Electrical Risk Score as a Predictor of Coronary Artery Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Electrocardiography
2.3. Coronary Angiography
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gokcek, I.; Sonmez, B.M.; Yucel, C.; Gokcek, M.B.; Ayyildiz, F.A. Diagnostic Value of Ischemia-Modified Albumin in Terms of Acute Coronary Syndromes in Chronic Renal Patients Admitted to Emergency Department with Chest Pain and/or Equivalent Symptoms. Eskisehir Med. J. 2022, 3, 70–78. [Google Scholar] [CrossRef]
- Yıldız, G.; Ayyıldız, F.A.; Yıldırım, Ö.T. Predictive Value of HALP Score in The Early Stage of NSTEMI. J. Cukurova Anesth. Surg. Sci. 2024, 7, 12–16. [Google Scholar] [CrossRef]
- Nikus, K.; Pahlm, O.; Wagner, G.; Birnbaum, Y.; Cinca, J.; Clemmensen, P.; Eskola, M.; Fiol, M.; Goldwasser, D.; Gorgels, A.; et al. Electrocardiographic classification of acute coronary syndromes: A review by a committee of the International Society for Holter and Non-Invasive Electrocardiology. J. Electrocardiol. 2010, 43, 91–103. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.L.; Reinier, K.; Rusinaru, C.; Uy-Evanado, A.; Darouian, N.; Phan, D.; Mack, W.J.; Jui, J.; Soliman, E.Z.; Tereshchenko, L.G.; et al. Electrical risk score beyond the left ventricular ejection fraction: Prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur. Heart J. 2017, 38, 3017–3025. [Google Scholar] [CrossRef]
- Elmas, A.N.; Fedai, H.; Toprak, K.; Taşcanov, M.B.; Altıparmak, İ.H.; Biçer, A.; Demirbağ, R.; Tanrıverdi, Z. The Association of Electrical Risk Score with Prognosis in Patients with Non-ST Elevation Myocardial Infarction Undergoing Coronary Angiography. Anatol. J. Cardiol. 2025, 29, 11–18. [Google Scholar] [CrossRef]
- Pham, H.N.; Holmstrom, L.; Chugh, H.; Uy-Evanado, A.; Nakamura, K.; Zhang, Z.; Salvucci, A.; Jui, J.; Reinier, K.; Chugh, S.S. Dynamic electrocardiogram changes are a novel risk marker for sudden cardiac death. Eur. Heart J. 2023, 45, 809–819. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Fabietti, M.; Di Iorio, C.; Mastropietri, F.; Sabatino, T.; Crapanzano, D.; Bertani, G.; Zaccagnini, G.; Lospinuso, I.; et al. Age, gender and drug therapy influences on Tpeak-tend interval and on electrical risk score. J. Electrocardiol. 2020, 59, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Onan, E.; Paydas, S.; Çağlayan, Ç.E.; Gök, G.; Balal, M.; Celik, A.I. The Relationship Between Patient Survival and ECG aVR T Wave Positivity in Hemodialysis Patients. J. Cukurova Anesth. Surg. Sci. 2023, 6, 19–27. [Google Scholar] [CrossRef]
