RIF [40]. Most definitions currently in use are based on the number of embryos transferred with no pregnancy. However, with changing practices in embryo transfer (ET), namely from multiple to single embryos, from cleavage to blastocyst stage, and from untested to chromosomally tested embryos, the implications of a single failed ET procedure have changed [41]. A recent comprehensive survey of the definitions in use that employ this paradigm has suggested that a consensus is emerging regarding RIF as the failure to achieve a clinical pregnancy after two to three transfers with good-quality embryos and that maternal age should also be taken into account [42,43]. In a study of 105 patients who failed to achieve pregnancy after three consecutive euploid blastocyst transfers, the success rates of the fourth and fifth transfers were evaluated. Live birth rates were similar between the two (40% vs. 53.3%, p = 0.14). The fourth transfer also showed a comparable success rate to the first (p = 0.29). Overall, the cumulative live birth rate after five euploid blastocyst transfers was 98.1%, demonstrating a continued potential for pregnancy success with additional transfers [44].
A study investigated the key metabolic processes and molecular pathways throughout the preimplantation embryo development, including PI3K-Akt, mTOR, AMPK, Wnt/β-catenin, TGF-β, Notch, and Jak-Stat signaling pathways. By examining these critical molecular pathways, a review sought to highlight the differences between in vitro and in vivo embryo development and to explore the physiological and clinical implications of these differences, aiming to enhance our understanding of embryonic development and clinical practices improvement in reproductive medicine [45]. Additionally, metabolic activity can influence implantation potential, as embryos with optimal energy metabolism are better powered for sustained growth and development [46].
The ability of an embryo to implant and develop into a viable fetus is primarily determined by its intrinsic characteristics. Morphological assessment plays a crucial role, as embryos that reach the advanced blastocyst stage exhibit higher implantation rates. Cleavage stage, cell number, symmetry, and the degree of fragmentation can be assessed to help evaluate embryo quality [47].
Beyond embryo quality, the interplay between gametes also affects early embryonic events. Both oocyte and sperm quality impact cleavage rates and genome activation, while epigenetic modifications stemming from gametes can influence embryonic gene expression and implantation. However, even high-quality embryos require a receptive endometrium for implantation, highlighting the importance of embryo–endometrial dialog. Quality embryos release signaling molecules, such as cytokines, that enhance communication with the endometrium, facilitating attachment and invasion [48].
3. Looking at the Uterine Cavity
Implantation is a special phenomenon characterized by a dynamic cross-talk between the blastocyst and the endometrium, resulting in the formation of the placenta, which serves as the interface between the developing fetus and the maternal circulation [49]. Table 1.

Table 1.
Overview of common uterine structural abnormalities.
Successful implantation depends on a well-coordinated interplay between the uterus, the embryo, and hormonal signals [50]. It is well known that abnormal uterus can directly or indirectly affect its ability to support embryo implantation, leading to RIF [51].
3.1. Uterine Anatomical Abnormalities
Congenital or acquired abnormalities of the uterus have been hypothesized to be linked with adverse fetal outcomes, pregnancy complications, and reduced fertility [51]. Various uterine abnormalities can impact implantation rates, including Müllerian anomalies, fibroids, polyps and intrauterine adhesions.
Müllerian duct anomalies (MDAs) are found in up to 7% of the general population and in nearly one-third of women with renal anomalies [52]. MDAs are hypothesized to contribute to adverse fetal outcomes, pregnancy complications, and reduced fertility [52]. Several imaging methods have been used in the assessing of MDAs, including ultrasound, hysterosalpingography, and magnetic resonance imaging (MRI) [53]. A study has demonstrated that MRI is the most effective imaging technique due to its superior capability of accurately visualizing complex uterovaginal anatomy [53].
Myomas, commonly known as uterine fibroids, are benign neoplasms of the uterine muscle that can significantly impact reproductive health [54]. In 2003, a study conducted by Baird et al. showed that the estimated prevalence of fibroids in women aged around 50 years was 70% for white women and over 80% for black women [55]. They are frequently associated with reproductive issues such as recurrent implantation failure, as they may alter uterine structure, disrupt endometrial receptivity, or impede embryo implantation [56]. Fibroids may account for 2–3% of infertility cases in women. Their impact on fertility varies based on their location in the uterus: subserosal (outside the uterus), intramural (inside the myometrium), and submucosal (inside the uterine cavity). Submucosal fibroids are associated with an increased risk of lower implantation, recurrent pregnancy loss, and less live birth rates in patients undergoing IVF [11,57]. Besides that, a septate uterus is believed to result from the incomplete resorption of the uterine septum around the 20th week of prenatal development [58]. The composition of the septum varies, ranging from a highly vascularized muscular structure to a less vascularized fibrous one, each with distinct implications for pregnancy. A more vascularized muscular septum may alter uterine motility, potentially leading to miscarriage or preterm delivery, while a less vascularized fibrous septum may impair implantation [52].
3.2. Intrauterine Pathology
Intrauterine adhesions, also known as Sherman’s syndrome, are formed by scar tissue within the uterus, often as a result of previous surgeries, infections, or trauma [59]. A systematic literature review, analyzing 58 articles published between 1974 and January 2022, demonstrated that this kind of adhesions can disrupt the normal uterine environment, affecting the endometrial lining and impair implantation, which may lead to infertility or recurrent pregnancy loss [60].
Beside this, endometrial polyps, considered to be benign glands, can also contribute to infertility and pregnancy complications, depending on their size and location within the uterine cavity [61]. A systematic review and meta-analysis conducted on 75 studies potentially eligible for inclusion concludes that women with endometrial polyps exhibit a higher prevalence of chronic endometritis compared to those without polyps [62]. In addition, a mini-review published in 2021 suggests that the polyps be removed in patients experiencing repeated IVF failure; however, further research is needed to confirm whether the procedure directly enhances pregnancy rates [63]. Moreover, a systematic review aims to assess the evidence on the effects of polypectomy on fecundity, implantation, and live birth rates [61]. Elias et al. (2015) [64] reported that the presence of polyps during controlled ovarian hyper-stimulation lead to an increased risk of biochemical pregnancy loss.
In the proliferative phase, the endometrium increases in thickness and becomes more vascularized, while in the secretory phase, endometrial glands grow, become tortuous, and boost their secretory activity. These changes reach their maximum about 10 to 20 days after ovulation [65]. Endocrine imbalance leads to altered oocyte maturation, uterine disorders, and endometriosis, leading to embryonic defects and decreased in vitro fertilization outcomes [17,52].
3.3. Intrauterine Inflammation and Chronic Endometritis
Chronic endometritis (CE) is a persistent inflammation of the endometrial mucosa, often caused by microbiological factors or mechanical and chemical irritants [66]. Currently, there is no consensus on standardized diagnostic criteria or tools for CE [67]. The prevalence of CE varies widely and is often underreported, ranging from 7.7% to 66% in cases of recurrent implantation failure and approximately 2.5% in the general infertile population. Some investigators have shown that the frequency of CE is 2.8–56.8% in infertility, 14–67.5% in recurrent implantation failure, and 9.3–67.6% in recurrent pregnancy loss [26]. From an immunological perspective, CE is characterized by the upregulation of IL-17 and the downregulation of TGFβ and IL-10 [68], and its severity is associated with a stronger Th1 and a weaker Th2 profiles [69].
CE is also more common in infertile patients with recurrent miscarriage. This condition negatively impacts the success of both spontaneous and in vitro fertilization (IVF) pregnancies and is associated with adverse perinatal outcomes, including intrauterine infections, preterm delivery, and postpartum endometritis. Diagnostic hysteroscopy should be considered for such patients to identify and manage CE effectively [70,71]. CE often presents without noticeable symptoms, making its prevalence in the general population challenging to be determined [72]. Proper investigation for CE is typically initiated only when clinical symptoms or related issues, such as infertility, arise [72].
Routinely two-dimensional ultrasonography may reveal several signs indicative of uterine abnormalities, including hematometra, hyperechogenic spots in the endometrium or along the border between the endometrium and myometrium, and varying endometrial thickness in longitudinal or transverse scans, which may suggest intracavitary synechiae [73]. Additionally, the appearance of an asynchronous endometrium with regard to the cycle phase can be observed, such as increased thickness even in the early proliferative phase, or a heteroechogenic endometrium. For patients experiencing infertility, particularly those with RIF or recurrent miscarriages, hysteroscopy should be considered as part of the diagnostic workup [74].
