Evaluating Tibial Tunnel Landmarks in Anterior Cruciate Ligament Reconstruction: Remnant Versus Lateral Meniscus Anterior Horn
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Surgical Groups
2.3. Surgical Technique
- Group 1 (ACL remnant group): The surgeon used the ACL tibial remnant as the primary reference. To identify the center of the remnant, partial debridement was performed and standardized using a scaled arthroscopic probe with a 4-mm hook length (1-mm markings), leaving ~5 mm of tissue. The guidewire was then inserted through the central portion of the remnant.
- Group 2 (LMAH group): Following complete debridement of the ACL remnant, surgeons used the posterior margin of the LMAH as a reference. The guide wire was aligned mediolaterally with the center of the intercondylar eminence (Note: The same tibial aiming device was used in both groups).
2.4. Post-Operative Rehabilitation
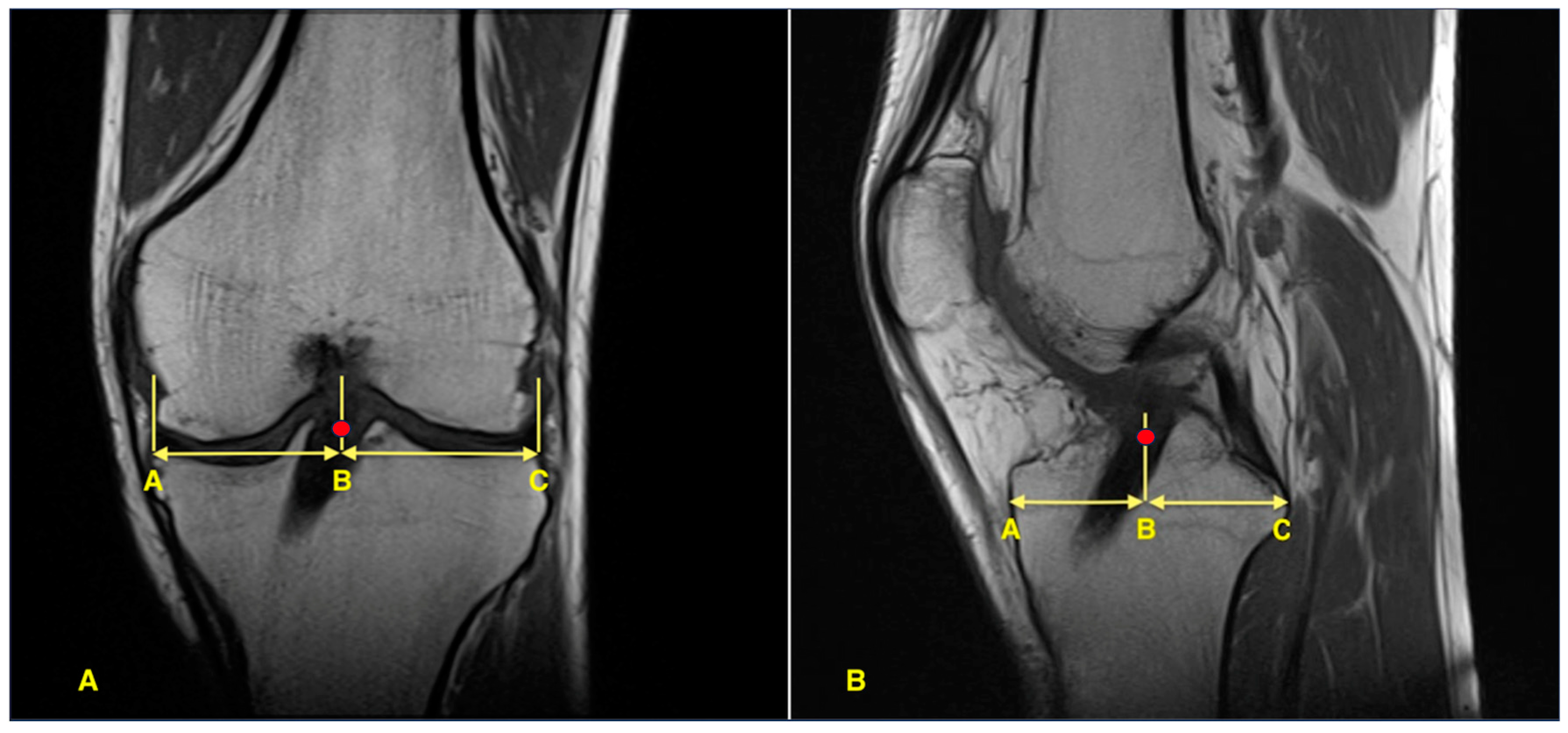
2.5. Imaging and Measurement
2.6. Data Collection
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACL | Anterior cruciate ligament |
| BMI | Body mass index |
| ICC | Intraclass correlation coefficient |
| IKDC | International Knee Documentation Committee |
| LMAH | Lateral meniscus anterior horn |
| MTS | Medial tibial spine |
| MRI | Magnetic resonance imaging |
| SD | Standard deviation |
References
- Dhawan, A.; Gallo, R.A.; Lynch, S.A. Anatomic tunnel placement in anterior cruciate ligament reconstruction. J. Am. Acad. Orthop. Surg. 2016, 24, 443–454. [Google Scholar] [CrossRef]
- Goldsmith, M.T.; Jansson, K.S.; Smith, S.D.; Engebretsen, L.; LaPrade, R.F.; Wijdicks, C.A. Biomechanical comparison of anatomic single- and double-bundle anterior cruciate ligament reconstructions: An in vitro study. Am. J. Sports Med. 2013, 41, 1595–1604. [Google Scholar] [CrossRef]
- Lucidi, G.A.; Agostinone, P.; Di Paolo, S.; Fabbro, G.D.; Serra, M.; Viotto, M.; Grassi, A.; Zaffagnini, S. Long-term outcomes after anterior cruciate ligament reconstruction with 3 different surgical techniques: A prospective randomized clinical and radio-graphic evaluation at a minimum of 20 years’ follow-up. Orthop. J. Sports Med. 2025, 13, 23259671241302348. [Google Scholar] [CrossRef]
- Bedi, A.; LaPrade, R.F.; Burrus, M.T. Radiographic and anatomic landmarks of the major knee ligaments. J. Bone Jt. Surg. Am. 2018, 100, 1241–1250. [Google Scholar] [CrossRef]
- Hutchinson, M.R.; Bae, T.S. Reproducibility of anatomic tibial landmarks for anterior cruciate ligament reconstructions. Am. J. Sports Med. 2001, 29, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Pedneault, C.; Laverdière, C.; Hart, A.; Boily, M.; Burman, M.; Martineau, P.A. Evaluating the accuracy of tibial tunnel place-ment after anatomic single-bundle anterior cruciate ligament reconstruction. Am. J. Sports Med. 2019, 47, 3187–3194. [Google Scholar] [CrossRef]