- Elmas, A.N.; Fedai, H.; Tanriverdi, Z. The relationship between circadian blood pressure patterns and electrical risk score in patients with hypertension. Medicine 2025, 104, e44511. [Google Scholar] [CrossRef]
- Bekler, Ö.; Kazan, S.D.; Harbalioğlu, H.; Kaypakli, O. Predictive Value of Electrocardiographic Markers Versus Echocardiographic and Clinical Measures for Appropriate ICD Shocks in Heart Failure Patients. J. Clin. Med. 2025, 14, 5506. [Google Scholar] [CrossRef]
- Sharif, S.; Kalmanovich, E.; Marcus, G.; Tsiporin, F.; Minha, S.; Barkagan, M.; Love, I.; Fuchs, S.; Zahavi, G.; Milman, A. QTc Prolongation as a Diagnostic Clue in Acute Pulmonary Embolism. J. Clin. Med. 2025, 14, 5005. [Google Scholar] [CrossRef]
- Roden, D.M. Long QT syndrome: Reduced repolarization reserve and the genetic link. J. Intern. Med. 2005, 259, 59–69. [Google Scholar] [CrossRef]
- Tse, G.; Gong, M.; Wong, W.T.; Georgopoulos, S.; Letsas, K.P.; Vassiliou, V.S.; Chan, Y.S.; Yan, B.P.; Wong, S.H.; Wu, W.K.; et al. The Tpeak − Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: A systematic review and meta-analysis. Heart Rhythm 2017, 14, 1131–1137. [Google Scholar] [CrossRef]
- Aro, A.L.; Phan, D.; Teodorescu, C.; Uy-Evanado, A.; Reinier, K.; Gunson, K.; Jui, J.; Huikuri, H.V.; Chugh, S.S. Cardiac structural and functional profile of patients with delayed QRS transition zone and sudden cardiac death. Europace 2016, 19, 629–635. [Google Scholar] [CrossRef]
- Aro, A.L.; Eranti, A.; Anttonen, O.; Kerola, T.; Rissanen, H.A.; Knekt, P.; Porthan, K.; Tikkanen, J.T.; Junttila, M.J.; Huikuri, H.V. Delayed QRS transition in the precordial leads of an electrocardiogram as a predictor of sudden cardiac death in the general population. Heart Rhythm 2014, 11, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, R.U.; Asif, M.; Uppara, S.; Sarkar, M.K.; Pasupulati, S.L.; Narmada, N.; Datla, S.S.V.; Murugesan, R.; Vaikkakara, S.; Guddeti, R.; et al. Influence of Age and Sex on the Clinical Profile of Metabolic Syndrome in Diabetic Patients. Cureus 2025, 17, e88376. [Google Scholar] [CrossRef] [PubMed]
- Duyuler, S.; Arslan, K.; Karabulut, R.C.; Aksoy, A.; Dağlı, M.; Duyuler, P.T. A Novel Electrocardiographic Index to Predict the Severity of Coronary Calcification. Anatol. J. Cardiol. 2025; in press. [Google Scholar] [CrossRef]