Histopathological evaluation involves an endometrial biopsy using a Novak curette or pipelle, with staining using hematoxylin and eosin to identify plasma cells (though less accurate), or the use of specific plasma cell stains/immunohistochemically methods such as Syndecan-1 or CD138 for a more precise diagnosis [75]. Microbiological assessment may include classical culture techniques, which are questionable and often lead to under diagnosis, or more specific molecular microbiology methods like PCR [75].
The ESHRE/ESGE CONgenital UTerine Anomalies (CONUTA) Working Group reported an initiative focused on establishing a consensus for the diagnosis of female genital anomalies. Various imaging techniques have been utilized to detect uterine malformations, each with its own potential and limitations in diagnosing the different types of malformations. These include sono-embryoscopy and magnetic resonance imaging [76].
The Cicinelli criteria, proposed in 2005, include several key features for diagnosing endometrial abnormalities: hyperemia, characterized by the expression of a vascular network at the peri-glandular level; stromal edema, resulting in a pale and thick endometrium; micro-polyps, which are small structures less than 1 mm in size; and inflammation, with the presence of inflammatory cells interspersed among normal stromal cells [77]. In 2019, Cicinelli et al. assessed the diagnostic accuracy of hysteroscopy in CE through a randomized controlled trial (RCT) observer study, aimed at evaluating the reproducibility of the proposed diagnostic criteria. The study results showed a positive impact of the criteria on physicians’ ability to identify CE [78].
Moreover, diagnosing CE is challenging for physicians. Classical histopathological staining, such as hematoxylin and eosin (HP), can identify plasma cells, but is less accurate. A more precise method involves CD138 immunohistochemistry staining or Syndecan-1 detection. Additionally, microbiological culture can be used, though it may be difficult and difficult to interpret, while PCR (Polymerase Chain Reaction) offers more specific and reliable diagnostic approach [66]. In another study, a total of 1189 hysteroscopies were performed, with biopsies and CD138 testing conducted. Among these, 735 cases (61.8%) showed no evidence of CE, while 454 cases (38.2%) were positive. CD138 testing was positive in 322 cases (27.1%). The hysteroscopic findings for CE included hyperemia in 34.7% of cases, micro-polyps in 2.1%, and interstitial edema in 3.5%. As for hysteroscopic signs in CD138-positive CE cases, the following results were observed: 17.8% had no signs, 39.5% exhibited hyperemia, 53.5% had micro-polyps, and 51.5% showed edema [79].
From the above studies, it can be concluded that infertile patients, particularly those with RIF or recurrent miscarriage (RM), exhibit a higher incidence of intrauterine pathologies, which significantly reduce the chances of achieving pregnancy, whether spontaneous or through ART. Various diagnostic modalities are available, including ultrasound, endometrial sampling (for histopathology or microbiology), and hysteroscopy. When treatment options are applicable, such as antibiotics, they should be administered based on the identified pathology. For patients who have never undergone a hysteroscopy, it is recommended as a diagnostic and therapeutic tool tailored to the specific pathology [11].
3.4. The Uterine Microbiota: A Key Predictor of Implantation Success
The association between uterine dysbiosis and recurrent implantation failure remains under exploration. It is a relatively recent area of investigation. Several studies have been performed to elucidate how uterine microbial imbalances might affect the ability of embryos to implant successfully during ART cycles. For many years, it was believed that a healthy fetus developed in a sterile environment [80]. However, a study published by Aagaard et al. (2014) discovered bacteria in the basal plate of the human placenta. The study outcome characterized a unique placental microbiome niche, consisting of nonpathogenic commensal microorganisms from the Firmicutes, Bacteroidetes, Proteobacteria, Tenericutes, and Fusobacteria phyla [81].
A study conducted in 130 women diagnosed with infertility aimed to evaluate the impact of uterine microbiota on embryo implantation success using metagenomics sequencing and microbiological examination. The population was divided into three distinct groups based on their IVF treatment history and outcome. The three groups included, respectively, women who were undergoing their first IVF attempt (n = 39), women who had experienced recurrent implantation failure following embryo transfer with ovarian stimulation (n = 27), and women who had recurrent implantation failure following frozen-thawed embryo transfer (n = 64). The study identified 44 species of microorganisms within the uterine cavity. Of these, 26 species were classified as opportunistic organisms, which have the potential to cause infections under certain conditions. The remaining 18 species were commensals without causing harm. These included 14 species of lactobacilli, bacteria commonly associated with maintaining a healthy microbiome, and 4 species of Bifidobacteria, another group of beneficial bacteria [82].
Accumulating evidence has suggested that an imbalance in the uterine microbiota could impact immune function and implantation [83], affect endometrial receptivity [84], and influence pro- and anti-inflammation balance [85]. A healthy microbiome plays a crucial role in the successful implantation of the embryo [86]. A balanced microbiome may interact directly with the embryo or with factors involved in implantation, such as the secretion of growth factors or cytokines. These interactions can help in creating a more favorable environment for the embryo’s successful attachment and development. However, when uterine dysbiosis occurs, it may lead to RIF [87,88]. According to the literature, the endometrial microbiome may act as a predictor of implantation success. The presence of specific beneficial microorganisms, especially Lactobacilli, is linked to a healthy endometrial environment that promotes embryo implantation. Increasing Lactobacillus levels to over 90% appears to enhance implantation outcomes [89,90]. Lactobacilli produce lactic acid, which helps maintain a low pH in the uterus, creating an environment that is inhospitable to pathogens while being conducive to implantation [91]. In contrast, pathogenic microorganisms, such as Gardnerella vaginalis or Mycoplasma, are often found in cases of dysbiosis and can contribute to a hostile uterine environment. These infections can trigger an inflammatory response that prevents proper implantation [82].
Another study, including 80 patients with RIF, compared uterine microbiome of 40 non-CE patients with that of 40 CE patients. Using Linear Discriminant Analysis (LDA), the study identified specific bacterial taxa that were characteristic of CE and non-CE patients. LDA revealed that Proteobacteria, Aminicenantales, and Chloroflexaceae were more commonly found in CE patients, while Lactobacillus, Acinetobacter, Herbaspirillum, Ralstonia, Shewanella, and Micrococcaceae were associated with non-CE patients. These findings highlight the distinct microbial profiles between the two groups [92]. In this context of chronic endometritis, a prospective study collected uterine endometrial specimens from 24 women with RIF, 27 RPL, and 29 fertile control women. The study found that the relative dominance rate of Lactobacillus iners was significantly higher in women with CE compared to women without CE. Additionally, the positive rate of Ureaplasma species was higher in women with CE (36.3%) than in those without CE. These results suggest that CE may play a role in the pathophysiology of both RPL and RIF, and that Lactobacillus iners and Ureaplasma species may be involved in the etiology of CE [93].
4. Looking at the Immune Profile
The implantation is a key event in the establishment of pregnancy. During the early stages of this process, the blastocyst embeds itself into the uterine wall [94]. This involves an interaction between the uterine epithelium and the trophoblast cells of the blastocyst, enabling attachment and invasion, both essential for the progression of pregnancy [95]. Implantation begins shortly (about 6 days) after fertilization and evolves into the formation of the placenta and the establishment of fetal–maternal interactions. This dynamic process continues to adapt and develop until approximately mid-gestation (around 22 weeks) [96]. During early pregnancy, the decidual (relating to the maternal–fetus interface) [97] immune system, such as NK cells, T cells, macrophages, and dendritic cells have specialized roles in maintaining immune tolerance to support implantation and fetal development [98]. The maternal immune system must balance tolerance and defense to allow embryo implantation [97]. The concept of immune tolerance in pregnancy refers to a healthy woman’s ability to carry a semi-allogeneic fetus without rejection by the mother’s immune system. This tolerance and the anti-inflammatory microenvironment institution are very crucial to protect the fetus and facilitate the interaction between the uterine immune cells and placental trophoblast cells [99]. The process of placental angiogenesis in pregnancy shares remarkable similarities with tumor development [100]. Like tumor cells, placental trophoblast cells are invasive, and the invasion is driven by uterine immune cells, particularly NK cells and regulatory T cells, which promote angiogenesis by secreting angiogenic factors, cytokines, and chemokines [99,100,101].