- van Eck, C.F.; Lesniak, B.P.; Schreiber, V.M.; Fu, F.H. Anatomic single- and double-bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy 2010, 26, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.P.; Telang, M.; Landzhov, B.; Olewnik, Ł.; Slavchev, S.A.; LaPrade, R.F.; Ruzik, K.; Tubbs, R.S. The novel epiligament theory: Differences in healing failure between the medial collateral and anterior cruciate ligaments. J. Exp. Orthop. 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.P.; Tubbs, R.S.; Olewnik, Ł.; Zielinska, N.; Telang, M.; Ananiev, J.; Dimitrova, I.N.; Slavchev, S.A.; Yordanov, Y.; LaPrade, R.F.; et al. A comparative study of the epiligament of the medial collateral and anterior cruciate ligaments in the human knee: Immunohistochemical analysis of CD 34, α-smooth muscle actin and vascular endothelial growth factor in relation to epiligament theory. Knee 2022, 39, 78–90. [Google Scholar] [CrossRef]
- Georgiev, G.P.; Yordanov, Y.; Olewnik, Ł.; Tubbs, R.S.; LaPrade, R.F.; Ananiev, J.; Slavchev, S.A.; Dimitrova, I.N.; Gaydarski, L.; Landzhov, B. Do the Differences in the Epiligament of the Proximal and Distal Parts of the Anterior Cruciate Ligament Explain Their Different Healing Capacities? Quantitative and Immunohistochemical Analysis of CD34 and α-SMA Expression in Relation to the Epiligament Theory. Biomedicines 2024, 12, 156. [Google Scholar] [CrossRef]
- Georgiev, G.P.; Gaydarski, L.; Landzhov, B. Should We Accept the Epiligament Theory About the Differences in the Healing Potential of the Medial Collateral and the Anterior Cruciate Ligament? Biomedicines 2025, 13, 522. [Google Scholar] [CrossRef]
- Jackson, D.W.; Gasser, S.I. Tibial tunnel placement in ACL reconstruction. Arthroscopy 1994, 10, 124–131. [Google Scholar] [CrossRef]
- Ferretti, M.; Doca, D.; Ingham, S.M.; Cohen, M.; Fu, F.H. Bony and soft tissue landmarks of the ACL tibial insertion site: An anatomical study. Knee Surg. Sports Traumatol. Arthrosc. 2011, 20, 62–68. [Google Scholar] [CrossRef]
- Hohmann, E.; Bryant, A.; Tetsworth, K. Tunnel positioning in anterior cruciate ligament reconstruction: How long is the learning curve? Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1576–1582. [Google Scholar] [CrossRef]
- Stäubli, H.U.; Rauschning, W. Tibial attachment area of the anterior cruciate ligament in the extended knee position. Anatomy and cryosections in vitro complemented by magnetic resonance arthrography in vivo. Knee Surg. Sports Traumatol. Arthrosc. 1994, 2, 138–146. [Google Scholar] [CrossRef]
