Using Active Standing Orthostatic Stress Test to Assess Physiological Responses in Individuals with Long COVID: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility and Screening Criteria
2.3. Data Extraction
2.4. Quality and Risk of Bias Assessment
2.5. Data Analysis and Synthesis
3. Results
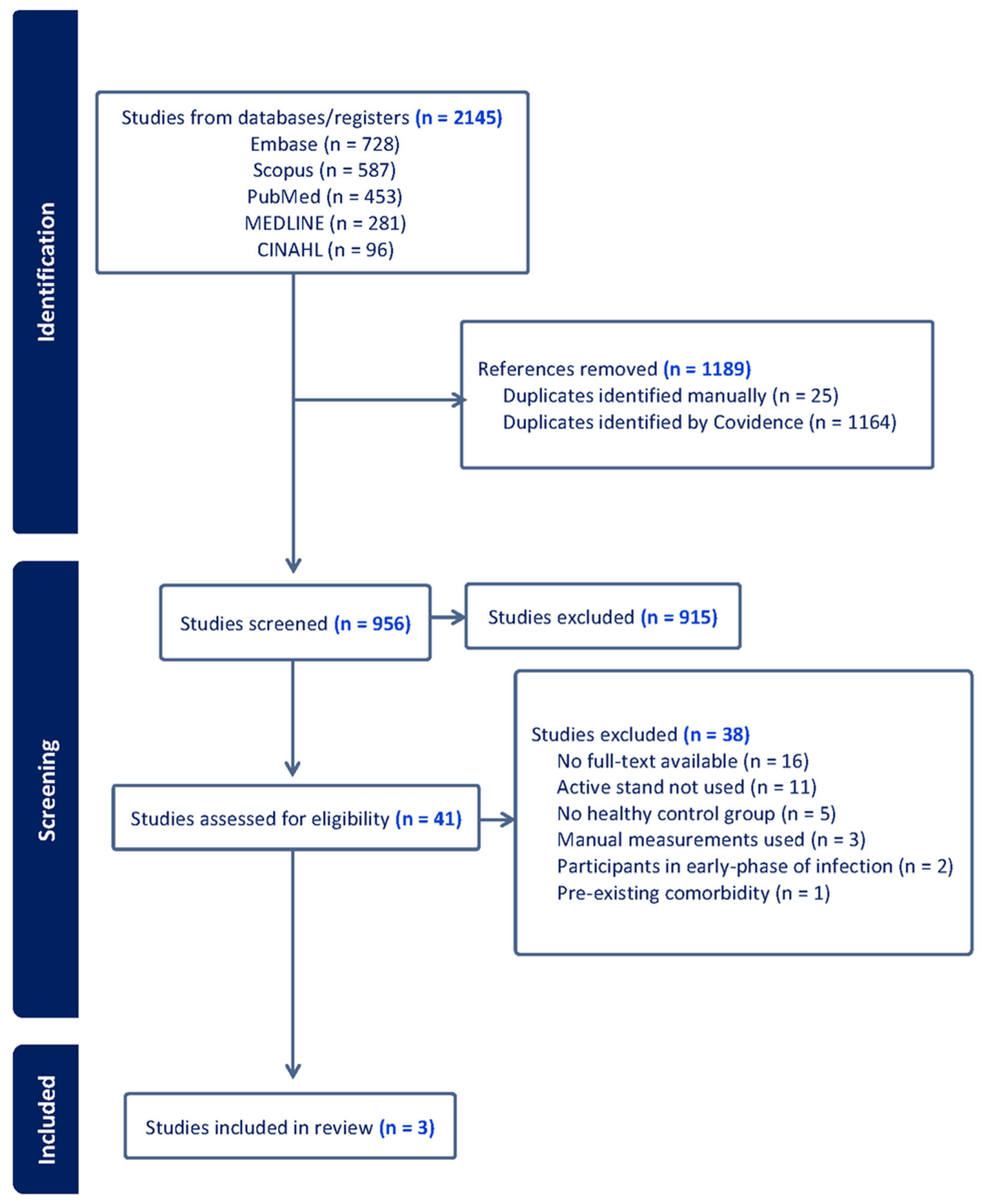
3.1. Study Selection and Search Strategy
3.2. Study Characteristics
3.3. Participant Cohort Definitions and Characteristics
3.4. Risk of Bias Assessment
3.5. Definitions and Diagnostic Criteria
3.6. Heart Rate Responses
3.7. Blood Pressure Responses
3.8. Time-Domain Heart Rate Variability Responses
3.9. Frequency-Domain Heart Rate Variability Responses
3.10. Respiratory Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BRSv | Vagal baroreflex sensitivity |
| CI | Confidence interval |
| DBP | Diastolic blood pressure |
| HC | Health controls |
| HF | High frequency |
| HR | Heart rate |
| HR | Heart rate variability |
| IQR | Inter-quartile range |
| LC | Long COVID |
| LC-IOH | Long COVID and initial orthostatic hypotension |
| LC-None | Long COVID but no abnormalities |
| LC-POTS | Long COVID and post orthostatic tachycardia syndrome |
| LF | Lower frequency |
| LF/HF ratio | Lower to higher frequency ratio |
| LFSBP | Low-frequency systolic blood pressure |
| MAP | Mean arterial pressure |
| NASEM | National Academies of Sciences, Engineering, and Medicine |
| NICE | National Institutes for Health and Care Excellence. |
| OH | Orthostatic hypotension |
| pNN50 | Percentage of successive R-R intervals differing by more than 50 ms |
| POTS | Post orthostatic tachycardia syndrome |
| RMSSD | Root-mean square of successive R-R interval differences |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| SDNN | Standard deviation of R-R intervals |
| TP | Total Power |
| WHO | World Health Organization |
References
- National Academies of Sciences, Engineering, and Medicine. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences; The National Academies Press: Washington, DC, USA, 2024. [Google Scholar]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; Normand, K.; Yang, Z.; Torres-Castro, R. Long-term impact of COVID-19: A systematic review of the literature and meta-analysis. Biomedicines 2021, 9, 900. [Google Scholar] [CrossRef]
- Ricci, F.; De Caterina, R.; Fedorowski, A. Orthostatic hypotension epidemiology, prognosis, and treatment. J. AM Coll. Cardiol. 2015, 66, 848–860. [Google Scholar] [CrossRef]
