The Programmed Placebo Effect in Patients with Syncope: Preliminary Clinical and Nanostructural Insights with a Hypothetical Quantum-Level Interpretation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.1.1. Participants
2.1.2. Ethical Considerations
2.2. Study Protocol
2.2.1. Nanotechnological Methodology
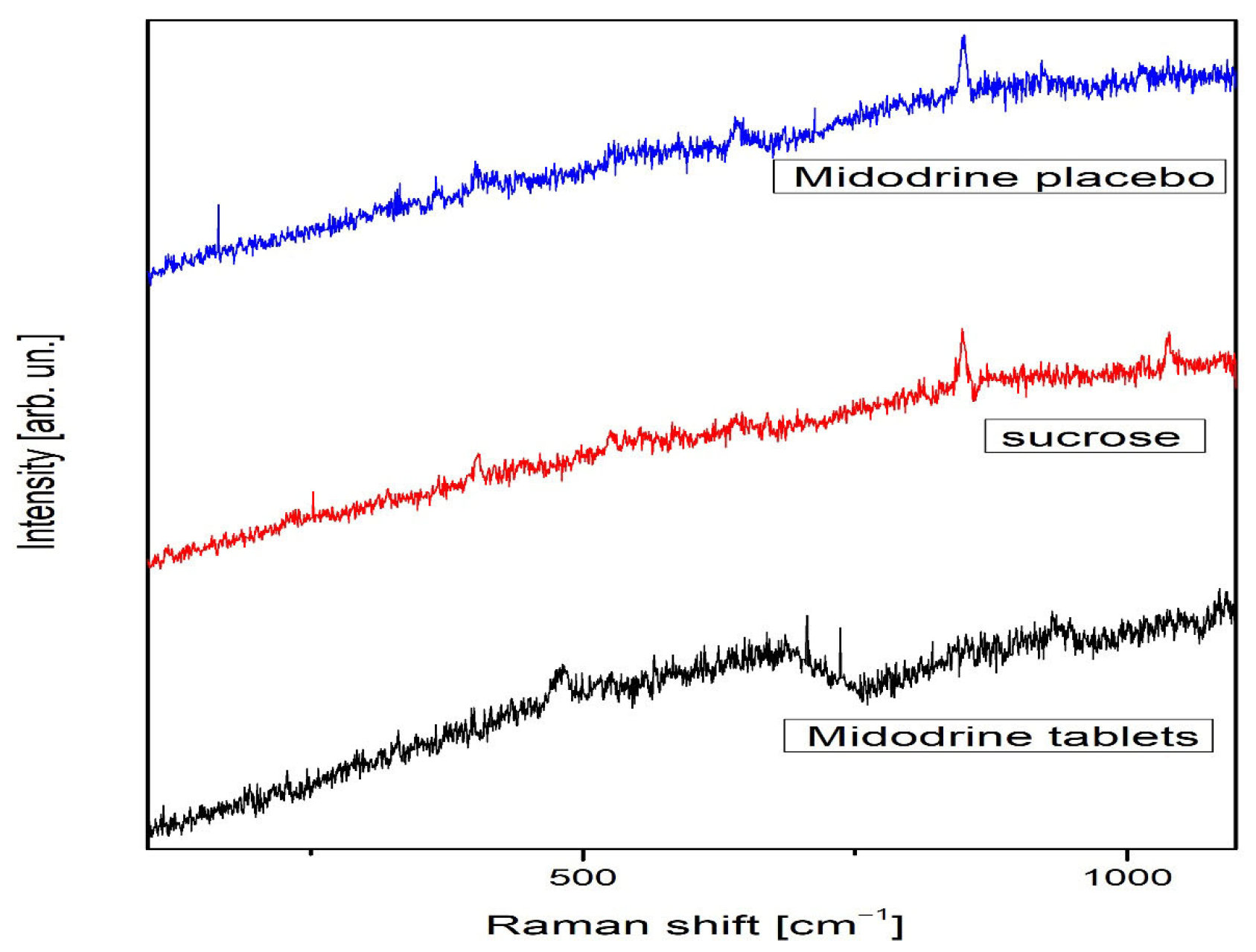
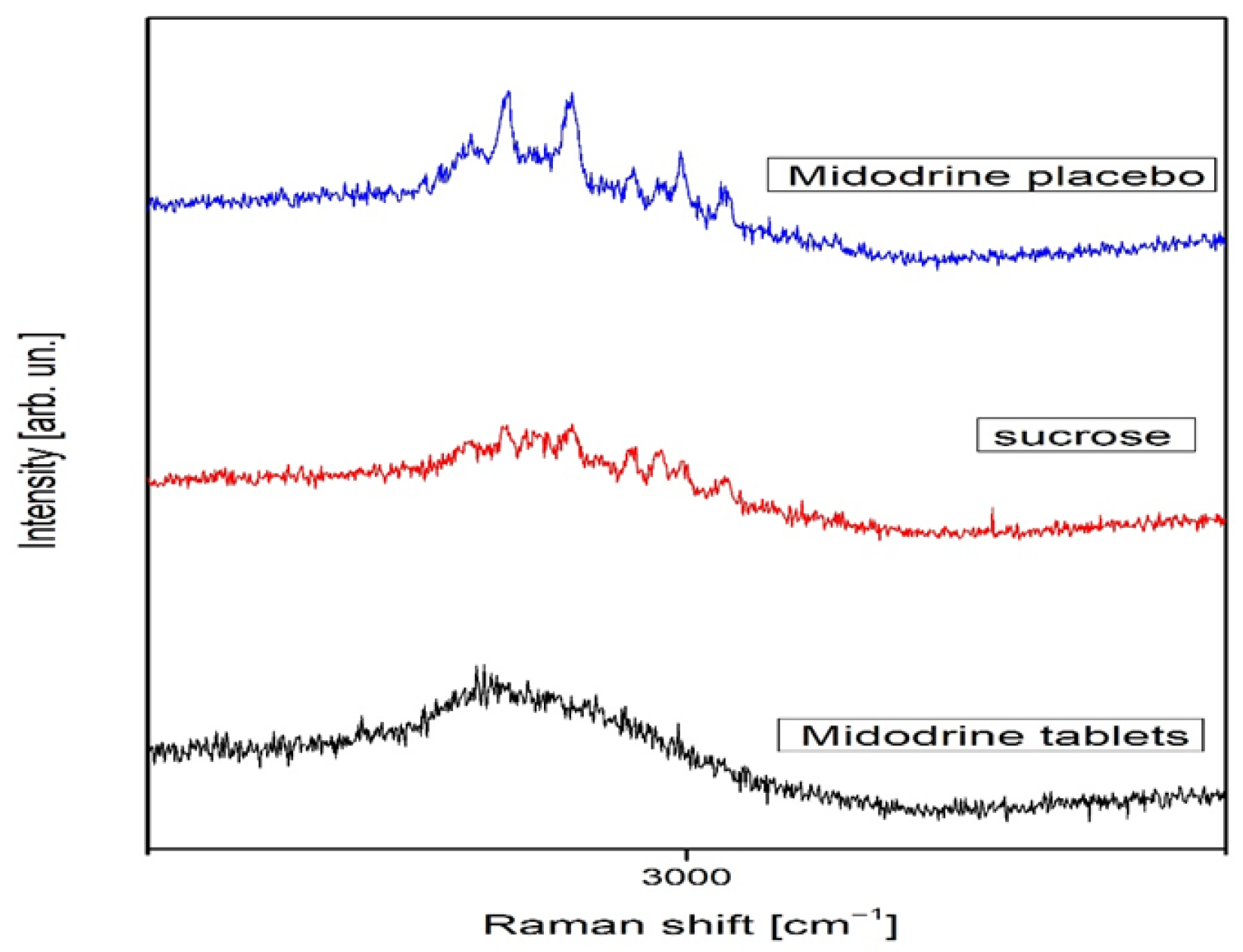
2.2.2. Raman Spectroscopy
2.2.3. X-Diffractometry in Nano-Medicine
2.3. Statistical Analysis
3. Results
3.1. Holter ECG and Ambulatory Blood Pressure Parameters
3.2. Effects on Nanotechnological Level
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC Guidelines for the Diagnosis and Management of Syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.M. Syncope: Epidemiology, Etiology, and Prognosis. Front. Physiol. 2014, 5, 471. [Google Scholar] [CrossRef] [PubMed]
- McTavish, D.; Goa, K.L. Midodrine: A Review of Its Pharmacological Properties and Therapeutic Use in Orthostatic Hypotension and Secondary Hypotensive Disorders. Drugs 1989, 38, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A.; Gilden, J.L.; Freeman, R.; Sheng, K.N.; McElligott, M.A. Efficacy of Midodrine vs Placebo in Neurogenic Orthostatic Hypotension: A Randomized, Double-Blind Multicenter Study. JAMA 1997, 277, 1046–1051. [Google Scholar] [CrossRef]
- Lei, L.Y.; Raj, S.R.; Sheldon, R.S. Midodrine for the Prevention of Vasovagal Syncope: A Systematic Review and Meta-Analysis. Europace 2022, 24, 1171–1178. [Google Scholar] [CrossRef]
- Flevari, P.; Livanis, E.G.; Theodorakis, G.N.; Zarvalis, E.; Mesiskli, T.; Kremastinos, D.T. Vasovagal Syncope: A Prospective, Randomized, Crossover Evaluation of the Effect of Propranolol, Nadolol and Placebo. J. Am. Coll. Cardiol. 2002, 40, 499–504. [Google Scholar] [CrossRef]
- Sheldon, R.; Connolly, S.; Rose, S.; Klingenheben, T.; Krahn, A.; Morillo, C.; Talajic, M.; Ku, T.; Fouad-Tarazi, F.; Ritchie, D.; et al. Prevention of Syncope Trial (POST): A Randomized, Placebo-Controlled Study of Metoprolol. Circulation 2006, 113, 1164–1170. [Google Scholar] [CrossRef]