4.1. Immune Cells in Pregnancy
4.1.1. uNK Cells
Uterine Natural Killer (uNK) cells are the most abundant immune cells in the decidua during early pregnancy. In humans, two distinct populations of circulating NK cells, the cytotoxic NK cells, characterized by CD56dim/CD16+ expression and representing approximately 90% of all circulating NK cells, and cytokine-producing NK cells, marked by CD56bright/CD16− expression [102,103]. uNK cells regulate trophoblast invasion and blood vessel remodeling but can hinder implantation if overactive [104]. The number of uNK cells varies throughout the menstrual cycle, with their proliferation being closely linked to the influence of sex steroid hormones, particularly estrogen and progesterone [4]. These hormones play a crucial role in regulating uNK cell expansion during the late secretory phase and within the decidualized endometrium, preparing the uterine environment for potential implantation and pregnancy [105]. uNK cells becomes abundant during the mid-luteal phase of the menstrual cycle due to the influence of progesterone. This latter acts on stromal cells in the uterine lining, prompting them to produce interleukin-15 (IL-15), IL-12, and IL-18. In particular, Il-18 is a cytokine crucial for uNK cell recruitment and activity. This mechanism is important for preparing the uterine environment for potential implantation and early pregnancy [104]. Additionally, uNK cells interact with extra villous trophoblast (EVT) cells through specific interactions between killer-cell immunoglobulin-like receptors (KIRs) on the uNK cells and human leukocyte antigen (HLA) molecules expressed on EVT cells [104].
Besides that, uNK cells play a vital role in facilitating trophoblast invasion by producing key molecules like the Interferon-gamma (IFN-γ) to promote remodeling of uterine spiral arteries, creating a favorable environment for trophoblast invasion and supply to the developing fetus. Vascular endothelial growth factor (VEGF) stimulates angiogenesis to support increased blood flow to the placenta, while Granulocyte–Macrophage Colony-Stimulating Factor (GM-CSF) enhances trophoblast survival, proliferation, and invasion [106,107]. These contributions by uNK cells are critical for maintaining immune tolerance, establishing a functional placenta and ensuring successful pregnancy [108,109].
4.1.2. T Cells
T cells, a type of lymphocyte, play a central role in the adaptive immune system. The T helper (Th) cell expresses CD4+ and displays dichotomy of Th1/Th2 immune responses. First proposed by Mosmann et al. in 1986, this concept was later adapted to explain maternal immune tolerance to fetal alloantigens [110,111]. Th1 cells drive pro-inflammatory responses by secreting cytokines like IFN-γ, TNF-α, and IL-2, which activate macrophages, enhance cytotoxic T cell activity, and promote cellular immunity. In contrast, Th2 cells are associated with anti-inflammatory and immune-tolerant responses, producing cytokines such as IL-4, IL-5, IL-10, and IL-13, which inhibit Th1-driven inflammation and support humoral immune functions [112].
The T helper Th1/Th2 theory remains a foundational concept in reproductive immunology that explains how the balance between two subsets of T helper cells, Th1, and Th2, affects immune responses [113], particularly during pregnancy. It emphasizes that a shift in the immune system’s balance is necessary to protect the semi-allogeneic fetus from maternal immune rejection while still maintaining overall immune function. This immune–microenvironment interaction also includes other T helper subsets such as Th17 cells, T cytotoxic (Tc), T regulatory (Treg), and B cells and is essential for the regulation of uterus immunity [111]. In fact, the dysregulation of Th cell immunity during pregnancy can lead to obstetrical complications, including recurrent pregnancy loss [111]. A study published by Makhseed in 2001 analyzed peripheral blood mononuclear cells from 54 women with a history of at least three normal pregnancies, 24 women with a history of recurrent spontaneous abortion (RSA), and 39 women with a history of RSA followed by normal pregnancy. The study results revealed that Th2 dominance reduces inflammation and prevents the maternal immune system from attacking the fetus. Meanwhile, excessive Th1 activity can lead to pregnancy complications such as recurrent miscarriages, preterm labor, or preeclampsia as it can trigger inflammation and rejection of the fetus [114]. Successful pregnancies are associated with a stronger Th2 bias, while spontaneous abortion and recurrent pregnancy loss are linked to a stronger Th1 bias [114].
Th17 and Treg cells play opposing but complementary roles in immune regulation, both crucial for maintaining immune homeostasis, immune tolerance and defense. This balance can be disrupted in certain situations, such as pregnancy and autoimmunity [115]. In pregnancy, the maternal immune system is challenged by fetal antigens, and Treg cells suppress Th17 cells to support fetal survival. Interestingly, autoimmune symptoms improve during pregnancy, highlighting the opposing roles of Th17 and Treg cells [116].
The study of Figueiredo and Schumacher explored the Th17/Treg ratio in both pregnancy and autoimmune conditions and suggested that understanding the balance between these cells could lead to new treatments for pregnancy loss and autoimmunity [115].
During the first trimester of pregnancy, Th17 cells, a subset of T helper cells, have been shown to play a role in the survival, proliferation, and invasion of human trophoblast cells [117]. The secretion of IL-17 by Th17 cells plays a crucial role in promoting processes essential for establishing a successful pregnancy. Th17 cells exhibit significant plasticity; while they are typically linked to inflammatory responses, they can adapt in the context of pregnancy and assume a more supportive role. Specifically, decidual Th17 cells (found in the uterine lining) appear to be associated with Th2 responses. The production of IL-4, a cytokine typically associated with Th2 cells, by decidual Th17 cells suggests that they may help create a more tolerant immune environment in the uterus, promoting fetal survival and preventing immune rejection [115,117]. Th17 and Th22 subsets, including classical and alternative forms, are distinguished from naïve CD4+ T cells by the cytokines that drive their differentiation. Once activated, Th17 and Th22 cells are vital for sustaining pregnancy while they also participate in the defense against extracellular pathogens at the maternal–fetal interface. Achieving the correct balance between Th1/Th2 and Treg/Th17 responses at the right time is critical for a successful pregnancy [111].
4.1.3. Macrophages
Macrophages are now recognized as crucial players in embryo implantation, a key step in establishing a successful pregnancy. As part of the innate immune system, macrophages M1 and M2 [118] contribute to creating an immune-tolerant and supportive environment in the uterus, ensuring proper communication between maternal tissues and the developing embryo and thus preventing maternal rejection of the semi-allogeneic fetus. During implantation, macrophages are involved in remodeling the uterine lining (endometrium) to facilitate the invasion and anchoring of trophoblast cells. They contribute to the breakdown of extracellular matrix components, allowing the embryo to embed in the uterine wall [98]. Macrophages secrete leukemia inhibitory factors that modify the glycosylation structures of epithelial cells necessary for embryo attachment [119]. They also play a role in facilitating implantation by supporting the development of the corpus luteum and enhancing progesterone secretion [120,121,122].
A study analysis revealed that implantation failure was associated with low blood progesterone levels [123]. Additionally, the removal of M2 macrophages leads to a predominance of M1 macrophages in the uterus and elevated tumor necrosis factor-α (TNF-α) mRNA expression, triggering inflammation. Intrauterine M2 macrophages play a crucial role in preventing excessive inflammation caused by M1 macrophages, regulating the proliferation of endometrial epithelial cells, and promoting their differentiation, which collectively contribute to successful implantation.
Macrophages play a vital role in implantation and placental development through two key mechanisms, angiogenesis and inflammation modulation. They promote angiogenesis by secreting vascular endothelial growth factor (VEGF), ensuring an adequate blood supply to the developing placenta and embryo [124]. Additionally, macrophages regulate the local inflammatory response necessary for implantation, preventing it from becoming excessive or harmful [125]. By releasing cytokines and growth factors, they create a supportive microenvironment. A delicate balance between pro-inflammatory (M1) and anti-inflammatory (M2) macrophage phenotypes is essential during this process. M1 macrophages drive the initial inflammatory response, while M2 macrophages facilitate tissue repair, angiogenesis, and immune tolerance, collectively supporting successful implantation and placental development [126].
4.2. Evidence of Immune Disorders in RIF
Women with RIF may have alterations in the number or function of endometrial immune cells. In those with RIF, the infiltration of 14 immune cell types, including natural killer T cells, macrophages, immature and activated dendritic cells, CD56dim natural killer cells, Th1 and Th2 cells, T follicular helper cells, regulatory T cells, immature B cells, as well as various subsets of CD8 and CD4 T cells, is significantly reduced [120]. Moreover, RIF is associated with specific immunological features, including a high expression of HLA-DR on natural killer cells and HLA-F in the endometrial stroma, as well as elevated levels of both HLA-F and soluble HLA-G (sHLA-G) in the endometrial glands, suggesting a potential immunological imbalance [127]. Sfakianoudis et al., 2021 reported that elevated numbers of uNK cells, associated with their defective activity leading to cytotoxicity, are linked to RIF and recurrent miscarriages. Moreover, proposed treatments, such as glucocorticoids, intra lipids, and intravenous immunoglobulins, lack robust evidence of safety and efficacy [128].
