Evaluation of the Effects of Eye Drops for Dry Eyes on Neuronal Pain Receptors in a Primary Culture Model of Trigeminal Ganglion Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
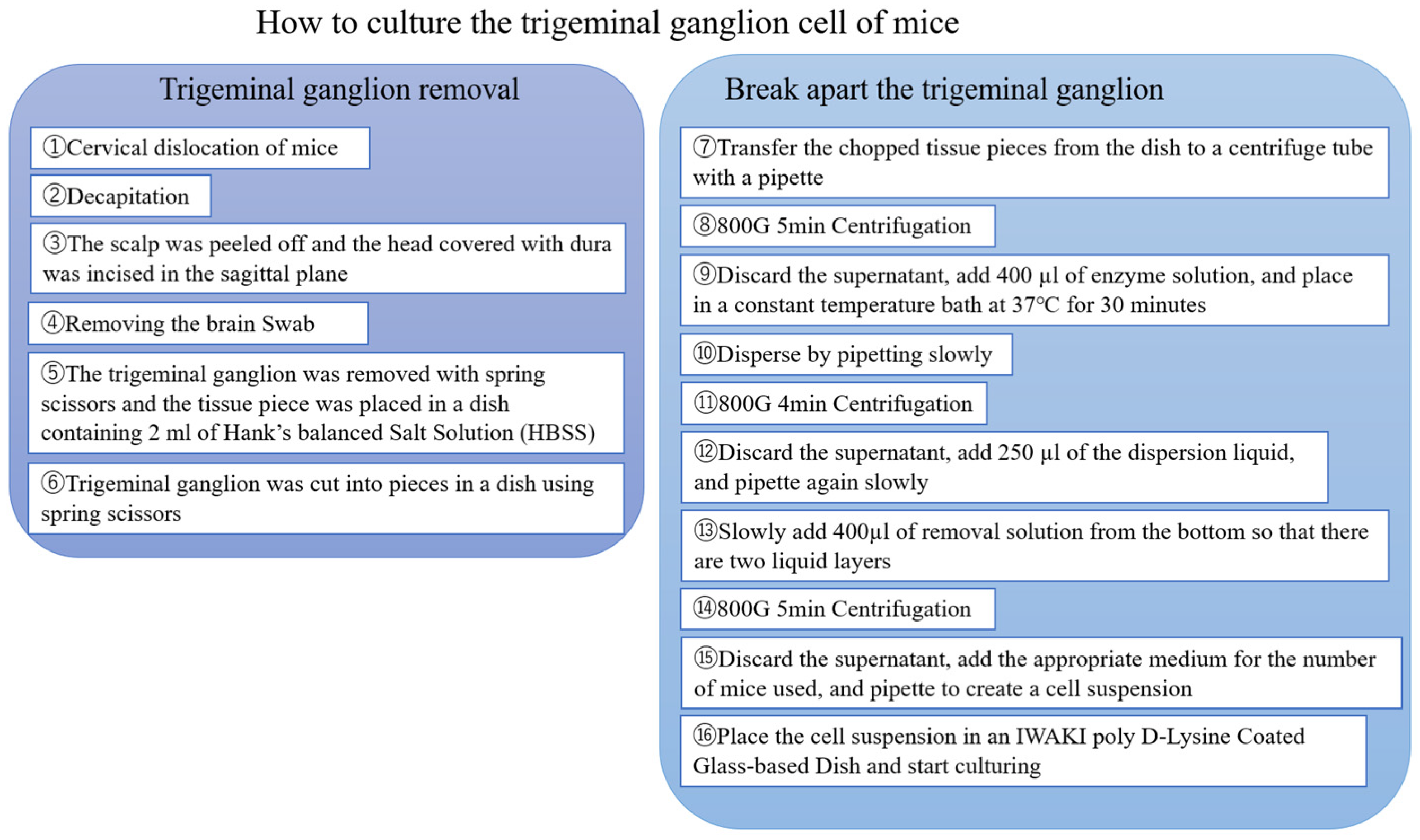
2.2. Primary Culture of Trigeminal Ganglion Neurons
2.3. Immunofluorescence Staining
2.4. Calcium Imaging of Primary Cultures
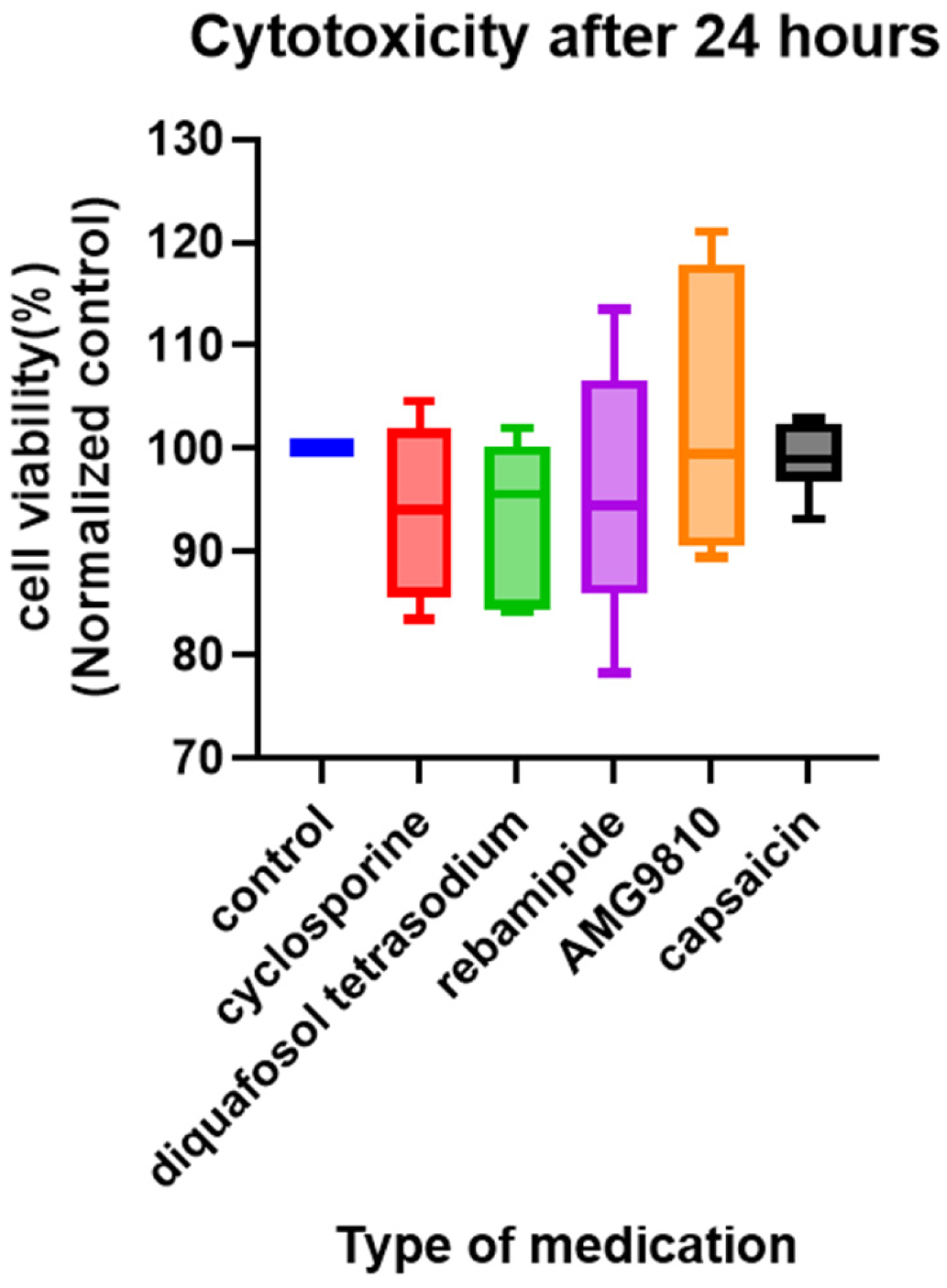
2.5. Cell Viability
2.6. Statistical Analysis
3. Results
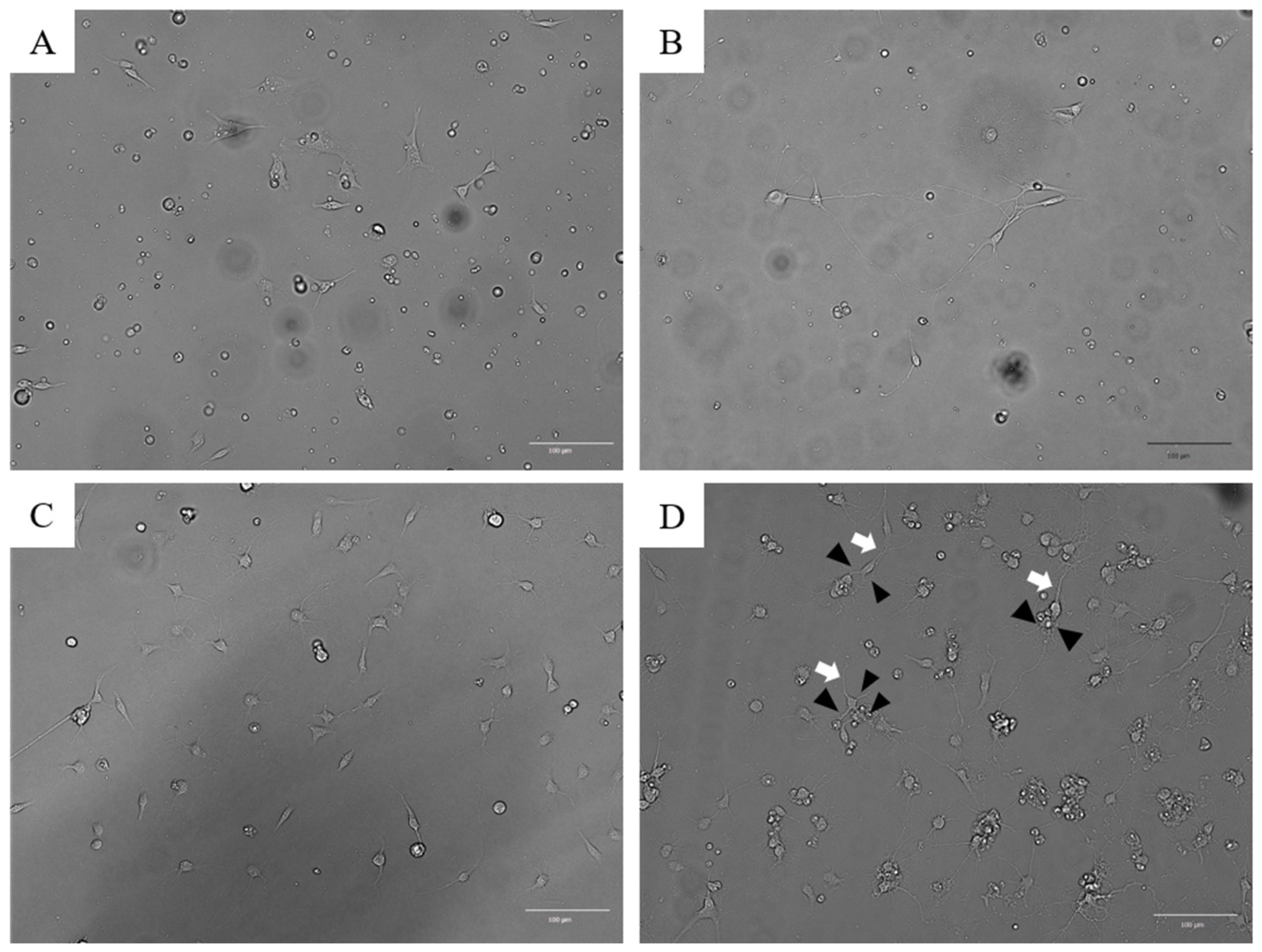
3.1. Establishment of PTGCs
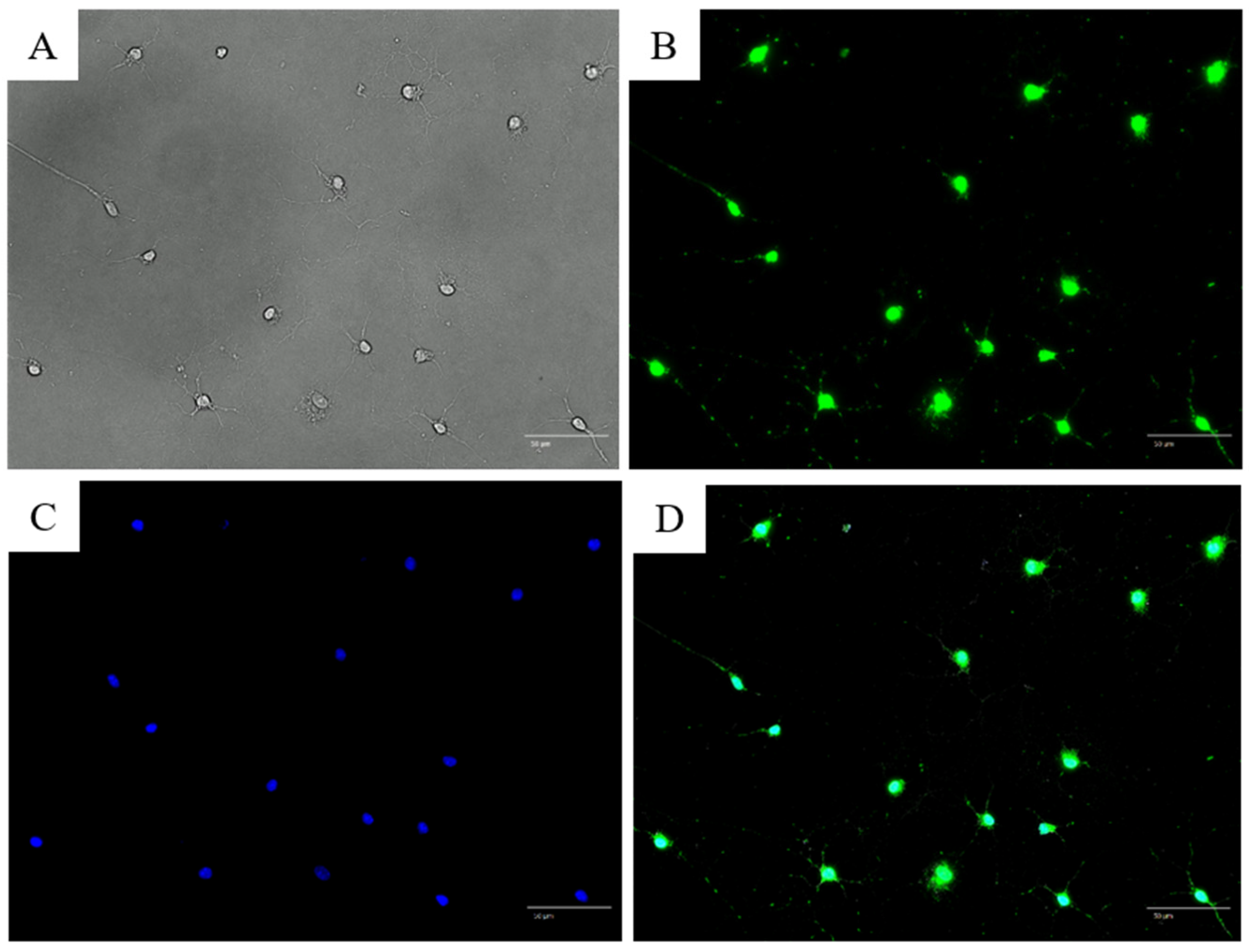
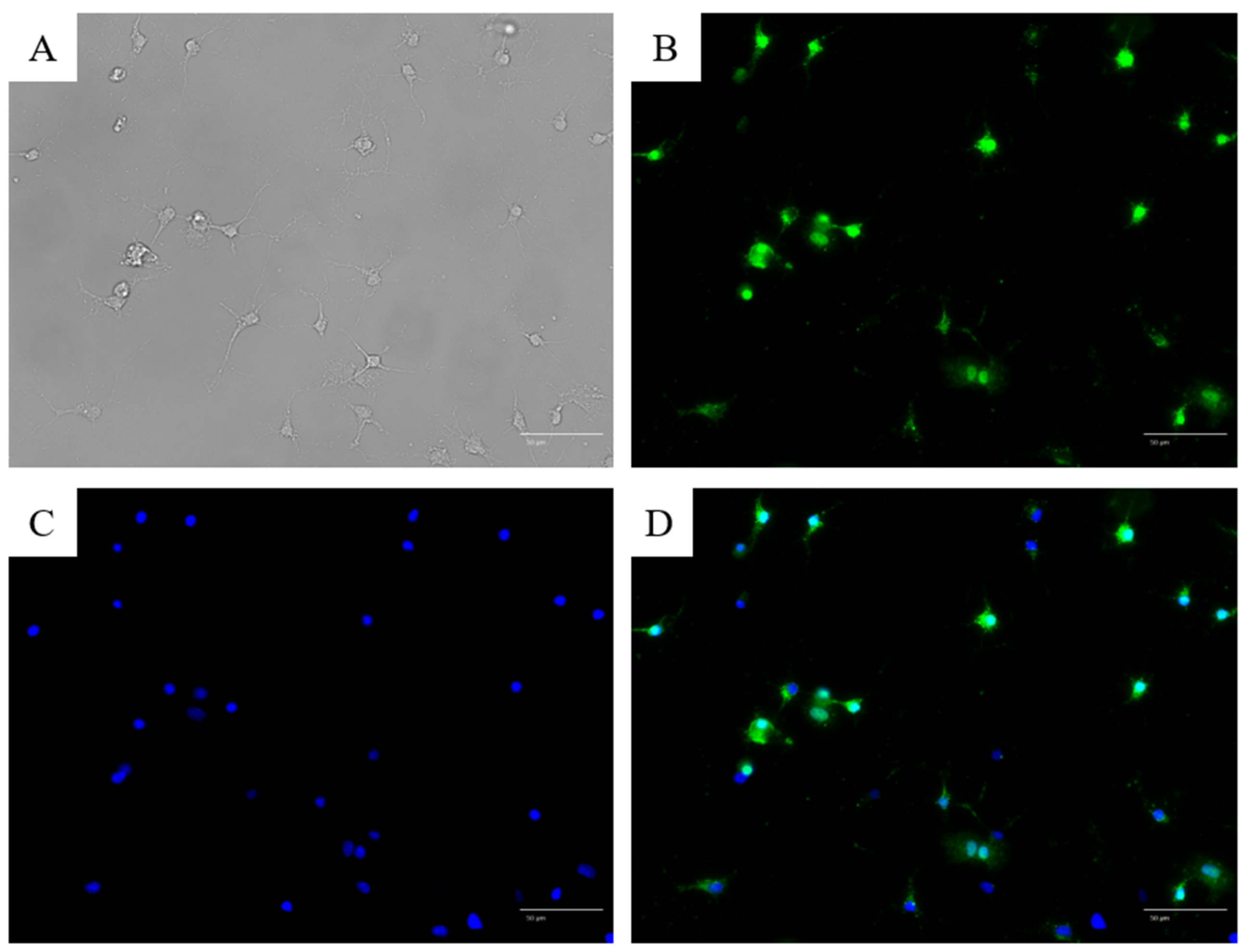
3.2. Immunofluorescence Staining with Anti-NeuN and Anti-TRPV1 Antibodies
