Sleep Stage Monitoring in Congenital Heart Disease (CHD) Using a Digital Health Application Programming Interface (API)
Abstract
1. Introduction
2. Participants and Methods
2.1. Study Participants
2.2. Sleep Tracking
2.3. Cardio Pulmonary Exercise Testing (CPET)
2.4. Statistical Analyses
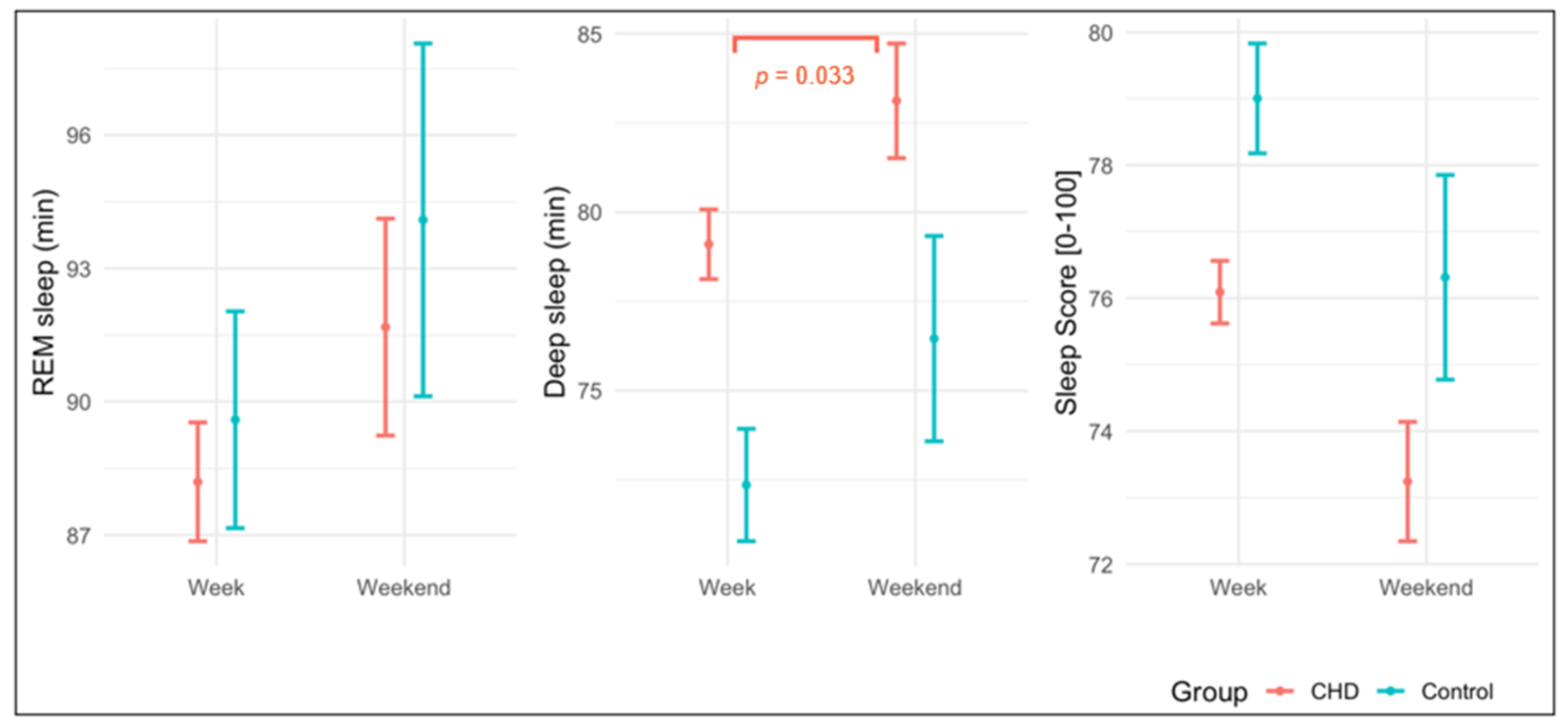
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dellborg, M.; Giang, K.W.; Eriksson, P.; Liden, H.; Fedchenko, M.; Ahnfelt, A.; Rosengren, A.; Mandalenakis, Z. Adults with congenital heart disease: Trends in event-free survival past middle age. Circulation 2023, 147, 930–938. [Google Scholar] [CrossRef]
- Tutarel, O.; Kempny, A.; Alonso-Gonzalez, R.; Jabbour, R.; Li, W.; Uebing, A.; Dimopoulos, K.; Swan, L.; Gatzoulis, M.A.; Diller, G.-P. Congenital heart disease beyond the age of 60: Emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur. Heart J. 2014, 35, 725–732. [Google Scholar] [CrossRef]
- Faizan, M.; Han, C.; Lee, S.W. Policy-Driven Digital Health Interventions for Health Promotion and Disease Prevention: A Systematic Review of Clinical and Environmental Outcomes. Healthcare 2025, 13, 2319. [Google Scholar] [CrossRef]
- Grandner, M.A. Sleep, health, and society. Sleep Med. Clin. 2022, 17, 117–139. [Google Scholar] [CrossRef]
- Grandner, M.A. Sleep and Health; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Chen, Y.; Kartsonaki, C.; Clarke, R.; Guo, Y.; Du, H.; Yu, C.; Yang, L.; Pei, P.; Stevens, R.; Burgess, S.; et al. Sleep duration and risk of stroke and coronary heart disease: A 9-year community-based prospective study of 0.5 million Chinese adults. BMC Neurol. 2023, 23, 327. [Google Scholar] [CrossRef]
- Kohyama, J. Which Is More Important for Health: Sleep Quantity or Sleep Quality? Children 2021, 8, 542. [Google Scholar] [CrossRef]
- Lipinska, G.; Thomas, K.G. The interaction of REM fragmentation and night-time arousal modulates sleep-dependent emotional memory consolidation. Front. Psychol. 2019, 10, 1766. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Hagen, E.W.; Finn, L.A.; Hla, K.M.; Carter, J.R.; Peppard, P.E. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: A longitudinal analysis of the Wisconsin Sleep Cohort. Thorax 2015, 70, 1062–1069. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, X.; Ma, J.; Wei, X.; Zhang, J.; Weiping, D. Effects of sleep deprivation on heart rate variability: A systematic review and meta-analysis. Front. Neurol. 2025, 16, 1556784. [Google Scholar] [CrossRef]