RIF in assisted reproduction technology is a significant challenge, often linked to immune structural disorders in the endometrium. A study enrolled 42 women with RIF and compared them to fertile gestational carriers. The study results identified a unique immune profile in one-third of RIF cases, termed the “not transformed endometrial immune phenotype.” This profile includes high HLA-DR expression on NK cells, an increased fraction of CD16+ NK cells, and a reduced fraction of CD56bright NK cells. Patients with RIF also exhibited altered cytokine expression, with higher IL18/TWEAK and IL15/Fn14 ratios, reduced levels of tumor necrosis factor-like weak inducer of apoptosis, and discrepancies in IL18 mRNA expression. Immune abnormalities were present in 66.7% of cases, potentially contributing to implantation failures despite genetically tested embryo transfers [129].
Dysregulation of the immune system in RIF also involves dendritic cells (DCs) and regulatory T cells, which are integral components of this immune environment [112,130]. ILT4 (Immunoglobulin-like transcript 4) is an inhibitory receptor expressed on dendritic cells, contributing to their tolerogenic properties [127]. In fertile women, ILT4+ dendritic cells help maintain immune tolerance at the maternal–fetal interface [130]. Studies suggest that in women with RIF, ILT4+ dendritic cells are significantly downregulated. This reduction may lead to an increase in immune activation and inflammation, disrupting the immune tolerance needed for implantation [130]. Besides that, FOXP3+ cells are crucial for suppressing excessive immune responses. In fertile women, ILT4+ dendritic cells are positively associated with FOXP3+ Tregs. In RIF cases, this association is disrupted, and the downregulation of ILT4+ dendritic cells impairs the recruitment, activation, or maintenance of FOXP3+ Tregs, leading to an imbalanced immune response. This disruption may contribute to the failure of implantation or recurrent miscarriages [127,130].
In the context of RIF, immune cells, including decidual M2 macrophages at the maternal–fetal interface and the activated dendritic cells, are two key players in this immune regulation [131]. The population of decidual M2 macrophages is significantly reduced in women with RIF. This decrease leads to a diminished anti-inflammatory environment, disrupting the delicate immune balance required for embryo implantation and placental development. Similarly to M2 macrophages, activated tolerogenic dendritic cells are significantly reduced in RIF and this reduction contributes to an impaired ability to recruit and activate Tregs, leading to heightened immune activation and increased inflammation [131]. This results in increased levels of pro-inflammatory cytokines and decreased suppression of harmful immune responses, creating an unfavorable environment for embryo implantation and early pregnancy maintenance [131].
Research demonstrated the association between RIF and cytokine profile imbalance at the maternal–fetal interface. While IL-6 (pro-inflammatory cytokine) is necessary for normal physiological processes, its excessive levels can lead to chronic inflammation and immune dysregulation [132]. In addition, high levels of IL-6 may also interfere with trophoblast invasion and promote an environment unsuitable for maintaining immune tolerance [133]. Similarly, IL-10 is a potent anti-inflammatory cytokine that plays a critical role in promoting immune tolerance. The reduced IL-10 levels in RIF patients result in a diminished anti-inflammatory response. This reduction leads to an inability to counterbalance the effects of pro-inflammatory cytokines like IL-6, further disrupting the immune environment [132].
Moreover, G-CSF (Granulocyte Colony-Stimulating Factor) is a growth factor involved in modulating the immune response and enhancing trophoblast function [134]. It supports the development and function of uNK cells and promotes angiogenesis, both of which are crucial for implantation [135]. Research revealed that a deficiency in G-CSF results in impaired endometrial receptivity, reduces trophoblast invasion, and leads to an inadequate support for embryo implantation. Targeting IL-6 with specific inhibitors to reduce its pro-inflammatory effects, and administering IL-10 or G-CSF to restore the immune balance, offers a promising avenue for improving implantation outcomes in RIF patients [132].
4.3. Immune Checkpoints
The involvement of immune checkpoints is essential for maintaining maternal immune tolerance during pregnancy. Their main role is to prevent allogenic fetal rejection [136] by modulating the maternal immune response to recognize the fetus, which carries paternal foreign antigens [137], while simultaneously preserving immune defense against infections. These checkpoints are expressed on immune cells to regulate the strength of immune responses while preserving self-tolerance [138]. Immune checkpoints, involving cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), T cell immunoglobulin and mucin-domain containing protein-3 (TIM-3), lymphocyte-activation gene 3 (LAG-3), and immune receptor tyrosine-based inhibitory motif (ITIM), are a group of inhibitory pathways, expressed at the maternal–fetal interface and acting in ligand–receptor fashions [138].
A review also discusses targeting of key immune cells, including T helper (TH1/TH2, TH17/Treg) and NK cells, and immune checkpoints to help maintain pregnancy and prevent complications such as miscarriage, preeclampsia, or preterm birth [139]. Preeclampsia is a severe and potentially fatal obstetric syndrome. It is strongly associated with immunological incompatibility between the mother and fetus, with genetic factors linking immune pathways to the risk of developing preeclampsia. A study by Lokki et al. investigated the immunogenic and immunomodulatory mechanisms that may contribute to the breakdown of tolerance, inflammation, and autoimmunity, all of which could lead to the development of preeclampsia [137,140].
A study by Schepanski et al., 2018 [141] discussed how prenatal challenges, integrating maternal hormones and immune markers, can influence fetal brain development and the offspring’s cognitive functions later in life. This review highlights the potential impact of maternal immune cells and cytokines on fetal brain development and the offspring’s cognitive and intellectual functioning that can persist across multiple generations reinforcing the concept of epigenetic inheritance [137,141]. Besides that, autophagy process is crucial in maintaining pregnancy by supporting fetal growth and survival. Its disruption, whether through upregulation or downregulation, can lead to pregnancy-related complications [138].
Limitation
To rescue RIF, it is important to improve the quality of gametes, implement adequate laboratory conditions and optimize staff skill to produce the most competent dormant blastocyst. The future challenge is how to be sure that the competent blastocyst will be able to switch via the epigenetic clock from dormant to active status and to send the appropriate signalization for the endometrium to initiate the cross-talk.
5. Conclusions
This review highlights modifiable factors and emerging interventions relevant to recurrent implantation failure, providing insights into mechanisms affecting implantation and endometrial receptivity. Clinically, findings emphasize the value of preconception counseling and individualized management, including weight optimization, smoking cessation, alcohol moderation, moderate physical activity, and stress reduction—interventions supported by moderate-to-strong evidence that can be readily applied in practice. While some novel therapies, such as immunomodulation or personalized embryo transfer timing, show promise, they are not yet incorporated into national or international infertility guidelines and should be considered experimental. Nevertheless, integrating evidence-based lifestyle and metabolic strategies may enhance ART outcomes and offer a practical framework for patient care.
Although significant progress has been made in understanding normal pregnancy, knowledge about RIF remains in development. Several factors have been implicated in its pathogenesis, including embryo quality, altered cytokine profiles, the involvement of checkpoint inhibitors, dysbiosis, changes in immune phenotype, and uterine abnormalities. Ongoing research continues to explore these aspects to uncover their roles in RIF and identify potential therapeutic strategies. In this context, several immune-based interventions have been proposed as supplementary treatments for RIF, based on its underlying pathophysiology. However, further research is essential to unravel the intricate interactions between endometrial immune cells. Well-designed clinical studies are necessary to validate these emerging concepts and establish their utility in clinical practice.