- Sahota, I.; Sheldon, R.; Pournazari, P. Clinical Improvement of Vasovagal Syncope in the Absence of Specific Therapies: The Seinfeld Effect. Cardiol. J. 2014, 21, 637–642. [Google Scholar] [CrossRef]
- Gavrić, B.; Romčević, M.; Kuryliszyn-Kudelska, I.; Dobrowolski, W.D.; Narkiewicz, U.; Sibera, D.; Romčević, N.; Hadžić, B. Effect of Laser Heating on Partial Decomposition of ZnCo2O4 Nanoparticles: Raman Study. Opt. Mater. 2024, 157, 116238. [Google Scholar] [CrossRef]
- Hadžić, B.; Kuryliszyn-Kudelska, I.; Ćurčić, M.; Romčević, M.; Ćirković, J.; Dobrowolski, W.D.; Romčević, N. The Dynamics of Phase Transitions and Symmetry Changes with Laser Heating in ZnO(Co) Nanoparticles. Phys. B Condens. Matter 2025, 711, 417308. [Google Scholar] [CrossRef]
- Sparén, A.; Svensson, O. Transmission Raman: Methods and Applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Sharma, B.; Moody, A. Raman Spectroscopy j Spatially Offset Raman Spectroscopy. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 9. [Google Scholar] [CrossRef]
- Conti, C.; Realini, M.; Colombo, C.; Matousek, P. Comparison of Key Modalities of Micro-Scale Spatially Offset Raman Spectroscopy. Analyst 2015, 140, 8127. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, N.; Zhu, M.; Qiu, X.; Sun, S.; Liu, Y.; Zhao, T.; Yao, J.; Shan, G. Application of Transmission Raman Spectroscopy with PLS for Paracetamol Quantification. Molecules 2022, 27, 1707. [Google Scholar] [CrossRef]
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially Offset Raman Spectroscopy for Biomedical Applications. Chem. Soc. Rev. 2021, 50, 556. [Google Scholar] [CrossRef]
- Karanović, L. Primenjena Kristalografija [Applied Crystallography]; Univerzitet u Beogradu: Beograd, Serbia, 1996. [Google Scholar]
- Chao, R.S.; Vail, K.C. Polymorphism of 1,2-Dihydro-6-Neopentyl-2-Oxonicotinic Acid. J. Pharm. Res. 1987, 4, 429–432. [Google Scholar]
- Tatini, L.K.; Reddy, K.V.S.R.K.; Rao, N.S. Vapor-Induced Phase Transformations in Docetaxel. AAPS PharmSciTech 2012, 13, 548–555. [Google Scholar] [CrossRef][Green Version]