- Vernon, S.D.; Funk, S.; Bateman, L.; Stoddard, G.J.; Hammer, S.; Sullivan, K.; Bell, J.; Abbaszadeh, S.; Lipkin, W.; Komaroff, A.L. Orthostatic challenge causes distinctive symptomatic, hemodynamic and cognitive responses in long COVID and myalgic encephalomyelitis/chronic fatigue syndrome. Front. Med. 2022, 9, 917019. [Google Scholar] [CrossRef]
- Ormiston, C.K.; Świątkiewicz, I.; Taub, P.R. Postural orthostatic tachycardia syndrome as a sequela of COVID-19. Heart Rhythm. 2022, 19, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, A.; Jennings, G.; Xue, F.; Byrne, L.; Duggan, E.; Romero-Ortuno, R. Orthostatic intolerance in adults reporting long COVID symptoms was not associated with postural orthostatic tachycardia syndrome. Front. Phys. 2022, 13, 833650. [Google Scholar]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.M.; Carleton, B.C.; Goralski, K.B. COVID-19 pathophysiology and pharmacology: What do we know and how did Canadians respond? A review of Health Canada authorized clinical vaccine and drug trials. Can. J. Physiol. Pharmacol. 2021, 99, 577–588. [Google Scholar] [CrossRef]
- Finucane, C.; van Wijnen, V.K.; Fan, C.W.; Soraghan, C.; Byrne, L.; Westerhof, B.E.; Freeman, R.; Fedorowski, A.; Harms, M.P.M.; Wieling, W.; et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin. Auton. Res. 2019, 29, 427–441. [Google Scholar] [CrossRef]
- McJunkin, B.; Rose, B.; Amin, O.; Shah, N.; Sharma, S.; Modi, S.; Kemper, S.; Yousaf, M. Detecting initial orthostatic hypotension: A novel approach. J. Am. Soc. Hypertens. 2015, 9, 365–369. [Google Scholar] [CrossRef]
- Dall, P.M.; Kerr, A. Frequency of the sit to stand task: An observational study of free-living adults. Appl. Ergon. 2010, 41, 58–61. [Google Scholar] [CrossRef]
- Wieling, W.; Kaufmann, H.; Claydon, V.E.; van Wijnen, V.K.; Harms, M.P.M.; Juraschek, S.P.; Thijs, R.D. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol. 2022, 21, 735–746. [Google Scholar] [CrossRef]
- Wieling, W.; Krediet, C.T.P.; van Dijk, N.; Linzer, M.; Tschakovsky, M.E. Initial orthostatic hypotension: Review of a forgotten condition. Clin. Sci. 2007, 112, 157–165. [Google Scholar] [CrossRef]
- Blitshteyn, S.; Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol. Res. 2021, 69, 205–211. [Google Scholar] [CrossRef]
- Galarza, E.J.; Fermín, O.V.; González, J.M.N.; Tostado, L.M.F.Á.; Lozano, M.A.E.; Rabasa, C.R.; Alvarado, M.D.R.M. Exaggerated blood pressure elevation in response to orthostatic challenge, a post-acute sequelae of SARS-CoV-2 infection (PASC) after hospitalization. Auton. Neurosci. 2023, 247, 103094. [Google Scholar]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Our Research; The Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000. [Google Scholar]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef]