- Agneskirchner, J.D.; Galla, M.; Landwehr, P.; Lobenhoffer, H.P. Simplified MRI sequences for postoperative control of hamstring anterior cruciate ligament reconstruction. Arch. Orthop. Trauma Surg. 2004, 124, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting ıntraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Büyükdoğan, K.; Laidlaw, M.S.; Fox, M.A.; Kew, M.E.; Miller, M.D. Effect of tibial tunnel placement using the lateral meniscus as a landmark on clinical outcomes of anatomic single-bundle anterior cruciate ligament reconstruction. Am. J. Sports Med. 2021, 49, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Parkar, A.P.; Adriaensen, M.E.; Vindfeld, S.; Solheim, E. The anatomic centers of the femoral and tibial insertions of the anterior cruciate ligament: A systematic review of imaging and cadaveric studies reporting normal center locations. Am. J. Sports Med. 2017, 45, 2180–2188. [Google Scholar] [CrossRef]
- Silva, A.; Sampaio, R.; Pinto, E. ACL reconstruction: Comparison between transtibial and anteromedial portal techniques. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 896–903. [Google Scholar] [CrossRef]
- Yau, W.P.; Fok, A.W.; Yee, D.K. Tunnel positions in transportal versus transtibial anterior cruciate ligament reconstruction: A case-control magnetic resonance imaging study. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Kassam, A.M.; Tillotson, L.; Schranz, P.J.; Mandalia, V.I. The lateral meniscus as a guide to anatomical tibial tunnel placement during anterior cruciate ligament reconstruction. Open Orthop. J. 2015, 9, 542–547. [Google Scholar] [CrossRef]
- Dimitriou, D.; Zou, D.; Wang, Z.; Tsai, T.Y.; Helmy, N. Anterior root of lateral meniscus and medial tibial spine are reliable intraoperative landmarks for the tibial footprint of anterior cruciate ligament. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Burrus, M.T.; Gwathmey, F.W.; Miller, M.D. A prospective evaluation of the anterior horn of the lateral meniscus as a landmark for tibial tunnel placement in anterior cruciate ligament (ACL) reconstruction. Knee 2016, 23, 478–481. [Google Scholar] [CrossRef]
- Keyhani, S.; Esmailiejah, A.A.; Mirhoseini, M.S.; Hosseininejad, S.-M.; Ghanbari, N. The Prevalence, Zone, and Type of the Meniscus Tear in Patients with Anterior Cruciate Ligament (ACL) Injury; Does Delayed ACL Reconstruction Affects the Meniscal Injury? Arch. Bone. Jt. Surg. 2020, 8, 432–438. [Google Scholar]