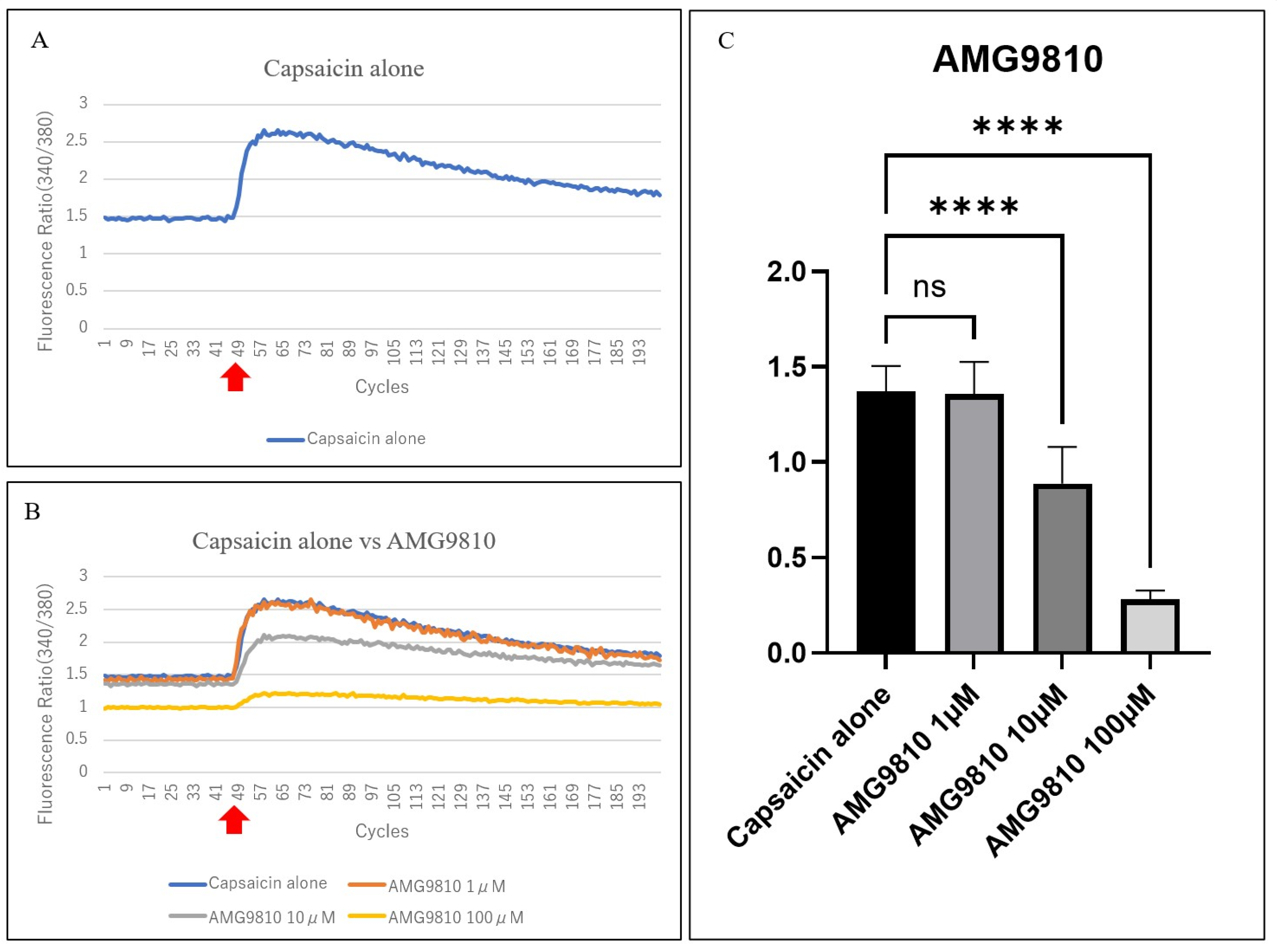
3.3. Calcium Imaging of Primary Trigeminal Ganglion-Derived Cultured Cells (TRGCs)
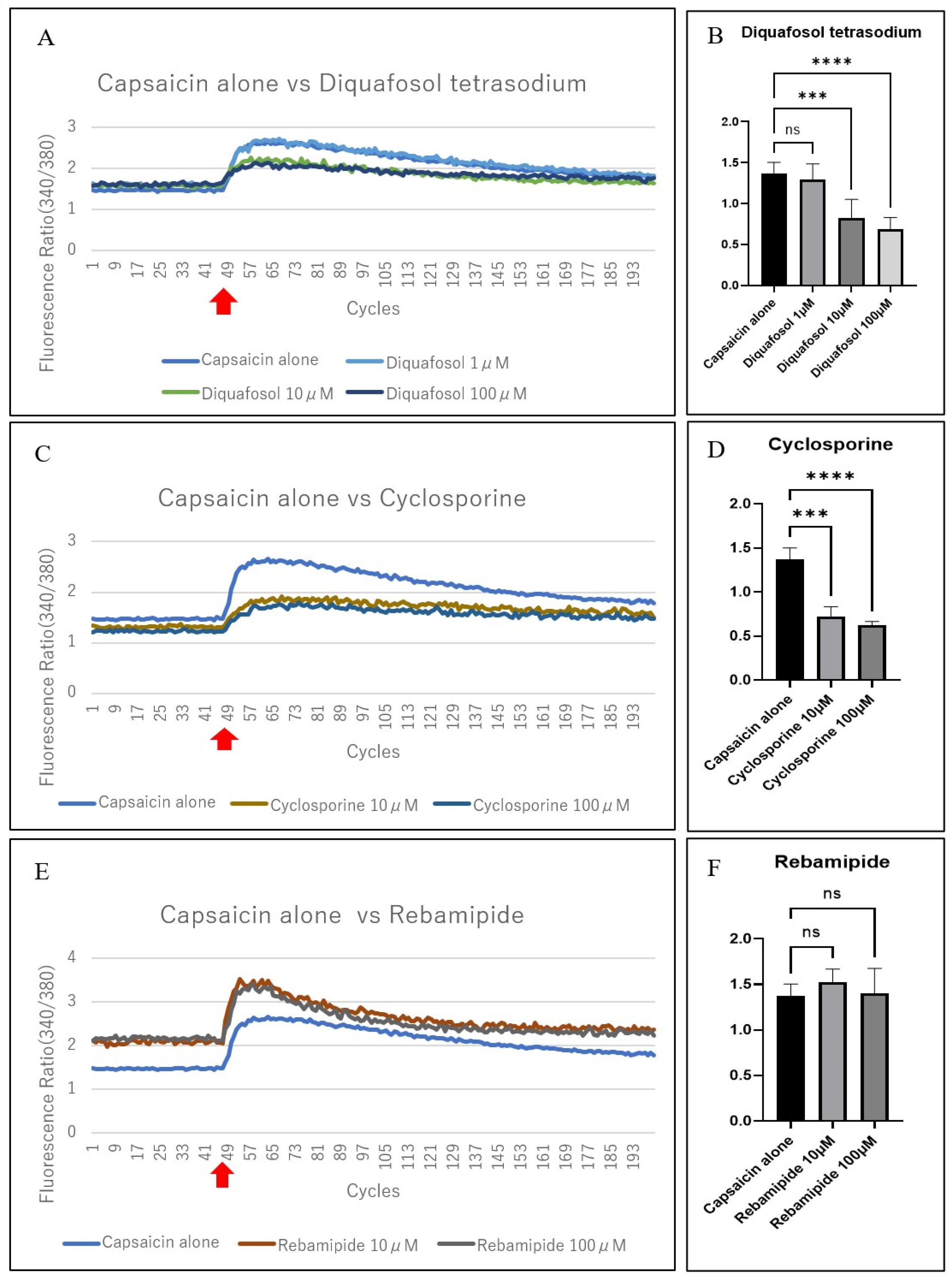
3.4. Evaluation of the Suppression of Capsaicin-Related Excitation in Primary TRGCs with Commercially Available Dry Eye Medications
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTGCs | Primary trigeminal ganglion cell cultures |
| TRGCs | Trigeminal ganglion-derived cells |
| TRPV1 | transient receptor potential vanilloid 1 |
References
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N.; et al. TFOS DEWS II introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Acosta, M.C.; Borderie, V.; Borrás, F.; Gallar, J.; Bury, T.; Laroche, L.; Belmonte, C. Decreased