- Brizuela, A.B.; Bichara, L.C.; Romano, E.; Yurquina, A.; Locatelli, S.; Brandan, S.A. A Complete Characterization of the Vibrational Spectra of Sucrose. Carbohydr. Res. 2012, 361, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Deveau, A.P.; Sheldon, R.; Maxey, C.; Ritchie, D.; Doucette, S.; Parkash, R. Sex Differences in Vasovagal Syncope: A POST I and II Analysis. Can. J. Cardiol. 2020, 36, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Witkowski, S.; Okazaki, K.; Levine, B.D. Gender and Hypovolemia Effects on Sympathetic Neural Responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R109–R116. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Lamarre-Cliche, M.; Souich, P.D.; Champlain, J.D.; Larochelle, P. Pharmacokinetics and Dynamics of Midodrine. Clin. Ther. 2008, 30, 1629–1638. [Google Scholar] [CrossRef]
- Darragh, M.; Vanderboor, T.; Booth, R.J.; Sollers, J.J., III; Consedine, N.S. Placebo ‘Serotonin’ Increases Heart Rate Variability. Physiol. Behav. 2015, 145, 45–49. [Google Scholar] [CrossRef]
- Ward, C.R.; Gray, J.C.; Gilroy, J.J.; Kenny, R.A. Midodrine in Neurocardiogenic Syncope. Heart 1998, 79, 45–49. [Google Scholar] [CrossRef]
- Jankovic, J.; Gilden, J.L.; Hiner, B.C.; Kaufmann, H.; Brown, D.C.; Coghlan, C.H.; Rubin, M.; Fouad-Tarazi, F.M. Neurogenic Orthostatic Hypotension: Midodrine vs Placebo. Am. J. Med. 1993, 95, 38–48. [Google Scholar] [CrossRef]
- Fouad-Tarazi, F.M.; Okabe, M.; Goren, H. Alpha Sympathomimetic Treatment of Autonomic Insufficiency. Am. J. Med. 1995, 99, 604–609. [Google Scholar] [CrossRef]
- Zimmermann-Viehoff, F.; Meissner, K.; Koch, J.; Weber, C.S.; Richter, S.; Deter, H.C. Autonomic Effects of Suggestive Placebo Interventions. J. Psychosom. Res. 2013, 75, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Sheldon, R.; Thorpe, K.E.; Roberts, R.S.; Ellenbogen, K.A.; Wilkoff, B.L.; Morillo, C.; Gent, M. VPS II: Pacemaker Therapy for Syncope. JAMA 2003, 289, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Meissner, K. The placebo effect and the autonomic nervous system: Evidence for an intimate relationship. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Kühnel, A.; Schweitzer, F.; Enge, S.; Gärtner, M. Neural underpinnings of open-label placebo effects in emotional distress. Neuropsychopharmacology 2023, 48, 560–566. [Google Scholar] [CrossRef]