- Siebold, R.; Ellert, T.; Metz, S.; Metz, J. Tibial insertions of the anteromedial and posterolateral bundles of the anterior cruciate ligament: Morphometry, arthroscopic landmarks, and orientation model for bone tunnel placement. Arthroscopy 2008, 24, 154–161. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, S.; Seong, S.C.; Lee, M.C. Anatomy of the anterior cruciate ligament insertion sites: Comparison of plain radiography and three-dimensional computed tomographic imaging to anatomic dissection. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2297–2305. [Google Scholar] [CrossRef]
- Pietrini, S.D.; Ziegler, C.G.; Anderson, C.J.; Wijdicks, C.A.; Westerhaus, B.D.; Johansen, S.; Engebretsen, L.; LaPrade, R.F. Radiographic landmarks for tunnel positioning in double-bundle ACL reconstructions. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 792–800. [Google Scholar] [CrossRef] [PubMed]
- de Abreu-E-Silva, G.M.; de Oliveira, M.H.; Maranhão, G.S.; Deligne, L.d.M.; Pfeilsticker, R.M.; Novais, E.N.; Nunes, T.A.; de Andrade, M.A. Three-dimensional computed tomography evaluation of anterior cruciate ligament footprint for anatomic single-bundle reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 770–776. [Google Scholar] [CrossRef]
| Group 1 (n = 35) | Group 2 (n = 39) | p | |
|---|---|---|---|
| Age (years) | 29.8 ± 7.9 | 27.7 ± 7.5 | 0.174 m |
| Follow-up (months) | 34.8 ± 12.2 | 33.74 ± 11.5 | 0.588 m |
| Gender (M/F) | 33/2 | 37/2 | 1.000 f |
| BMI (kg/m2) | 25.5 ± 2.3 | 25.0 ± 3.2 | 0.462 t |
| Operated side (Right/Left) | 15/20 | 23/16 | 0.166 X2 |
| Group 1 (n = 35) | Group 2 (n = 39) | p | |
|---|---|---|---|
| Tegner score | |||
| Preinjury | 7.29 ± 0.96 | 7.18 ± 0.94 | 0.409 m |
| Preoperative | 3.97 ± 1.04 | 4.08 ± 1.04 | 0.624 m |
| Postoperative | 6.49 ± 1.36 | 6.23 ± 1.69 | 0.568 m |
| p † (overall) | 8.50 × 10−15 F | 3.28 × 10−15 F | |
| p † (Preinjury-Preoperative) | 1.92 × 10−7 w | 3.86 × 10−8 w | |
| p † (Preoperative-Postoperative) | 3.04 × 10−7 w | 2.15 × 10−7 w | |
| p † (Postoperative-Preinjury) | 0.004 w | 0.0009 w | |
| Lysholm score | |||
| Preoperative | 68.46 ± 7.92 | 68.64 ± 10.05 | 0.578 m |
| Postoperative | 86.37 ± 12.55 | 83.31 ± 13.67 | 0.191 m |
| p † | 3.47 × 10−7 w | 5.50 × 10−8 w | |
| IKDC score | |||
| Preoperative | 46.71 ± 9.60 | 45.49 ± 9.04 | 0.573 t |
| Postoperative | 83.26 ± 16.33 | 82.64 ± 16.36 | 0.637 m |
| p † | 2.45 × 10−7 w | 5.19 × 10−8 w |
| Group 1 (n = 35) | Group 2 (n = 39) | p | ||
|---|---|---|---|---|
| Lachman test | ||||
| Preoperative | ||||
| Grade 1 | 6 (17.1%) | 9 (23.1%) | 0.587 m | |
| Grade 2 | 21 (60.0%) | 22 (56.4%) | ||
| Grade 3 | 8 (22.9%) | 8 (20.5%) | ||
| Postoperative | ||||
| Grade 0 | 27 (77.1%) | 31 (79.5%) | 0.856 m | |
| Grade 1 | 8 (22.9%) | 7 (17.9%) | ||
| Grade 2 | 0 (0.0%) | 1 (2.6%) | ||