corneal sensitivity in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2341–2345. [Google Scholar] [CrossRef]
- Yang, A.Y.; Chow, J.; Liu, J. Corneal innervation and sensation: The eye and beyond. Yale J. Biol. Med. 2018, 91, 13–21. [Google Scholar]
- Belmonte, C.; Viana, F. Molecular and cellular limits to somatosensory specificity. Mol. Pain 2008, 4, 1744–8069. [Google Scholar] [CrossRef]
- Schöbel, N.; Radtke, D.; Lübbert, M.; Gisselmann, G.; Lehmann, R.; Cichy, A.; Schreiner, B.S.P.; Altmüller, J.; Spector, A.C.; Spehr, J.; et al. Trigeminal ganglion neurons of mice show intracellular chloride accumulation and chloride-dependent amplification of capsaicin-induced responses. PLoS ONE 2012, 7, e48005. [Google Scholar] [CrossRef]
- Rossi, H.L.; Broadhurst, K.A.; Luu, A.S.K.; Lara, O.; Kothari, S.D.; Mohapatra, D.P.; Recober, A. Abnormal trigeminal sensory processing in obese mice. Pain 2016, 157, 235–246. [Google Scholar] [CrossRef][Green Version]
- Launay, P.S.; Reboussin, E.; Liang, H.; Kessal, K.; Godefroy, D.; Rostene, W.; Sahel, J.A.; Baudouin, C.; Melik Parsadaniantz, S.; Le Goazigo, A.R. Ocular inflammation induces trigeminal pain, peripheral and central neuroinflammatory mechanisms. Neurobiol. Dis. 2016, 88, 16–28. [Google Scholar] [CrossRef]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Danielson, P.; Zhou, Q. Corneal