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G.; ESH Working Group on CV Risk in Low Resource Settings. Panethnic differences in blood pressure in Europe: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef]
- Hira, R.; Baker, J.R.; Siddiqui, T.; Patel, A.; Valani, F.G.A.; Lloyd, M.G.; Floras, J.S.; Morillo, C.A.; Sheldon, R.S.; Raj, S.R.; et al. Attenuated cardiac autonomic function in patients with long COVID with impaired orthostatic hemodynamics. Clin. Auton. Res. 2025, 35, 301–314. [Google Scholar] [CrossRef]
- Seeley, M.C.; Gallagher, C.; Ong, E.; Langdon, A.; Chieng, J.; Bailey, D.; Page, A.; Lim, H.S.; Lau, D.H. High incidence of autonomic dysfunction and postural orthostatic tachycardia syndrome in patients with long COVID: Implications for management and health care planning. Am. J. Med. 2025, 138, 354–361. [Google Scholar] [CrossRef]
- Shah, B.; Kunal, S.; Bansal, A.; Jain, J.; Poundrik, S.; Shetty, M.K.; Batra, V.; Chaturvedi, V.; Yusuf, J.; Mukhopadhyay, S.; et al. Heart rate variability as a marker of cardiovascular dysautonomia in post-COVID-19 syndrome using artificial intelligence. Indian Pacing Electrophysiol. J. 2022, 22, 70–76. [Google Scholar] [CrossRef]
- Serviente, C.; Decker, S.T.; Layec, G. From heart to muscle: Pathophysiological mechanisms underlying long-term physical sequelae from SARS-CoV-2 infection. J. Appl. Phys. 2022, 132, 581–592. [Google Scholar] [CrossRef]
- Cairo, B.; Gelpi, F.; Bari, V.; Anguissola, M.; Singh, P.; De Maria, B.; Ranucci, M.; Porta, A. A model-based spectral directional approach reveals the long-term impact of COVID-19 on cardiorespiratory control and baroreflex. Biomed. Eng. Online 2025, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodríguez, Y.; Zapata, E.; Ramírez-Santana, C.; Anaya, J.M. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J. Inf. Dis. 2022, 225, 2155–2162. [Google Scholar] [CrossRef]
- Bielecka, E.; Sielatycki, P.; Pietraszko, P.; Zapora-Kurel, A.; Zbroch, E. Elevated arterial blood pressure as a delayed complication following COVID-19—A narrative review. Int. J. Mol. Sci. 2024, 25, 1837. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Rowe, P.C.; Visser, F.C. Two different hemodynamic responses in ME/CFS patients with postural orthostatic tachycardia syndrome during head-up tilt testing. J. Clin. Med. 2024, 13, 7726. [Google Scholar] [CrossRef]
- Rinaldi, L.; Rigo, S.; Pani, M.; Bisoglio, A.; Khalaf, K.; Minonzio, M.; Shiffer, D.; Romeo, M.A.; Verzeletti, P.; Ciccarelli, M.; et al. Long-COVID autonomic syndrome in working age and work ability impairment. Sci. Rep. 2024, 14, 11835. [Google Scholar] [CrossRef]