- Ashar, Y.K.; Sun, M.; Knight, K.; Flood, T.F.; Anderson, Z.; Kaptchuk, T.J.; Wager, T.D. Open-Label Placebo Injection for Chronic Back Pain With Functional Neuroimaging: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2432427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schienle, A.; Unger, I.; Schwab, D. Changes in neural processing and evaluation of negative facial expressions after administration of an open-label placebo. Sci. Rep. 2022, 12, 6577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howick, J.; Hoffmann, T. How placebo characteristics can influence estimates of intervention effects in trials. Can. Med. Assoc. J. 2018, 190, E908–E911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colloca, L. Preface: The Fascinating Mechanisms and Implications of the Placebo Effect. Int. Rev. Neurobiol. 2018, 138, xv–xx. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammami, M.M.; Yusuf, A.; Shire, F.S.; Hussein, R.; Al-Swayeh, R. Does the placebo effect modulate drug bioavailability? Randomized cross-over studies of three drugs. J. Negat. Results Biomed. 2017, 16, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| (1) Midodrine N = 26 | (2) Placebo N = 21 | (3) Midodrine + Placebo N = 20 | p Value | |
|---|---|---|---|---|
| Female (n,%) | 18 (69.2%) | 16 (76.2%) | 16 (80%) | 0.693 a |
| Age (yrs.) (mean ± SD) | 41.6 ± 15.6 | 39.6 ± 16.2 | 35.8 ± 12.1 | 0.374 b |
|
(1) Midodrine
N = 26 |
(2) Placebo
N = 21 |
(3) Midodrine + Placebo
N = 20 | p-Value | |
|---|---|---|---|---|
| Before the treatment | ||||
| HR (bpm) (mean ± SD) | 75.7 ±10.8 | 79.7 ± 9.7 | 75.1 ± 6.6 | 0.175 a |
| SDNN (ms) (mean ± SD) | 150.4 ± 34.4 | 148.8 ± 37.0 | 147.3 ± 28.2 | 0.953 a |
| RMSSD (ms) (mean ± SD) | 34 ± 15 | 31 ±11.1 | 35.2 ± 10.3 | 0.489 a |
| pNN50 (%) (mean ± SD) | 11.7 ± 10.9 | 9.9 ± 7.6 | 13.4 ± 9 | 0.434 a |
| TP (ms2) (mean ± SD) | 3309.3 ± 1403.7 | 3358.2 ±1726.3 | 3764.9 ± 1355.1 | 0.571 a |
| VLF (ms2) (mean ± SD) | 2161.2 ± 759.8 | 2189.5 ± 1239.7 | 2400.6 ± 854.3 | 0.699 a |
| LF (ms2) (mean ± SD) | 731.5 ± 343.5 | 815.2 ± 393.3 | 974.6 ± 363.4 | 0.113 a |
| HF (ms2) (mean ± SD) | 388.8 ± 387.6 | 330.5 ± 255.5 | 367.2 ± 197.9 | 0.795 a |