Author Contributions
A.M., T.M., M.B., and R.C. were responsible for the conceptualization, validation, investigation, resources, supervision, and project administration. M.L. and F.I.M. handled the writing—original draft preparation. A.M., J.T., and M.B. were in charge of the writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef]
- Simon, A.; Laufer, N. Repeated implantation failure: Clinical approach. Fertil. Steril. 2012, 97, 1039–1043. [Google Scholar] [CrossRef]
- Shufaro, Y.; Schenker, J.G. Implantation Failure, Etiology, Diagnosis and Treatment. Int. J. Infertil. Fetal Med. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza-Tesarik, R. Molecular Clues to Understanding Causes of Human-Assisted Reproduction Treatment Failures and Possible Treatment Options. Int. J. Mol. Sci. 2022, 23, 10357. [Google Scholar] [CrossRef]
- Muter, J.; Lynch, V.J.; McCoy, R.C.; Brosens, J.J. Human embryo implantation. Dev. Camb. Engl. 2023, 150, dev201507. [Google Scholar] [CrossRef]
- Popovic, M.; Chuva de Sousa Lopes, S.M. Emerging in vitro platforms and omics technologies for studying the endometrium and early embryo-maternal interface in humans. Placenta 2022, 125, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Aplin, J.D. Adhesion molecules in endometrial epithelium: Tissue integrity and embryo implantation. J. Anat. 2009, 215, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Saghian, R.; Bogle, G.; James, J.L.; Clark, A.R. Establishment of maternal blood supply to the placenta: Insights into plugging, unplugging and trophoblast behaviour from an agent-based model. Interface Focus 2019, 9, 20190019. [Google Scholar] [CrossRef] [PubMed]
- Mrozikiewicz, A.E.; Ożarowski, M.; Jędrzejczak, P. Biomolecular Markers of Recurrent Implantation Failure—A Review. Int. J. Mol. Sci. 2021, 22, 10082. [Google Scholar] [CrossRef]
- Nenonen, H.; Kondic, A.; Henic, E.; Hjelmér, I. Recurrent implantation failure and inflammatory markers in serum and follicle fluid of women undergoing assisted reproduction. J. Reprod. Immunol. 2024, 162, 104209. [Google Scholar] [CrossRef]
- Ma, J.; Gao, W.; Li, D. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front. Endocrinol. 2023, 13, 1061766. [Google Scholar] [CrossRef]
- Schulte, M.M.B.; Tsai, J.; Moley, K.H. Obesity and PCOS. Reprod. Sci. 2015, 22, 6–14. [Google Scholar] [CrossRef]
- Rahman, M.S.; Park, Y.; Hosseinirad, H.; Shin, J.-H.; Jeong, J.-W. The interplay between endometriosis and obesity. Trends Endocrinol. Metab. 2025. [Google Scholar] [CrossRef]
- Montjean, D.; Godin Pagé, M.-H.; Bélanger, M.-C.; Benkhalifa, M.; Miron, P. An Overview of E-Cigarette Impact on Reproductive Health. Life 2023, 13, 827. [Google Scholar] [CrossRef]
- Tsiapakidou, S.; Mahmood, T.; Savona-Ventura, C. The potential impact of tobacco use on female fertility and pregnancy outcomes: An invited scientific review by EBCOG. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 290, 85–87. [Google Scholar] [CrossRef]
- Fan, D.; Liu, L.; Xia, Q.; Wang, W.; Wu, S.; Tian, G.; Liu, Y.; Ni, J.; Wu, S.; Guo, X. Female alcohol consumption and fecundability: A systematic review and dose-response meta-analysis. Sci. Rep. 2017, 7, 13815. [Google Scholar] [CrossRef] [PubMed]
- Tricotteaux-Zarqaoui, S.; Lahimer, M.; Abou Diwan, M.; Corona, A.; Candela, P.; Cabry, R.; Bach, V.; Khorsi-Cauet, H.; Benkhalifa, M. Endocrine disruptor chemicals exposure and female fertility declining: From pathophysiology to epigenetic risks. Front. Public Health 2024, 12, 1466967. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef]
- Lédée, N.; Prat-Ellenberg, L.; Chevrier, L.; Balet, R.; Simon, C.; Lenoble, C.; Irani, E.E.; Bouret, D.; Cassuto, G.; Vitoux, D.; et al. Uterine immune profiling for increasing live birth rate: A one-to-one matched cohort study. J. Reprod. Immunol. 2017, 119, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Moldenhauer, L.M. Immunological determinants of implantation success. Int. J. Dev. Biol. 2014, 58, 205–217. [Google Scholar] [CrossRef]
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef]
- Bustamante, M.; Balagué-Dobón, L.; Buko, Z.; Sakhi, A.K.; Casas, M.; Maitre, L.; Andrusaityte, S.; Grazuleviciene, R.; Gützkow, K.B.; Brantsæter, A.-L.; et al. Common genetic variants associated with urinary phthalate levels in children: A genome-wide study. Environ. Int. 2024, 190, 108845. [Google Scholar] [CrossRef]
- Song, D.; Feng, X.; Zhang, Q.; Xia, E.; Xiao, Y.; Xie, W.; Li, T.C. Prevalence and confounders of chronic endometritis in premenopausal women with abnormal bleeding or reproductive failure. Reprod. Biomed. Online 2018, 36, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Pinto, V.; Marinaccio, M.; Indraccolo, U.; De Ziegler, D.; Resta, L. Chronic Endometritis Due to Common Bacteria Is Prevalent in Women With Recurrent Miscarriage as Confirmed by Improved Pregnancy Outcome After Antibiotic Treatment. Reprod. Sci. 2014, 21, 640–647. [Google Scholar] [CrossRef]
- Ichiyama, T.; Kuroda, K.; Nagai, Y.; Urushiyama, D.; Ohno, M.; Yamaguchi, T.; Nagayoshi, M.; Sakuraba, Y.; Yamasaki, F.; Hata, K.; et al. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod. Med. Biol. 2021, 20, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; Inversetti, A.; Marraffa, S.; Campagnolo, L.; Arthur, J.; Zambella, E.; Di Simone, N. Chronic endometritis and recurrent reproductive failure: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1427454. [Google Scholar] [CrossRef]
- Reader, K.L.; Stanton, J.-A.L.; Juengel, J.L. The Role of Oocyte Organelles in Determining Developmental Competence. Biology 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Kalafat, E.; Somigliana, E. A new definition of recurrent implantation failure on the basis of anticipated blastocyst aneuploidy rates across female age. Fertil. Steril. 2021, 116, 1320–1327. [Google Scholar] [CrossRef]
- Dcunha, R.; Hussein, R.S.; Ananda, H.; Kumari, S.; Adiga, S.K.; Kannan, N.; Zhao, Y.; Kalthur, G. Current Insights and Latest Updates in Sperm Motility and Associated Applications in Assisted Reproduction. Reprod. Sci. 2020, 29, 7–25. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, W.; Cao, Z.; Zhang, Y.; Liu, G. Screening of functional maternal-specific chromatin regulators in early embryonic development of zebrafish. Commun. Biol. 2024, 7, 1354. [Google Scholar] [CrossRef]
- Tesarik, J.; Galán-Lázaro, M.; Mendoza-Tesarik, R. Ovarian Aging: Molecular Mechanisms and Medical Management. Int. J. Mol. Sci. 2021, 22, 1371. [Google Scholar] [CrossRef]
- Tesarik, J. Endocrinology of Primary Ovarian Insufficiency: Diagnostic and Therapeutic Clues. Endocrines 2025, 6, 18. [Google Scholar] [CrossRef]
- Lahimer, M.; Montjean, D.; Cabry, R.; Capelle, S.; Lefranc, E.; Bach, V.; Ajina, M.; Ben Ali, H.; Khorsi-Cauet, H.; Benkhalifa, M. Paternal Age Matters: Association with Sperm Criteria’s- Spermatozoa DNA Integrity and Methylation Profile. J. Clin. Med. 2023, 12, 4928. [Google Scholar] [CrossRef]
- Elkhatib, I.; Nogueira, D.; Bayram, A.; Abdala, A.; Ata, B.; Melado, L.; Lawrenz, B.; Kalafat, E.; Gianaroli, L.; Fatemi, H.M. The influence of male age and sperm parameters on blastulation and euploidy rates. Fertil. Steril. 2025, 124, 1006–1015. [Google Scholar] [CrossRef]
- Lahimer, M.; Capelle, S.; Lefranc, E.; Cabry, R.; Montjean, D.; Bach, V.; Ajina, M.; Ali, H.B.; Benkhalifa, M.; Khorsi-Cauet, H. Effect of pesticide exposure on human sperm characteristics, genome integrity, and methylation profile analysis. Environ. Sci. Pollut. Res. 2023, 30, 77560–77567. [Google Scholar] [CrossRef]