- Fedorowski, A.; Olsén, M.F.; Nikesjö, F.; Janson, C.; Bruchfeld, J.; Lerm, M.; Hedman, K. Cardiorespiratory dysautonomia in post-COVID-19 condition: Manifestations, mechanisms and management. J. Intern. Med. 2023, 294, 548–562. [Google Scholar] [CrossRef]
- Novak, P.; Mukerji, S.S.; Alabsi, H.S.; Systrom, D.; Marciano, S.P.; Felsenstein, D.; Mullally, W.J.; Pilgrim, D.M. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann. Neurol. 2022, 91, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Marques, K.C.; Quaresma, J.A.S.; Falcão, L.F.M. Cardiovascular autonomic dysfunction in “Long COVID”: Pathophysiology, heart rate variability, and inflammatory markers. Front. Cardiovasc. Med. 2023, 10, 1256512. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Miller, A.J.; Ejaz, A.; Molinger, J.; Goyal, P.; MacLeod, D.B.; Swavely, A.; Wilson, E.; Pergola, M.; Tandri, H.; et al. Cerebral blood flow in orthostatic intolerance. J. Am. Heart Assoc. 2025, 14, e036752. [Google Scholar] [CrossRef] [PubMed]
- De Matos, D.G.; De Santana, J.L.; Aidar, F.J.; Cornish, S.M.; Giesbrecht, G.G.; Mendelson, A.A.; Duhamel, T.A.; Villar, R. Cardiovascular regulation during active standing orthostatic stress in older adults living with frailty: A systematic review. Arch. Gerontol. Geriatr. 2025, 136, 105894. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Study (Year) | Country | Study Design | Sample Size | Proportion of Females (%) | Age (Mean ± SD, Median [IQR])/[CI] (Years) | Definition of Long COVID | Active Standing Protocol (Timeframe) | Outcome Measures |
|---|---|---|---|---|---|---|---|---|
| Shah et al. (2022) [25] | India | Prospective single center | LC = 92 HC = 120 | 41% 46% | LC = 50.6 ± 12.1 HC = 51.8 ± 4.2 | Descriptive definition; no organizational source | 5 min supine, 3 min stand | Heart rate variability |
| Seeley et al. (2025) [24] | Australia | Prospective comparative | LC = 30 POTS = 33 HC = 33 | 82% 94% 82% | LC = 37 [15] POTS = 28 [14] HC = 28 [23] | WHO’s Delphi Consensus | 10 min supine, 10 min standing | Respiratory sinus arrhythmia, heart rate and blood pressure responses |
| Hira et al. (2025) [23] | Canada | Cross-sectional | LC = 94 HC = 33 | 81% 76% | LC = 42 [36, 53] HC = 49 [30, 62] | NICE guideline | 10 min supine, 10 min standing | Heart rate variability, blood pressure variability, Baroreflex sensitivity, Blood pressure and heart rate responses |
| Study (Year) | Supine HR Findings | Standing HR Findings |
|---|---|---|