- Salzillo, C.; La Verde, M.; Imparato, A.; Molitierno, R.; Lucà, S.; Pagliuca, F.; Marzullo, A. Cardiovascular diseases in public health: Chromosomal abnormalities in congenital heart disease causing sudden cardiac death in children. Medicina 2024, 60, 1976. [Google Scholar] [CrossRef]
- Vincenti, M.; Guillaumont, S.; Clarivet, B.; Macioce, V.; Mura, T.; Boulot, P.; Cambonie, G.; Amedro, P. Prognosis of severe congenital heart diseases: Do we overestimate the impact of prenatal diagnosis? Arch. Cardiovasc. Dis. 2019, 112, 261–269. [Google Scholar] [CrossRef]
- Vakali, M.; Memon, M.; Gatzoulis, M.; Polkey, M. Sleep disordered breathing and adult congenital heart disease. Int. J. Cardiol. Congenit. Heart Dis. 2024, 18, 100532. [Google Scholar] [CrossRef]
- Ykeda, D.S.; Lorenzi-Filho, G.; Lopes, A.A.; Alves, R.S. Sleep in infants with congenital heart disease. Clinics 2009, 64, 1205–1210. [Google Scholar] [CrossRef]
- Caissie, D.M.; Dollimount, A.; Tomczak, C.R.; Erlandson, M.C.; Pockett, C.; Adamko, D.J.; Olver, T.D.; Switzer, H.; Wright, K.D. Adaptive Functioning and Sleep Quality: Associations in Young Children with Congenital Heart Disease. Pediatr. Cardiol. 2024, 46, 1514–1522. [Google Scholar] [CrossRef]
- Johnson, J.T.; Yetman, A.T. Cardiopulmonary exercise testing in adults with congenital heart disease. Prog. Pediatr. Cardiol. 2012, 34, 47–52. [Google Scholar] [CrossRef]
- Mantegazza, V.; Apostolo, A.; Hager, A. Cardiopulmonary exercise testing in adult congenital heart disease. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S1), S93–S101. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1494–1563. [Google Scholar] [CrossRef]
- Warnes, C.A.; Liberthson, R.; Danielson, G.K.; Dore, A.; Harris, L.; Hoffman, J.I.; Somerville, J.; Williams, R.G.; Webb, G.D. Task force 1: The changing profile of congenital heart disease in adult life. J. Am. Coll. Cardiol. 2001, 37, 1170–1175. [Google Scholar] [CrossRef]
- Patel, A.K.; Reddy, V.; Shumway, K.R.; Araujo, J.F. Physiology, sleep stages. In StatPearls [Internet]; StatPearls Publishing: Orlando, PR, USA, 2024. [Google Scholar]
- Garmin Ltd. Schlafüberwachung. Available online: https://www.garmin.com/de-DE/garmin-technology/health-science/sleep-tracking/ (accessed on 5 August 2025).
- Lau, T.; Ong, J.L.; Ng, B.K.L.; Chan, L.F.; Koek, D.; Tan, C.S.; Müller-Riemenschneider, F.; Cheong, K.; Massar, S.A.A.; Chee, M.W.L. Minimum number of nights for reliable estimation of habitual sleep using a consumer sleep tracker. Sleep Adv. 2022, 3, zpac026. [Google Scholar] [CrossRef]
- Cooper, D.M.; Weiler-Ravell, D. Gas exchange response to exercise in children. Am. Rev. Respir. Dis. 1984, 129, S47–S48. [Google Scholar] [CrossRef]