| LF/HF (mean ± SD) | 2.9 ± 1.5 | 3.5 ± 2.3 | 3 ± 1.2 | 0.432 a |
| After the treatment | ||||
| HR (bpm) (mean ± SD) | 76.8 ± 9.4 | 77.1 ± 10.2 | 76.6 ± 7.9 | 0.993 a |
| SDNN (ms) (mean ± SD) | 150.4 ± 34.4 | 148.8 ± 37.0 | 147.3 ± 28.2 | 0.953 a |
| RMSSD (ms) (mean ± SD) | 34 ± 15 | 31 ±11.1 | 35.2 ± 10.3 | 0.489 a |
| pNN50 (%) (mean ± SD) | 12.7 ± 10.1 | 12.2 ± 9.1 | 10.2 ± 6.1 | 0.617 a |
| TP (ms2) (mean ± SD) | 3433.4 ± 1153 | 3626.1 ± 1779.7 | 3592.8 ± 1232.5 | 0.902 a |
| VLF (ms2) (mean ± SD) | 2271.2 ± 672.2 | 2378.4 ± 1258.7 | 2392.2 ± 839.4 | 0.913 a |
| LF (ms2) (mean ± SD) | 775.7 ± 304.6 | 835.1 ± 405 | 907.8 ± 339.1 | 0.505 a |
| HF (ms2) (mean ± SD) | 359.3 ± 314.7 | 388.1 ± 296.8 | 275.2 ± 129.4 | .337 a |
| LF/HF (mean ± SD) | 3.1 ± 1.4 | 3.1 ± 2.2 | 3.8 ± 1.6 | 0.296 a |
| Δ values (after–before) * | ||||
| HR (bpm) (mean ± SD) | 1.1 | −2.6 | 1.8 | 0.060 a |
| SDNN (ms) (mean ± SD) | −1.3 | −3.1 | −5.2 | 0.873 a |
| RMSSD (ms) (mean ± SD) | 0.9 3 | 3.3 3 | −4.3 1,2 | 0.002 a |
| pNN50 (%) (mean ± SD) | 1 3 | 2.3 3 | −3.2 1,2 | 0.005 a |
| TP (ms2) (mean ± SD) | 124.2 | 268 | −171.6 | 0.149 a |
| VLF (ms2) (mean ± SD) | 110 | 188.9 | −8.3 | 0.451 a |
| LF (ms2) (mean ± SD) | 44.1 | 19.8 | −66.8 | 0.107 a |
| HF (ms2) (mean ± SD) | −29.5 2 | 57.5 1,3 | −92 2 | 0.002 a |
| LF/HF (mean ± SD) | 0.2 | −0.5 3 | 0.8 2 | 0.002 a |
|
(1) Midodrine
N = 26 |
(2) Placebo
N = 20 |
(3) Midodrine + Placebo
N = 21 | p-Value | |
|---|---|---|---|---|
| Before the treatment | ||||
| SBP average (mmHg) (mean ± SD) | 118.5 ± 8.5 | 117.8 ± 8.7 | 115.7 ± 7.1 | 0.476 a |
| SBP day (mmHg) (mean ± SD) | 118 ± 23.1 | 120.5 ± 8.9 | 118.9 ± 7.8 | 0.837 a |
| SBP night (mmHg) (mean ± SD) | 108 ± 9.6 | 108.9± 10.9 | 107.6 ± 7.6 | 0.882 a |
| DBP average (mmHg) (mean ± SD) | 71.9± 6.1 | 72.4 ± 6.3 | 70.3 ± 5.7 | 0.490 a |
| DBP day (mmHg) (mean ± SD) | 72.1 ± 11.6 | 74.6 ± 6.8 | 72.7 ± 6.5 | 0.551 a |
| DBP night (mmHg) (mean ± SD) | 64.4 ± 7.2 | 65.5 ± 7.8 | 64.8 ± 4.7 | 0.843 a |
| After the treatment | ||||
| SBP average (mmHg) (mean ± SD) | 119.7 ± 7.5 | 115.4 ± 9.3 | 118.5 ± 9.6 | 0.192 a |