| Shah et al. (2022) [25] | LC: 88 ± 15 bpm HC: 78 ± 11 bpm p = 0.0001 | LC-OH: 99 ± 18 bpm No-OH: 86 ± 14 bpm p = 0.006 HC: not reported. |
| Seeley et al. (2025) [24] | LC: 72 ± 13 bpm POTS: 79 ± 12 bpm HC: 68 ± 9 bpm Group (p = 0.003) LC > HC (p = 0.05) LC < POTS p: not reported | ∆HR (0–10 min) LC: 36 [30–47] bpm POTS: 46 [34–59] bpm HC: 15 [9–20] bpm Group (p = 0.001) LC > HC (p = 0.001) LC~POTS (p = 0.1) |
| Hira et al. (2025) [23] | LC: 67 [62–75] bpm HC: 61 [56–70] bpm p = 0.01 Subgroups: LC-IOH: 67 [61–77] bpm LC-POTS: 68 [65–75] bpm LC-none: 63 [57–73] bpm HC: 61 [56–70] bpm Group (p = 0.023) LC-none~HC (p = 0.999) LC-none~LC-POTS (p = 0.283) LC-none~LC-IOH (p = 0.063) LC-POTS~LC-IOH (p = 0.999) | LC: 89 [77–106] bpm HC: 78 [69–87] bpm p = 0.001 Subgroups: LC-IOH: 85 [77–93] bpm LC-POTS: 114 [103–131] bpm LC-none: 80 [70–88] bpm HC: 78 [69–87] bpm Group (p < 0.001) LC-none~HC (p = 0.999) LC-none < LC-POTS (p = 0.001) LC-none~LC- IOH (p = 0.279) LC-POTS > LC-IOH (p = 0.001) |
| Variable | Shah et al. (2022) [25] | Seeley et al. (2025) [24] | Hira et al. (2025) [23] |
|---|---|---|---|
| SBP-supine (mmHg) | Absolute values not reported. | No significant group differences (p = 0.12). No absolute values reported. | LC: 119 [109–132] mmHg HC: 119 [114–124] mmHg p = 0.683. Subgroup: LC-IOH: 130 [123–143] mmHg LC-none: 113 [105–119] mmHg LC-POTS: 112 [103–123] mmHg LC-IOH > LC-none (p = 0.001). LC-IOH > LC-POTS (p = 0.001). |
| DBP-supine (mmHg) | Absolute values not reported. | No data reported for DBP in supine. | LC: 71 [64–77] mmHg HC: 67 [64–72] mmHg p = 0.065 Subgroup: LC-IOH: 76 [67–79] mmHg LC-none: 67 [63–73] mmHg LC-POTS: 66 [63–73] mmHg LC-IOH > LC-none (p = 0.001). LC-IOH > LC-POTS (p = 0.009). |
| MAP-supine (mmHg) | Not reported. | Not reported. | LC: 87 [80–95] mmHg HC: 85 [80–91] mmHg p = 0.268 Subgroup: LC-IOH: 94 [87–100] mmHg LC-none: 81 [78–89] mmHg LC-POTS: 81 [77–92] mmHg LC-IOH > LC-none (p = 0.001). LC-IOH > LC-POTS (p = 0.001). |
| LFSBP supine (mmHg2) | Not reported. | Not reported. | LC: 4.06 [2.69–6.62] mmHg2 HC: 5.15 [3.77–9.37] mmHg2 LC-none < HC (p = 0.001) |
| SBP-standing (mmHg) | Absolute values not reported. OH identified in 13% of participants. | No significant differences (group*time interaction; p = 0.14). | LC vs. Controls: 113 [99–128] vs. 115 [107–132] mmHg; p = 0.241. Group main effect: p < 0.001. Subgroup: LC-IOH (124 [112–136] > LC-none (109 [95–126]; p = 0.031) and LC-POTS (102 [96–117]; p < 0.001). |