- Gläser, S.; Ittermann, T.; Schäper, C.; Obst, A.; Dörr, M.; Spielhagen, T.; Felix, S.; Völzke, H.; Bollmann, T.; Opitz, C. The Study of Health in Pomerania (SHIP) reference values for cardiopulmonary exercise testing. Pneumologie 2012, 67, 58–63. [Google Scholar]
- Fritz, C.; Hock, J.; Oberhoffer, R.; Hager, A.; Ewert, P.; Müller, J. Reduced Parasympathetic Activity in Patients With Different Types of Congenital Heart Disease and Associations to Exercise Capacity. J. Cardiopulm. Rehabil. Prev. 2021, 41, 35–39. [Google Scholar] [CrossRef]
- Müller, J.; Amberger, T.; Berg, A.; Goeder, D.; Remmele, J.; Oberhoffer, R.; Ewert, P.; Hager, A. Physical activity in adults with congenital heart disease and associations with functional outcomes. Heart 2017, 103, 1117–1121. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Reading/Addison-Wesley: Boston, MA, USA, 1977. [Google Scholar]
- Kelly, R.M.; McDermott, J.H.; Coogan, A.N. Differences in Sleep Offset Timing between Weekdays and Weekends in 79,161 Adult Participants in the UK Biobank. Clocks Sleep 2022, 4, 658–674. [Google Scholar] [CrossRef]
- Vestergaard, C.L.; Simpson, M.R.; Sivertsen, B.; Kallestad, H.; Langsrud, K.; Scott, J.; Vedaa, Ø. Weekday-to-weekend sleep duration patterns among young adults and outcomes related to health and academic performance. Sleep Sci. Pract. 2024, 8, 15. [Google Scholar] [CrossRef]
- Adachi, H.; Yamamoto, R.; Fujino, R.; Kanayama, D.; Sakagami, Y.; Akamine, S.; Marutani, N.; Yanagida, K.; Mamiya, Y.; Koyama, M.; et al. Association of weekday-to-weekend sleep differences and stress response among a Japanese working population: A cross-sectional study. Sleep Med. 2021, 82, 159–164. [Google Scholar] [CrossRef]
- Zhang, R.; Tomasi, D.; Shokri-Kojori, E.; Wiers, C.E.; Wang, G.-J.; Volkow, N.D. Sleep inconsistency between weekends and weekdays is associated with changes in brain function during task and rest. Sleep 2020, 43, zsaa076. [Google Scholar] [CrossRef]
- Drake, M.; Ginde, S.; Cohen, S.; Bartz, P.; Sowinski, J.; Reinhardt, E.; Saleska, T.; Earing, M.G. Prevalence, Risk Factors, and Impact of Obstructive Sleep Apnea in Adults with Congenital Heart Disease. Pediatr. Cardiol. 2020, 41, 724–728. [Google Scholar] [CrossRef]
- Ayas, N.T.; Taylor, C.M.; Laher, I. Cardiovascular consequences of obstructive sleep apnea. Curr. Opin. Cardiol. 2016, 31, 599–605. [Google Scholar] [CrossRef]
- Combs, D.; Fernandez, V.; Barber, B.J.; Morgan, W.J.; Hsu, C.-H.; Andrews, J.; Parthasarathy MD, S.; Klewer, S.E.; Seckeler, M.D. Obstructive Sleep Apnea is Associated With Cardiac Dysfunction in Children With Congenital Heart Disease. Circulation 2020, 142 (Suppl. S3), A14771. [Google Scholar] [CrossRef]