- Kaarouch, I.; Bouamoud, N.; Madkour, A.; Louanjli, N.; Saadani, B.; Assou, S.; Aboulmaouahib, S.; Amzazi, S.; Copin, H.; Benkhalifa, M.; et al. Paternal age: Negative impact on sperm genome decays and IVF outcomes after 40 years. Mol. Reprod. Dev. 2018, 85, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmar, N.; Peinado, V.; Vera, M.; Remohí, J.; Pellicer, A.; Simón, C.; Hassold, T.; Rubio, C. Chromosomal abnormalities in embryos from couples with a previous aneuploid miscarriage. Fertil. Steril. 2012, 98, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Cornelisse, S.; Zagers, M.; Kostova, E.; Fleischer, K.; Wely, M.; Mastenbroek, S. Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation. Cochrane Database Syst. Rev. 2020, 2020, CD005291. [Google Scholar] [CrossRef]
- Stojanovic Gavrilovic, A.Z.; Cekovic, J.M.; Parandilovic, A.Z.; Nikolov, A.B.; Sazdanovic, P.S.; Velickovic, A.M.; Andjelkovic, M.V.; Sorak, M.P. IL-6 of follicular fluid and outcome of in vitro fertilization. Medicine 2022, 101, e29624. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Bai, S.; Zhang, J.; Sun, X.; Meng, A.; Chen, H. Understanding recurrent pregnancy loss: Recent advances on its etiology, clinical diagnosis, and management. Med. Rev. 2022, 2, 570–589. [Google Scholar] [CrossRef]
- Kamath, M.S.; Mascarenhas, M.; Kirubakaran, R.; Bhattacharya, S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst. Rev. 2020, 2020, CD003416. [Google Scholar] [CrossRef]
- Cimadomo, D.; Sosa Fernandez, L.; Soscia, D.; Fabozzi, G.; Benini, F.; Cesana, A.; Dal Canto, M.B.; Maggiulli, R.; Muzzì, S.; Scarica, C.; et al. Inter-centre reliability in embryo grading across several IVF clinics is limited: Implications for embryo selection. Reprod. Biomed. Online 2022, 44, 39–48. [Google Scholar] [CrossRef]
- Cimadomo, D.; Craciunas, L.; Vermeulen, N.; Vomstein, K.; Toth, B. Definition, diagnostic and therapeutic options in recurrent implantation failure: An international survey of clinicians and embryologists. Hum. Reprod. 2021, 36, 305–317. [Google Scholar] [CrossRef]
- Gill, P.; Ata, B.; Arnanz, A.; Cimadomo, D.; Vaiarelli, A.; Fatemi, H.M.; Ubaldi, F.M.; Garcia-Velasco, J.A.; Seli, E. Does recurrent implantation failure exist? Prevalence and outcomes of five consecutive euploid blastocyst transfers in 123 987 patients. Hum. Reprod. 2024, 39, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Rinaudo, P.F. Metabolic regulation of preimplantation embryo development in vivo and in vitro: Molecular mechanisms and insights. Biochem. Biophys. Res. Commun. 2024, 726, 150256. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Soc. Reprod. Fertil. 2012, 143, 417–427. [Google Scholar] [CrossRef]
- Luna, M.; Copperman, A.B.; Duke, M.; Ezcurra, D.; Sandler, B.; Barritt, J. Human blastocyst morphological quality is significantly improved in embryos classified as fast on day 3 (≥10 cells), bringing into question current embryological dogma. Fertil. Steril. 2008, 89, 358–363. [Google Scholar] [CrossRef]
- Dvoran, M.; Nemcova, L.; Kalous, J. An Interplay between Epigenetics and Translation in Oocyte Maturation and Embryo Development: Assisted Reproduction Perspective. Biomedicines 2022, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, J.-S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Kayisli, U.A.; Taylor, H.S. The Role of Growth Factors and Cytokines during Implantation: Endocrine and Paracrine Interactions. Semin. Reprod. Med. 2009, 27, 62–79. [Google Scholar] [CrossRef]
- Hosseinirad, H.; Yadegari, P.; Falahieh, F.M.; Shahrestanaki, J.K.; Karimi, B.; Afsharzadeh, N.; Sadeghi, Y. The impact of congenital uterine abnormalities on pregnancy and fertility: A literature review. JBRA Assist. Reprod. 2021, 25, 608–616. [Google Scholar] [CrossRef]
- Sugi, M.D.; Penna, R.; Jha, P.; Pōder, L.; Behr, S.C.; Courtier, J.; Mok-Lin, E.; Rabban, J.T.; Choi, H.H. Müllerian Duct Anomalies: Role in Fertility and Pregnancy. RadioGraphics 2021, 41, 1857–1875. [Google Scholar] [CrossRef]
- Olpin, J.D.; Heilbrun, M. Imaging of Müllerian Duct Anomalies. Clin. Obstet. Gynecol. 2009, 52, 40. [Google Scholar] [CrossRef]
- Barjon, K.; Mikhail, L.N. Uterine Leiomyomata. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Baird, D.D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Garneau, A.S.; Young, S.L. Defining recurrent implantation failure: A profusion of confusion or simply an illusion? Fertil. Steril. 2021, 116, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Barjon, K.; Kahn, J.; Singh, M. Uterine Leiomyomata. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Blanco-Breindel, M.F.; Kahn, J.; Singh, M. Septate Uterus. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Deans, R.; Abbott, J. Review of Intrauterine Adhesions. J. Minim. Invasive Gynecol. 2010, 17, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Hooker, A.B.; de Leeuw, R.A.; Emanuel, M.H.; Mijatovic, V.; Brolmann, H.A.M.; Huirne, J.A.F. The link between intrauterine adhesions and impaired reproductive performance: A systematic review of the literature. BMC Pregnancy Childbirth 2022, 22, 837. [Google Scholar] [CrossRef]
- Afifi, K.; Anand, S.; Nallapeta, S.; Gelbaya, T.A. Management of endometrial polyps in subfertile women: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 151, 117–121. [Google Scholar] [CrossRef]
- Vitagliano, A.; Cialdella, M.; Cicinelli, R.; Santarsiero, C.M.; Greco, P.; Buzzaccarini, G.; Noventa, M.; Cicinelli, E. Association between Endometrial Polyps and Chronic Endometritis: Is It Time for a Paradigm Shift in the Pathophysiology of Endometrial Polyps in Pre-Menopausal Women? Results of a Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 2182. [Google Scholar] [CrossRef]
- Jee, B.C.; Jeong, H.G. Management of endometrial polyps in infertile women: A mini-review. Clin. Exp. Reprod. Med. 2021, 48, 198–202. [Google Scholar] [CrossRef]
- Elias, R.T.; Pereira, N.; Karipcin, F.S.; Rosenwaks, Z.; Spandorfer, S.D. Impact of newly diagnosed endometrial polyps during controlled ovarian hyperstimulation on in vitro fertilization outcomes. J. Minim. Invasive Gynecol. 2015, 22, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Deligdisch, L. Hormonal Pathology of the Endometrium. Mod. Pathol. 2000, 13, 285–294. [Google Scholar] [CrossRef]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018, 218, 602.e1–602.e16. [Google Scholar] [CrossRef]
- Murtinger, M.; Wirleitner, B.; Spitzer, D.; Bralo, H.; Miglar, S.; Schuff, M. Diagnosing chronic endometritis: When simplification fails to clarify. Hum. Reprod. Open 2022, 2022, hoac023. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Chen, Z.; Zhang, W.; Liu, X.; Fang, J.; Liu, F.; Kwak-Kim, J. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod. Biol. Endocrinol. 2019, 17, 2. [Google Scholar] [CrossRef]
- Kitazawa, J.; Kimura, F.; Nakamura, A.; Morimune, A.; Hanada, T.; Amano, T.; Tsuji, S.; Kasahara, K.; Satooka, H.; Hirata, T.; et al. Alteration in endometrial helper T-cell subgroups in chronic endometritis. Am. J. Reprod. Immunol. 2021, 85, e13372. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Xie, X.; Chen, Y.; Wang, Z.; Zhang, J.; Liu, C.; Lin, G.; Wang, Y.; Guo, Y. How does chronic endometritis influence pregnancy outcomes in endometriosis associated infertility? A retrospective cohort study. Reprod. Health 2024, 21, 162. [Google Scholar] [CrossRef]