| DBP-standing (mmHg) | Absolute values not reported. | DBP increased over time in LC and POTS vs. controls (p = 0.03). POTS vs. controls p < 0.03; LC vs. control p = 0.74. | LC vs. Controls: 72 [64–79] vs. 71 [66–76] mmHg; p = 0.999. Group main effect not significant (p = 0.135). |
| MAP-standing (mmHg) | Not reported. | Not reported. | LC vs. Controls: 87 [75–96] vs. 86 [81–97] mmHg; p = 0.534. Group main effect p = 0.007. Subgroup: LC-IOH (90 [84–100] > LC-none (84 [72–91]; p = 0.049) and LC-POTS (79 [74–92]; (p = 0.019). |
| LFSBP standing (mmHg2) | Not reported. | Not reported. | LC vs. Controls: 10.5 [6.19–14.8] vs. 14.5 [9.05–30] mmHg2; p = 0.001. Subgroup: LC-none (6.59 [4.22–12] < controls (14.5 [9.05–30] mmHg2; p = 0.001) and LC-POTS (10.2 [4.49–17]; p = 0.042). |
| Variable | (Shah et al., 2022) [25] | (Hira et al., 2025) [23] |
|---|---|---|
| RMSSD | LC:13.9 ± 11.8 ms HC: 19.9 ± 19.5 ms LC < Controls (p = 0.01) Subgroup: Graded reduction by COVID severity (p = 0.0001) Asymptomatic: 24.2 ms Mild: 16.3 ms Moderate: 9.3 ms Severe: 7.2 ms | Supine: No significant group-level differences. Standing: LC: 15 [8.9–22] ms HC: 18 [13–32] ms LC < HC (p = 0.011). Subgroup: LC-IOH: 15 [9.60–27] ms LC-none: 19 [16–38] ms LC-POTS: 9.72 [7.37–13] ms LC-POTS < LC-none (p = 0.001) LC-POTS < LC-IOH (p = 0.024) |
| SDNN | LC: 16.9 ± 12.9 ms HC: 22.5 ± 17.6 ms LC < Controls (p = 0.01) | Supine: No significant group-level differences. Standing: LC: 31 [22–43] ms HC: 40 [32–55] ms; LC < HC (p = 0.001). Subgroup: LC-none: 37 [28–51] ms LC-POTS: 27 [20–33] ms LC-POTS < LC-none (p = 0.018) |
| pNN50 | Not reported | Supine: No significant group-level differences. Standing: LC: 0.23 [0–0.87] ms HC: 0.78 [0.11–2.72] ms LC < HC (p = 0.011). Subgroup: LC-IOH: 2.46 [0.32–7.44]% LC-none: 0.83 [0–2.50]% LC-POTS: 8.12 [3.10–13]% LC-POTS > LC-none (p = 0.039) LC-POTS > LC-IOH (p = 0.026) |
| Variable | Supine Findings | Standing Findings |
|---|---|---|
| HF | No significant difference between LC and HC. Subgroup: HC: 1136 [262–3121] ms2 LC-none: 1919 [725–4921] ms2 LC-IOH: 1384 [339–2036] ms2 LC-POTS: 2166 [1112–4419] ms2 Group (p = 0.026) LC-POTS > LC-IOH (p = 0.028) | LC: 315 [95–730] ms2 HC: 349 [260–1190] ms2 LC < HC (p = 0.042) Subgroup: HC: 349 [260–1190] ms2 LC-none: 578 [337–1881] ms2 LC-IOH: 296 [98–871] ms2 LC-POTS: 100 [51–339] ms2 Group (p < 0.001) LC-none > LC-POTS (p = 0.001). |
| LF | No significant differences between LC and HC. Subgroup: HC: 1484 [603–4210] ms2 LC-none: 2130 [1142–4393] ms2 LC-IOH: 1429 [643–2778] ms2 LC-POTS: 2680 [1889–4938] ms2 Group (p = 0.015) LC-POTS > LC-IOH (p = 0.012) | LC: 1203 [483–2212] ms2 HC: 1901 [935–4008] ms2 LC < HC (p = 0.008) Subgroup: HC: 1901 [935–4008] ms2 LC-none: 1806 [1106–3932] ms2 LC-IOH: 974 [428–1941] ms2 LC-POTS: 1080 [298–1671] ms2 Group (p = 0.001) LC-POTS < LC-none (p = 0.020) |