- Holman, M.L. Obstructive Sleep Apnea Syndrome: Implications for Primary Care. Nurse Pract. 2005, 30, 38–43. [Google Scholar] [CrossRef]
- Geer, J.H.; Hilbert, J. Gender Issues in Obstructive Sleep Apnea. Yale J. Biol. Med. 2021, 94, 487–496. [Google Scholar]
- Robertson, K.; Seckeler, M.; Klewer, S.; Hsu, C.-H.; Edgin, J.; Provencio-Dean, N.; Lopez, S.; Parthasarathy, S.; Combs, D. 605 Sleep problems are associated with behavioral problems and decreased quality of life in children with Fontan circulation. Sleep 2021, 44 (Suppl. S2), A238. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Cuellar, J.A.; Huwa, K.E.; Jameson, J.T.; Watson, C.H.; Bessman, S.C.; Hirsch, D.A.; Cooper, A.D.; Drummond, S.P.; Markwald, R.R. Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep 2021, 44, zsaa291. [Google Scholar] [CrossRef]
- Evenson, K.R.; Spade, C.L. Review of Validity and Reliability of Garmin Activity Trackers. J. Meas. Phys. Behav. 2020, 3, 170–185. [Google Scholar] [CrossRef]
- Lee, X.K.; Chee, N.; Ong, J.L.; Teo, T.B.; van Rijn, E.; Lo, J.C.; Chee, M.W.L. Validation of a Consumer Sleep Wearable Device With Actigraphy and Polysomnography in Adolescents Across Sleep Opportunity Manipulations. J. Clin. Sleep Med. 2019, 15, 1337–1346. [Google Scholar] [CrossRef]
- Roberts, D.M.; Schade, M.M.; Mathew, G.M.; Gartenberg, D.; Buxton, O.M. Detecting sleep using heart rate and motion data from multisensor consumer-grade wearables, relative to wrist actigraphy and polysomnography. Sleep 2020, 43, zsaa045. [Google Scholar] [CrossRef]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-Ananta, T.; Ajmal; Ramella-Roman, J.C.; McShane, M.J.; Cote, G.L. Sources of inaccuracy in photoplethysmography for continuous cardiovascular monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef]
- Yang, P.-Y.; Ho, K.-H.; Chen, H.-C.; Chien, M.-Y. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. J. Physiother. 2012, 58, 157–163. [Google Scholar] [CrossRef]
- Miller, D.J.; Sargent, C.; Roach, G.D. A validation of six wearable devices for estimating sleep, heart rate and heart rate variability in healthy adults. Sensors 2022, 22, 6317. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Associations of physical activity with sleep satisfaction, perceived stress, and problematic Internet use in Korean adolescents. BMC Public Health 2014, 14, 1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Boros, S. The effect of physical activity on sleep quality: A systematic review. Eur. J. Physiother. 2021, 23, 11–18. [Google Scholar] [CrossRef]