- Poselyaninov, A.S.; Tsvetkova, A.S.; Khomenko, P.V.; Grubbe, M.E.; Ovechkin, A.O.; Bernikova, O.G.; Demidova, M.M.; Azarov, J.E.; Platonov, P.G. Duration of electrocardio-graphic TPEAK-TEND interval in ischemia: Dispersion of repolarization vs. myocardium area at risk. J. Electrocardiol. 2025, 93, 154129. [Google Scholar] [CrossRef]
- Zorlu, Ç.; Açıkel, B.; Ömür, S.E. Frontal Plane QRS-T Angle Is a Predictor of Ventricular Arrhythmia in Heart Failure with Preserved Ejection Fraction. Ann. Noninvasive Electrocardiol. 2025, 30, e70062. [Google Scholar] [CrossRef] [PubMed]
- Nasr, G.; Mahfouz, A.T.; El Husseny, M.W.A.; Kamel, O.; Shaban, G.; Ghaleb, R. Clinical and echocardiographic outcomes of sync-atrioventricular versus nominal optimization in correlation with QRS narrowing among CRT patients. BMC Cardiovasc. Disord. 2025, 25, 635. [Google Scholar] [CrossRef] [PubMed]
| Control Group (n = 156) | Coronary Artery Disease Group (n = 158) | p | |
|---|---|---|---|
| Gender n,% | 80 (51.3%) | 113 (71.5%) | <0.001 |
| Hypertension n,% | 84 (53.8%) | 58 (36.7%) | 0.002 |
| Diabetes mellitus n,% | 48 (30.8%) | 48 (30.4%) | 0.940 |
| Peripheral artery disease n,% | 3 (1.9%) | 2 (1.3%) | 0.642 |
| Chronic obstructive pulmonary disease n,% | 8 (5.1%) | 6 (3.8%) | 0.568 |
| Stroke n,% | 0 (0%) | 2 (1.3%) | 0.159 |
| Chronic kidney failure n,% | 1 (0.6%) | 6 (3.8%) | 0.058 |
| Malignancy n,% | 1 (0.6%) | 1 (0.6%) | 0.993 |
| Smoking n,% | 65 (41.7%) | 91 (57.6%) | 0.005 |
| Creatinine, mg/dL | 0.8 (0.7–0.97) | 0.9 (0.8–1.08) | <0.001 |
| Glomerular filtration rate | 90.3 ± 19.1 | 79.6 ± 22.0 | <0.001 |
| Total Cholesterol, mg/dL | 197.2 ± 38.3 | 193.1 ± 49.2 | 0.441 |
| High-density lipoprotein cholesterol, mg/dL | 47.8 ± 12.2 | 40.9 ± 9.3 | <0.001 |
| Low-density lipoprotein cholesterol, mg/dL | 118.2 ± 32.8 | 121.6 ± 40.9 | 0.430 |
| Triglyceride, mg/dL | 137.5 (90–202) | 128 (87.5–193) | 0.303 |
| Hemoglobin g/dL | 13.8 ± 1.7 | 13.9 ± 2.1 | 0.359 |
| Leucocyte count, ×103/μL | 7.8 (6.6–9.3 | 9.7 (7.4–12.1) | <0.001 |
| Platelet count, ×103/μL | 230.5 (199–269.2) | 237 (191–285.5) | 0.503 |
| Ejection fraction, % | 60 (58–62) | 55 (50–60) | <0.001 |
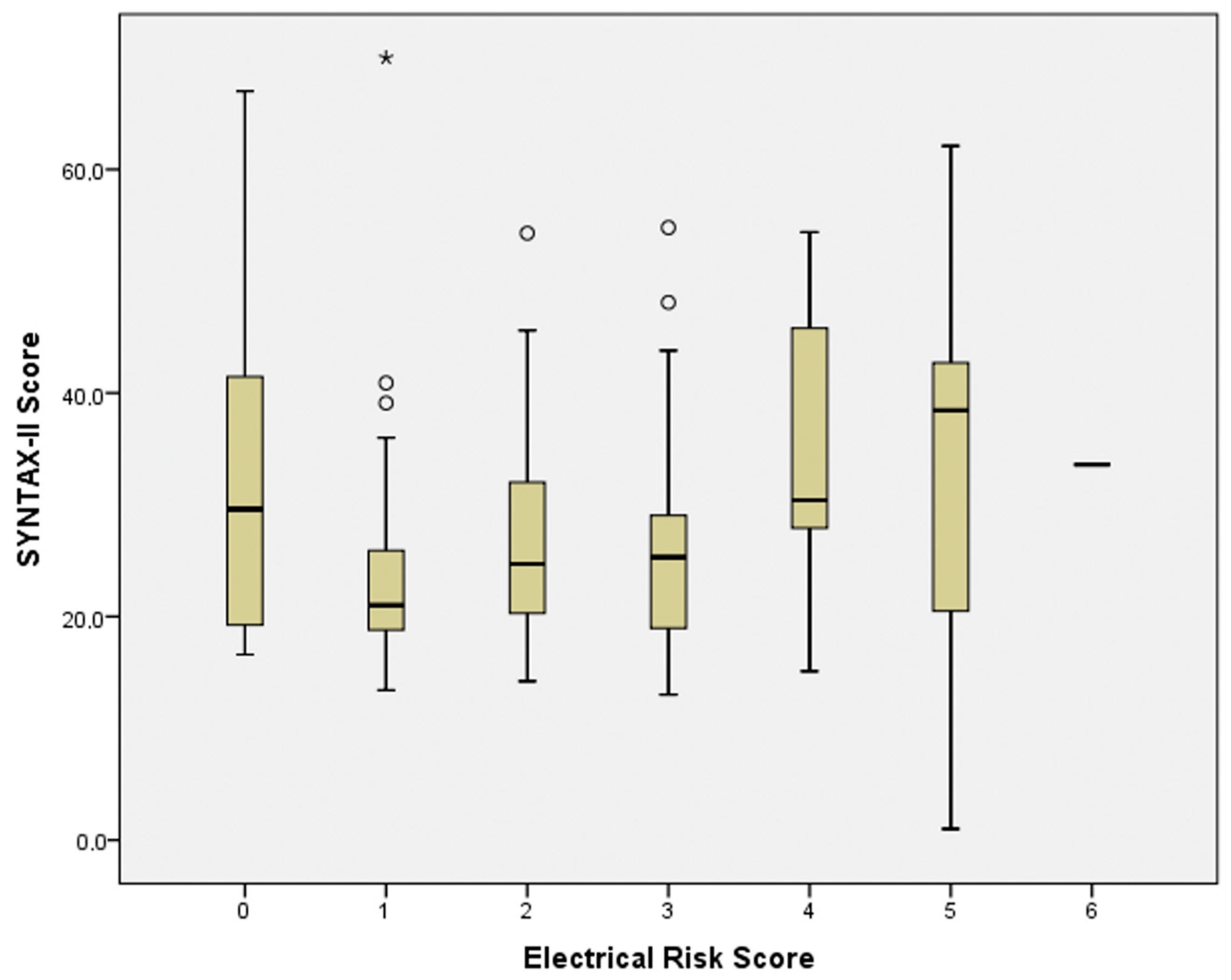
| Electrical Risk Score | Control Group (n = 156) | Coronary Artery Disease Group (n = 158) | p |
|---|---|---|---|
| 0 | 20 | 11 | 0.006 |
| 1 | 46 | 33 | |
| 2 | 51 | 49 | |
| 3 | 28 | 37 | |
| 4 | 10 | 14 | |
| 5 | 1 | 13 | |
| 6 | 0 | 1 |
| Electrical Risk Score | Control Group (n = 156) | Coronary Artery Disease Group (n = 158) | p |