| SBP day (mmHg) (mean ± SD) | 123.8 ± 8.1 | 118.1 ± 10.1 | 121.9 ± 10.9 | 0.098 a |
| SBP night (mmHg) (mean ± SD) | 108 ± 9.3 | 107.3 ± 9.1 | 109.4 ± 8.9 | 0.723 a |
| DBP average (mmHg) (mean ± SD) | 71 ± 5.7 | 70.2 ± 6.8 | 72.4 ± 7.4 | 0.495 a |
| DBP day (mmHg) (mean ± SD) | 73.8 ± 8.7 | 72.1 ± 7.2 | 74.9 ± 8 | 0.383 a |
| DBP night (mmHg) (mean ± SD) | 62.9 ± 6.1 | 64.2 ± 7 | 66.1 ± 7 | 0.253 a |
| Δ values (after–before) * | ||||
| SBP average (mmHg) (mean ± SD) | −0.2 3 | −2.3 3 | 4.1 1,2 | 0.007 a |
| SBP day (mmHg) (mean ± SD) | −1.7 | −0.8 | 3 | 0.192 a |
| SBP night (mmHg) (mean ± SD) | −1.2 3 | −1.6 3 | 3 1,2 | 0.001 a |
| DBP average (mmHg) (mean ± SD) | −1.2 3 | −1.6 3 | 3 1,2 | 0.001 a |
| DBP day (mmHg) (mean ± SD) | 1.5 | −1.8 | 3 | 0.131 a |
| DBP night (mmHg) (mean ± SD) | −1.8 | −0.7 | 2.1 | 0.116 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadžić, B.; Romčević, N.; Marković, N.; Petrović, M.; Bojić, M.; Milovanović, B. The Programmed Placebo Effect in Patients with Syncope: Preliminary Clinical and Nanostructural Insights with a Hypothetical Quantum-Level Interpretation. J. Clin. Med. 2025, 14, 6386. https://doi.org/10.3390/jcm14186386
Hadžić B, Romčević N, Marković N, Petrović M, Bojić M, Milovanović B. The Programmed Placebo Effect in Patients with Syncope: Preliminary Clinical and Nanostructural Insights with a Hypothetical Quantum-Level Interpretation. Journal of Clinical Medicine. 2025; 14(18):6386. https://doi.org/10.3390/jcm14186386
Chicago/Turabian StyleHadžić, Branka, Nebojša Romčević, Nikola Marković, Maša Petrović, Milovan Bojić, and Branislav Milovanović. 2025. "The Programmed Placebo Effect in Patients with Syncope: Preliminary Clinical and Nanostructural Insights with a Hypothetical Quantum-Level Interpretation" Journal of Clinical Medicine 14, no. 18: 6386. https://doi.org/10.3390/jcm14186386
APA StyleHadžić, B., Romčević, N., Marković, N., Petrović, M., Bojić, M., & Milovanović, B. (2025). The Programmed Placebo Effect in Patients with Syncope: Preliminary Clinical and Nanostructural Insights with a Hypothetical Quantum-Level Interpretation. Journal of Clinical Medicine, 14(18), 6386. https://doi.org/10.3390/jcm14186386