- Vitagliano, A.; Laganà, A.S.; De Ziegler, D.; Cicinelli, R.; Santarsiero, C.M.; Buzzaccarini, G.; Chiantera, V.; Cicinelli, E.; Marinaccio, M. Chronic Endometritis in Infertile Women: Impact of Untreated Disease, Plasma Cell Count and Antibiotic Therapy on IVF Outcome—A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2250. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sethi, A. Endometritis—Diagnosis, Treatment and its impact on fertility—A Scoping Review. JBRA Assist. Reprod. 2022, 26, 538–546. [Google Scholar] [CrossRef]
- Van den Bosch, T. Ultrasound in the diagnosis of endometrial and intracavitary pathology: An update. Australas. J. Ultrasound Med. 2012, 15, 7–12. [Google Scholar] [CrossRef]
- Russell, P.; Hey-Cunningham, A.; Berbic, M.; Tremellen, K.; Sacks, G.; Gee, A.; Cheerala, B. Asynchronous glands in the endometrium of women with recurrent reproductive failure. Pathology 2014, 46, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Elnashar, A.T.; Sabry, M.; Elnashar, A.T.; Sabry, M. Chronic endometritis in In-Vitro fertilization failure patients. Glob. J. Fertil. Res. 2021, 6, 001–005. [Google Scholar] [CrossRef]
- Grimbizis, G.F.; Di Spiezio Sardo, A.; Saravelos, S.H.; Gordts, S.; Exacoustos, C.; Van Schoubroeck, D.; Bermejo, C.; Amso, N.N.; Nargund, G.; Timmerman, D.; et al. The Thessaloniki ESHRE/ESGE consensus on diagnosis of female genital anomalies. Hum. Reprod. 2016, 31, 2–7. [Google Scholar] [CrossRef]
- Cicinelli, E.; Resta, L.; Nicoletti, R.; Tartagni, M.; Marinaccio, M.; Bulletti, C.; Colafiglio, G. Detection of chronic endometritis at fluid hysteroscopy. J. Minim. Invasive Gynecol. 2005, 12, 514–518. [Google Scholar] [CrossRef]
- Cicinelli, E.; Vitagliano, A.; Kumar, A.; Lasmar, R.B.; Bettocchi, S.; Haimovich, S.; Kitaya, K.; de Ziegler, D.; Simon, C.; Moreno, I.; et al. Unified diagnostic criteria for chronic endometritis at fluid hysteroscopy: Proposal and reliability evaluation through an international randomized-controlled observer study. Fertil. Steril. 2019, 112, 162–173.e2. [Google Scholar] [CrossRef]
- Song, D.; Li, T.-C.; Zhang, Y.; Feng, X.; Xia, E.; Huang, X.; Xiao, Y. Correlation between hysteroscopy findings and chronic endometritis. Fertil. Steril. 2019, 111, 772–779. [Google Scholar] [CrossRef]
- Banchi, P.; Colitti, B.; Opsomer, G.; Rota, A.; Van Soom, A. The dogma of the sterile uterus revisited: Does microbial seeding occur during fetal life in humans and animals? Reprod. Camb. Engl. 2023, 167, e230078. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Keburiya, L.K.; Smolnikova, V.Y.; Priputnevich, T.V.; Muravieva, V.V.; Gordeev, A.B.; Trofimov, D.Y.; Shubina, E.S.; Kochetkova, T.O.; Rogacheva, M.S.; Kalinina, E.A.; et al. Does the uterine microbiota affect the reproductive outcomes in women with recurrent implantation failures? BMC Womens Health 2022, 22, 168. [Google Scholar] [CrossRef] [PubMed]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Gao, X.; Louwers, Y.V.; Laven, J.S.E.; Schoenmakers, S. Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 622. [Google Scholar] [CrossRef]
- Medina-Bastidas, D.; Camacho-Arroyo, I.; García-Gómez, E. Current findings in endometrial microbiome: Impact on uterine diseases. Reproduction 2022, 163, R81–R96. [Google Scholar] [CrossRef]
- Bardos, J.; Fiorentino, D.; Longman, R.E.; Paidas, M. Immunological Role of the Maternal Uterine Microbiome in Pregnancy: Pregnancies Pathologies and Alterated Microbiota. Front. Immunol. 2020, 10, 2823. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; He, F.; Lin, Z.; Liu, S.; Tang, L.; Huang, Y.; Hu, Z. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int. J. Cancer 2021, 148, 1708–1716. [Google Scholar] [CrossRef]
- Rokhsartalab Azar, P.; Karimi, S.; Haghtalab, A.; Taram, S.; Hejazi, M.; Sadeghpour, S.; Pashaei, M.R.; Ghasemnejad-Berenji, H.; Taheri-Anganeh, M. The role of the endometrial microbiome in embryo implantation and recurrent implantation failure. J. Reprod. Immunol. 2024, 162, 104192. [Google Scholar] [CrossRef]
- Balla, B.; Illés, A.; Tobiás, B.; Pikó, H.; Beke, A.; Sipos, M.; Lakatos, P.; Kósa, J.P. The Role of the Vaginal and Endometrial Microbiomes in Infertility and Their Impact on Pregnancy Outcomes in Light of Recent Literature. Int. J. Mol. Sci. 2024, 25, 13227. [Google Scholar] [CrossRef]
- Lebedeva, O.P.; Popov, V.N.; Syromyatnikov, M.Y.; Starkova, N.N.; Maslov, A.Y.; Kozarenko, O.N.; Gryaznova, M.V. Female reproductive tract microbiome and early miscarriages. APMIS 2023, 131, 61–76. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, H.; Zhang, C.; Zhang, S. Chronic endometritis and the endometrial microbiota: Implications for reproductive success in patients with recurrent implantation failure. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 49. [Google Scholar] [CrossRef]
- Takimoto, K.; Yamada, H.; Shimada, S.; Fukushi, Y.; Wada, S. Chronic Endometritis and Uterine Endometrium Microbiota in Recurrent Implantation Failure and Recurrent Pregnancy Loss. Biomedicines 2023, 11, 2391. [Google Scholar] [CrossRef]
- Su, R.-W.; Fazleabas, A.T. Implantation and Establishment of Pregnancy in Human and Nonhuman Primates. Adv. Anat. Embryol. Cell Biol. 2015, 216, 189–213. [Google Scholar] [CrossRef]
- Aplin, J.D.; Kimber, S.J. Trophoblast-uterine interactions at implantation. Reprod. Biol. Endocrinol. 2004, 2, 48. [Google Scholar] [CrossRef]
- Benagiano, G.; Mancuso, S.; Guo, S.-W.; Di Renzo, G.C. Events Leading to the Establishment of Pregnancy and Placental Formation: The Need to Fine-Tune the Nomenclature on Pregnancy and Gestation. Int. J. Mol. Sci. 2023, 24, 15420. [Google Scholar] [CrossRef]
- Hsu, P.; Nanan, R.K.H. Innate and Adaptive Immune Interactions at the Fetal–Maternal Interface in Healthy Human Pregnancy and Pre-Eclampsia. Front. Immunol. 2014, 5, 125. [Google Scholar] [CrossRef]
- Saito, S. Role of immune cells in the establishment of implantation and maintenance of pregnancy and immunomodulatory therapies for patients with repeated implantation failure and recurrent pregnancy loss. Reprod. Med. Biol. 2024, 23, e12600. [Google Scholar] [CrossRef]
- SHARMA, S. Natural killer cells and regulatory T cells in early pregnancy loss. Int. J. Dev. Biol. 2014, 58, 219–229. [Google Scholar] [CrossRef]
- Boulanger, H.; Bounan, S.; Mahdhi, A.; Drouin, D.; Ahriz-Saksi, S.; Guimiot, F.; Rouas-Freiss, N. Immunologic aspects of preeclampsia. AJOG Glob. Rep. 2024, 4, 100321. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shima, T.; Inada, K.; Nakashima, A. Which Types of Regulatory T cells Play Important Roles in Implantation and Pregnancy Maintenance? Am. J. Reprod. Immunol. 2013, 69, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Hernández, I.; Alecsandru, D.; García-Velasco, J.A.; Domínguez, F. Uterine natural killer cells: From foe to friend in reproduction. Hum. Reprod. Update 2021, 27, 720–746. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Li, D.; Li, M. An Estrogen–NK Cells Regulatory Axis in Endometriosis, Related Infertility, and Miscarriage. Int. J. Mol. Sci. 2024, 25, 3362. [Google Scholar] [CrossRef]