| p † | 4.39 × 10−8 w | 1.66 × 10−8 w | ||
| Pivot-shift test | ||||
| Preoperative | ||||
| Grade 1 | 7 (20.0%) | 10 (25.6%) | 0.496 m | |
| Grade 2 | 18 (51.4%) | 20 (51.3%) | ||
| Grade 3 | 10 (28.6%) | 9 (23.1%) | ||
| Postoperative | ||||
| Grade 0 | 29 (82.9%) | 33 (84.6%) | 0.799 m | |
| Grade 1 | 5 (14.3%) | 6 (15.4%) | ||
| Grade 2 | 1 (2.9%) | 0 (0.0%) | ||
| p † | 8.65 × 10−8 w | 2.69 × 10−8 w | ||
| KT-2000 (mm) ‡ | Mean ± SD | 2.03 ± 1.48 | 2.18 ± 1.94 | 0.978 m |
| Group 1 (n = 35) | Group 2 (n = 39) | p | |
|---|---|---|---|
| Return to sports (Yes/No) | 30/5 | 32/7 | 0.759 f |
| Pivoting sports | 23/5 | 25/7 | |
| Non-pivoting sports | 7/0 | 7/0 | |
| Level of return to sports | |||
| Lower level | 4 | 6 | 0.733 f |
| Same level | 26 | 26 | |
| Higher level | 0 | 0 | |
| Time to return to sports (months) | 8.83 ± 2.17 | 8.88 ± 1.90 | 0.791 m |
| Group 1 (n = 35) | Group 2 (n = 39) | p | |
|---|---|---|---|
| Sagittal Tunnel Position (%) | 44.57 ± 4.85 | 46.87 ± 4.41 | 0.036 t |
| Anterior Cortex to Graft Center (mm) | 24.69 ± 3.23 | 26.20 ± 2.93 | 0.038 t |
| Anterior Cortex to Posterior Cortex (mm) | 55.39 ± 3.02 | 55.90 ± 3.81 | 0.466 t |
| Coronal Tunnel Position (%) | 47.99 ± 2.84 | 48.77 ± 2.68 | 0.215 m |
| Medial Cortex to Graft Center (mm) | 36.70 ± 2.57 | 37.50 ± 3.21 | 0.249 t |
| Medial Cortex to Lateral Cortex (mm) | 76.47 ± 3.54 | 76.89 ± 4.49 | 0.719 t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pehlivanoglu, G.; Yildiz, K.I.; Albayrak, K.; Cakir, T.; Aykut, U.S.; Ozkul, B. Evaluating Tibial Tunnel Landmarks in Anterior Cruciate Ligament Reconstruction: Remnant Versus Lateral Meniscus Anterior Horn. J. Clin. Med. 2025, 14, 8096. https://doi.org/10.3390/jcm14228096
Pehlivanoglu G, Yildiz KI, Albayrak K, Cakir T, Aykut US, Ozkul B. Evaluating Tibial Tunnel Landmarks in Anterior Cruciate Ligament Reconstruction: Remnant Versus Lateral Meniscus Anterior Horn. Journal of Clinical Medicine. 2025; 14(22):8096. https://doi.org/10.3390/jcm14228096
Chicago/Turabian StylePehlivanoglu, Gokhan, Kadir Ilker Yildiz, Kutalmis Albayrak, Tolga Cakir, Umit Selcuk Aykut, and Baris Ozkul. 2025. "Evaluating Tibial Tunnel Landmarks in Anterior Cruciate Ligament Reconstruction: Remnant Versus Lateral Meniscus Anterior Horn" Journal of Clinical Medicine 14, no. 22: 8096. https://doi.org/10.3390/jcm14228096
APA StylePehlivanoglu, G., Yildiz, K. I., Albayrak, K., Cakir, T., Aykut, U. S., & Ozkul, B. (2025). Evaluating Tibial Tunnel Landmarks in Anterior Cruciate Ligament Reconstruction: Remnant Versus Lateral Meniscus Anterior Horn. Journal of Clinical Medicine, 14(22), 8096. https://doi.org/10.3390/jcm14228096