epithelium-derived neurotrophic factors promote nerve regeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4695–4702. [Google Scholar] [CrossRef]
- Hu, J.; Hu, X.; Kan, T. MiR-34c participates in diabetic corneal neuropathy via regulation of autophagy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Im, S.T.; Jo, Y.Y.; Han, G.; Jo, H.J.; Kim, Y.H.; Park, C.K. Dexmedetomidine inhibits voltage-gated sodium channels via α2-adrenoceptors in trigeminal ganglion neurons. Mediat. Inflamm. 2018, 2018, 1782719. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.N.; Warwick, R.; Duroux, M.; Hanani, M.; Gazerani, P. Oxaliplatin enhances gap junction-mediated coupling in cell cultures of mouse trigeminal ganglia. Exp. Cell Res. 2015, 336, 94–99. [Google Scholar] [CrossRef]
- Kayama, Y.; Shibata, M.; Takizawa, T.; Ibata, K.; Shimizu, T.; Ebine, T.; Toriumi, H.; Yuzaki, M.; Suzuki, N. Functional interactions between transient receptor potential M8 and transient receptor potential V1 in the trigeminal system: Relevance to migraine pathophysiology. Cephalalgia 2018, 38, 833–845. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, Y.P.; Hou, Z.; Huang, C.; Zhu, H.; Zhang, C.Q.; Yin, Q. Novel insights into NeuN: From neuronal marker to splicing regulator. Mol. Neurobiol. 2016, 53, 1637–1647. [Google Scholar] [CrossRef]