- Kanter, J.R.; Mani, S.; Gordon, S.M.; Mainigi, M. Uterine natural killer cell biology and role in early pregnancy establishment and outcomes. FS Rev. 2021, 2, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Kwak-Kim, J.; Gilman-Sachs, A. REVIEW ARTICLE: Clinical Implication of Natural Killer Cells and Reproduction. Am. J. Reprod. Immunol. 2008, 59, 388–400. [Google Scholar] [CrossRef]
- Shmeleva, E.V.; Colucci, F. Maternal natural killer cells at the intersection between reproduction and mucosal immunity. Mucosal Immunol. 2021, 14, 991–1005. [Google Scholar] [CrossRef]
- Wang, F.; Qualls, A.E.; Marques-Fernandez, L.; Colucci, F. Biology and pathology of the uterine microenvironment and its natural killer cells. Cell. Mol. Immunol. 2021, 18, 2101–2113. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [CrossRef]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiang, X.; Nie, L.; Guo, X.; Zhang, F.; Wen, C.; Xia, Y.; Mao, L. The emerging role of Th1 cells in atherosclerosis and its implications for therapy. Front. Immunol. 2023, 13, 1079668. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Hisano, M.; Isojima, S.; Irie, S.; Arata, N.; Watanabe, N.; Kubo, T.; Kato, T.; Murashima, A. Relationship of Th1/Th2 cell balance with the immune response to influenza vaccine during pregnancy. J. Med. Virol. 2009, 81, 1923–1928. [Google Scholar] [CrossRef]
- Makhseed, M.; Raghupathy, R.; Azizieh, F.; Omu, A.; Al-Shamali, E.; Ashkanani, L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum. Reprod. Oxf. Engl. 2001, 16, 2219–2226. [Google Scholar] [CrossRef]
- Figueiredo, A.S.; Schumacher, A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 2016, 148, 13–21. [Google Scholar] [CrossRef]
- Graham, J.J.; Longhi, M.S.; Heneghan, M.A. T helper cell immunity in pregnancy and influence on autoimmune disease progression. J. Autoimmun. 2021, 121, 102651. [Google Scholar] [CrossRef]
- Tang, C.; Hu, W. The role of Th17 and Treg cells in normal pregnancy and unexplained recurrent spontaneous abortion (URSA): New insights into immune mechanisms. Placenta 2023, 142, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Jasper, M.J.; Hull, M.L.; Aplin, J.D.; Robertson, S.A. Macrophages regulate expression of α1,2-fucosyltransferase genes in human endometrial epithelial cells. Mol. Hum. Reprod. 2012, 18, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, W.; Li, Y.; Wang, Y.; Liu, L.; Zhang, X. Potential Biomarkers and Endometrial Immune Microenvironment in Recurrent Implantation Failure. Biomolecules 2023, 13, 406. [Google Scholar] [CrossRef]
- Robertson, S.A.; Moldenhauer, L.M.; Green, E.S.; Care, A.S.; Hull, M.L. Immune determinants of endometrial receptivity: A biological perspective. Fertil. Steril. 2022, 117, 1107–1120. [Google Scholar] [CrossRef]
- Care, A.S.; Diener, K.R.; Jasper, M.J.; Brown, H.M.; Ingman, W.V.; Robertson, S.A. Macrophages regulate corpus luteum development during embryo implantation in mice. J. Clin. Investig. 2013, 123, 3472–3487. [Google Scholar] [CrossRef]
- Cimadomo, D.; de Los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.J.; Montjean, D.; Toth, B.; Vermeulen, N.; Macklon, N.; et al. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar] [CrossRef]
- Corliss, B.A.; Azimi, M.S.; Munson, J.; Peirce, S.M.; Murfee, W.L. Macrophages: An Inflammatory Link between Angiogenesis and Lymphangiogenesis. Microcirculation 2016, 23, 95–121. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Du, Y. Macrophages in immunoregulation and therapeutics|Signal Transduction and Targeted Therapy. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Luo, Y.; Yuan, P.; Hu, S.; Wang, H.; Zhang, H.; Ma, L. Inflammatory environment-adaptive patterned surface for spatiotemporal immunomodulation of macrophages. Acta Biomater. 2022, 153, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Papúchová, H.; Saxtorph, M.H.; Hallager, T.; Jepsen, I.E.; Eriksen, J.O.; Persson, G.; Funck, T.; Weisdorf, I.; Macklon, N.S.; Larsen, L.G.; et al. Endometrial HLA-F expression is influenced by genotypes and correlates differently with immune cell infiltration in IVF and recurrent implantation failure patients. Hum. Reprod. 2022, 37, 1816–1834. [Google Scholar] [CrossRef] [PubMed]
- Sfakianoudis, K.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Maziotis, E.; Kokkini, G.; Tsirligkani, C.; Bolaris, S.; Nikolettos, K.; Chronopoulou, M.; et al. The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomedicines 2021, 9, 1425. [Google Scholar] [CrossRef]
- Sudoma, I.; Goncharova, Y.; Dons’koy, B.; Mykytenko, D. Immune phenotype of the endometrium in patients with recurrent implantation failures after the transfer of genetically tested embryos in assisted reproductive technology programs. J. Reprod. Immunol. 2023, 157, 103943. [Google Scholar] [CrossRef]
- Liu, S.; Wei, H.; Li, Y.; Huang, C.; Lian, R.; Xu, J.; Chen, L.; Zeng, Y. Downregulation of ILT4+ dendritic cells in recurrent miscarriage and recurrent implantation failure. Am. J. Reprod. Immunol. 2018, 80, e12998. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, M.; Li, X.; Huang, H.; Sun, Y. Gene profiling reveals the role of inflammation, abnormal uterine muscle contraction and vascularity in recurrent implantation failure. Front. Genet. 2023, 14, 1108805. [Google Scholar] [CrossRef]
- Guo, L.; Guo, A.; Yang, F.; Li, L.; Yan, J.; Deng, X.; Dai, C.; Li, Y. Alterations of Cytokine Profiles in Patients With Recurrent Implantation Failure. Front. Endocrinol. 2022, 13, 949123. [Google Scholar] [CrossRef]
- Nieves, C.; Victoria da Costa Ghignatti, P.; Aji, N.; Bertagnolli, M. Immune Cells and Infectious Diseases in Preeclampsia Susceptibility. Can. J. Cardiol. 2024, 40, 2340–2355. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, J.; Cai, X.; Yin, T.; Zhang, Y.; Yang, C.; Yang, J. Granulocyte colony-stimulating factor in reproductive-related disease: Function, regulation and therapeutic effect. Biomed. Pharmacother. 2022, 150, 112903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yan, P.; Ma, W.; Li, J. Cytokine modulation and immunoregulation of uterine NK cells in pregnancy disorders. Cytokine Growth Factor Rev. 2024, 81, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.S.; Lee, D.; Hong, J.Y. Multi-Layered Mechanisms of Immunological Tolerance at the Maternal-Fetal Interface. Immune Netw. 2024, 24, e30. [Google Scholar] [CrossRef]
- Than, N.G.; Hahn, S.; Rossi, S.W.; Szekeres-Bartho, J. Editorial: Fetal-Maternal Immune Interactions in Pregnancy. Front. Immunol. 2019, 10, 2729. [Google Scholar] [CrossRef]
- Komijani, E.; Parhizkar, F.; Abdolmohammadi-Vahid, S.; Ahmadi, H.; Nouri, N.; Yousefi, M.; Aghebati-Maleki, L. Autophagy-mediated immune system regulation in reproductive system and pregnancy-associated complications. J. Reprod. Immunol. 2023, 158, 103973. [Google Scholar] [CrossRef]
- Esparvarinha, M.; Madadi, S.; Aslanian-Kalkhoran, L.; Nickho, H.; Dolati, S.; Pia, H.; Danaii, S.; Taghavi, S.; Yousefi, M. Dominant immune cells in pregnancy and pregnancy complications: T helper cells (TH1/TH2, TH17/Treg cells), NK cells, MDSCs, and the immune checkpoints. Cell Biol. Int. 2023, 47, 507–519. [Google Scholar] [CrossRef]
- Lokki, A.I.; Heikkinen-Eloranta, J.K.; Laivuori, H. The Immunogenetic Conundrum of Preeclampsia. Front. Immunol. 2018, 9, 2630. [Google Scholar] [CrossRef]
- Schepanski, S.; Buss, C.; Hanganu-Opatz, I.L.; Arck, P.C. Prenatal Immune and Endocrine Modulators of Offspring’s Brain Development and Cognitive Functions Later in Life. Front. Immunol. 2018, 9, 2186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).