| Variable | CHD | Control | p-Value * |
|---|---|---|---|
| Total (n) | 175 | 52 | |
| Sex (female) in n (%) | 86 (49%) | 21 (40%) | 0.340 |
| Age (Mean ± SD) in years | 33.1 ± 10.3 | 34.4 ± 12.4 | 0.498 |
| BMI (Mean ± SD) in kg/m2 | 24.3 ± 4.0 | 24.0 ± 3.7 | 0.575 |
| Predictor | β REM Univ. | p-Value | β REM Multiv. | p-Value | β Deep Univ. | p-Value | β Deep Multiv. | p-Value | β Sleep Score Univ. | p-Value | β Sleep Score Multiv. | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | −11.55 | 0.314 | – | – | −3.7 | 0.657 | – | – | 0.03 | 0.569 | – | – |
| Sex (female) | 889.9 | <0.001 | 797.3 | <0.001 | 27.6 | 0.871 | – | – | 3.32 | 0.018 | 2.52 | 0.063 |
| peak (mL/kg/mi) | 5.07 | 0.418 | – | – | 0.1 | 0.977 | – | – | 0.07 | 0.058 | – | – |
| Severity: moderate (complex) | 537.4 | 0.029 | 309.1 | 0.216 | −98.2 | 0.586 | – | – | 4.09 | 0.005 | 3.75 | 0.017 |
| Severity: simple (complex) | 925.4 | 0.025 | 516.4 | 0.254 | 40.2 | 0.894 | – | – | 7.07 | 0.004 | 6.27 | 0.028 |
| BMI (kg/m2) | −109.1 | <0.001 | −104.1 | <0.001 | 40.6 | 0.059 | – | – | −0.56 | 0.002 | −0.56 | 0.001 |
| Surgeries: 1–2 (none) | −439.2 | 0.111 | −243.2 | 0.410 | 323.7 | 0.108 | – | – | −2.99 | 0.075 | −0.98 | 0.595 |
| Surgeries: 3+ (none) | −1064 | 0.005 | −793.1 | 0.056 | 219.4 | 0.425 | – | – | −4.54 | 0.047 | −0.96 | 0.707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöneburg, C.; Uphoff, I.; Ludwig, V.; Oberhoffer-Fritz, R.; Ewert, P.; Müller, J. Sleep Stage Monitoring in Congenital Heart Disease (CHD) Using a Digital Health Application Programming Interface (API). J. Clin. Med. 2025, 14, 8097. https://doi.org/10.3390/jcm14228097
Schöneburg C, Uphoff I, Ludwig V, Oberhoffer-Fritz R, Ewert P, Müller J. Sleep Stage Monitoring in Congenital Heart Disease (CHD) Using a Digital Health Application Programming Interface (API). Journal of Clinical Medicine. 2025; 14(22):8097. https://doi.org/10.3390/jcm14228097
Chicago/Turabian StyleSchöneburg, Charlotte, Isabel Uphoff, Viktoria Ludwig, Renate Oberhoffer-Fritz, Peter Ewert, and Jan Müller. 2025. "Sleep Stage Monitoring in Congenital Heart Disease (CHD) Using a Digital Health Application Programming Interface (API)" Journal of Clinical Medicine 14, no. 22: 8097. https://doi.org/10.3390/jcm14228097
APA StyleSchöneburg, C., Uphoff, I., Ludwig, V., Oberhoffer-Fritz, R., Ewert, P., & Müller, J. (2025). Sleep Stage Monitoring in Congenital Heart Disease (CHD) Using a Digital Health Application Programming Interface (API). Journal of Clinical Medicine, 14(22), 8097. https://doi.org/10.3390/jcm14228097