| TP | No significant differences between LC and HC. Subgroup: HC: 689 [2547–17,839] ms2 LC-none: 7702 [4681–14,480] ms2 LC-IOH: 5975 [3321–11,206] ms2 LC-POTS: 9415 [5096–16,398] ms2 Group (p = 0.057) LC-POTS > LC-IOH (p = 0.030) | LC: 3313 [1773–5559] ms2 HC: 5342 [3410–10,550] ms2 LC < HC (p < 0.001) Subgroup: HC: 5342 [3410–10,550] ms2 LC-none: 4938 [301–9406] ms2 LC-IOH: 3269 [1757–5130] ms2 LC-POTS: 2619 [1413–3949] ms2 Group (p = 0.001) LC-POTS < LC-none (p = 0.006) |
| BRSv | No significant differences between LC and HC. Subgroup: HC: 6.89 [4.94–11.4] ms/mmHg LC-none: 10.7 [5.35–17.0] ms/mmHg LC-IOH: 6.24 [4.11–10.0] ms/mmHg LC-POTS: 9.81 [8.47–16.0] ms/mmHg Group (p = 0.001) LC-POTS > LC-IOH (p = 0.010) LC-IOH > LC-none (p = 0.001) | No significant differences between LC and controls. Subgroup: Controls: 3.97 [2.93–6.41] ms/mmHg] LC-none: 5.33 [3.34–7.06] ms/mmHg LC-IOH: 2.77 [1.76–5.11] ms/mmHg LC-POTS: 2.85 [1.44–3.93] ms/mmHg Group p = 0.001 LC-POTS < LC-none (p = 0.001) LC-IOH < LC-none (p = 0.005) |
| LF/HF ratio | No significant differences between groups or subgroups. | No significant differences between groups or subgroups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olarinde, F.; Nunes-Silva, A.; Sanchez-Ramirez, D.C.; Molgat-Seon, Y.; Villar, R. Using Active Standing Orthostatic Stress Test to Assess Physiological Responses in Individuals with Long COVID: A Systematic Review. J. Clin. Med. 2025, 14, 8139. https://doi.org/10.3390/jcm14228139
Olarinde F, Nunes-Silva A, Sanchez-Ramirez DC, Molgat-Seon Y, Villar R. Using Active Standing Orthostatic Stress Test to Assess Physiological Responses in Individuals with Long COVID: A Systematic Review. Journal of Clinical Medicine. 2025; 14(22):8139. https://doi.org/10.3390/jcm14228139
Chicago/Turabian StyleOlarinde, Faith, Albená Nunes-Silva, Diana C. Sanchez-Ramirez, Yannick Molgat-Seon, and Rodrigo Villar. 2025. "Using Active Standing Orthostatic Stress Test to Assess Physiological Responses in Individuals with Long COVID: A Systematic Review" Journal of Clinical Medicine 14, no. 22: 8139. https://doi.org/10.3390/jcm14228139
APA StyleOlarinde, F., Nunes-Silva, A., Sanchez-Ramirez, D. C., Molgat-Seon, Y., & Villar, R. (2025). Using Active Standing Orthostatic Stress Test to Assess Physiological Responses in Individuals with Long COVID: A Systematic Review. Journal of Clinical Medicine, 14(22), 8139. https://doi.org/10.3390/jcm14228139