|---|---|---|---|
| Heart rate n,% | 76 (48.7%) | 89 (56.3%) | 0.177 |
| Left ventricle hypertrophy n,% | 32 (20.5%) | 23 (14.6%) | 0.165 |
| Delayed QRS transition zone n,% | 55 (35.3%) | 76 (48.1%) | 0.023 |
| Wide frontal QRS T angle n,% | 38 (24.3%) | 51 (32.3%) | 0.119 |
| Prolonged QTc n,% | 28 (17.9%) | 51 (32.3%) | 0.004 |
| Prolonged T peak to end interval n,% | 48 (30.8%) | 79 (50.0%) | 0.001 |
| Odds Ratio | 95% Confidence Interval | p | |
|---|---|---|---|
| Electrical risk score | 1.338 | 1.036–1.728 | 0.026 |
| Age | 1.076 | 1.040–1.114 | <0.001 |
| Gender | 0.473 | 0.226–0.991 | 0.047 |
| Hypertension | 2.159 | 1.083–4.302 | 0.029 |
| Diabetes Mellitus | 0.735 | 0.366–1.475 | 0.386 |
| Peripheral Artery Disease | 0.624 | 0.051–7.671 | 0.712 |
| Chronic Renal Failure | 1.460 | 0.1.4–20.425 | 0.779 |
| Malignancy | 3.006 | 0.035–260.225 | 0.629 |
| Smoking | 0.518 | 0.246–1.093 | 0.084 |
| Creatinine | 0.976 | 0.608–1.566 | 0.919 |
| High-density lipoprotein cholesterol | 0.943 | 0.911–0.976 | 0.001 |
| Low-density lipoprotein cholesterol | 1.011 | 1.002–1.020 | 0.012 |
| Triglyceride | 0.997 | 0.993–1.000 | 0.051 |
| Hemoglobin | 0.873 | 0.722–1.056 | 0.163 |
| Leukocyte count | 1.156 | 1.032–1.295 | 0.012 |
| Platelet count | 1.003 | 0.998–1.007 | 0.279 |
| Ejection fraction | 0.909 | 0.863–0.957 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turgay Yıldırım, Ö.; Dişikırık, T.; Arslan, G.Y.; Belpınar, M.S.; Beral, A.; Özden, B.; Özgeyik, M. Electrical Risk Score as a Predictor of Coronary Artery Disease. J. Clin. Med. 2025, 14, 8106. https://doi.org/10.3390/jcm14228106
Turgay Yıldırım Ö, Dişikırık T, Arslan GY, Belpınar MS, Beral A, Özden B, Özgeyik M. Electrical Risk Score as a Predictor of Coronary Artery Disease. Journal of Clinical Medicine. 2025; 14(22):8106. https://doi.org/10.3390/jcm14228106
Chicago/Turabian StyleTurgay Yıldırım, Özge, Tuğba Dişikırık, Gamze Yeter Arslan, Mehmet Semih Belpınar, Ayberk Beral, Barış Özden, and Mehmet Özgeyik. 2025. "Electrical Risk Score as a Predictor of Coronary Artery Disease" Journal of Clinical Medicine 14, no. 22: 8106. https://doi.org/10.3390/jcm14228106
APA StyleTurgay Yıldırım, Ö., Dişikırık, T., Arslan, G. Y., Belpınar, M. S., Beral, A., Özden, B., & Özgeyik, M. (2025). Electrical Risk Score as a Predictor of Coronary Artery Disease. Journal of Clinical Medicine, 14(22), 8106. https://doi.org/10.3390/jcm14228106