- Dredge, B.K.; Jensen, K.B. NeuN/Rbfox3 nuclear and cytoplasmic isoforms differentially regulate alternative splicing and nonsense-mediated decay of Rbfox2. PLoS ONE 2011, 6, e21585. [Google Scholar] [CrossRef]
- Toivari, E.; Manninen, T.; Nahata, A.K.; Jalonen, T.O.; Linne, M.L. Effects of transmitters and amyloid-beta peptide on calcium signals in rat cortical astrocytes: Fura-2AM measurements and stochastic model simulations. PLoS ONE 2011, 6, e17914. [Google Scholar] [CrossRef]
- Takeuchi, K.; Sato, S.I.; Abe, K.; Kimura, M.; Abe, T.A.; Yoshinaga, K.; Inaba, H. Intracellular compartmentalization of fura-2 dye demonstrated by laser-excitation fluorescence microscopy: A problem in measuring cytosolic free calcium concentration using fura-2 fluorescence in vascular smooth muscle cells. Tohoku J. Exp. Med. 1989, 159, 23–35. [Google Scholar] [CrossRef]
- Drummond, I.A.; Lee, A.S.; Resendez, E., Jr.; Steinhardt, R.A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem. 1987, 262, 12801–12805. [Google Scholar] [CrossRef] [PubMed]
- Poenie, M.; Alderton, J.; Steinhardt, R.; Tsien, R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science 1986, 233, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Cortina, P.; Navea, A.; Terencio, M.C.; Diaz-Llopis, M.; Gómez-Lechón, M.J.; Menezo, J.L. Diclofenac sodium and cyclosporin A inhibit human lens epithelial cell proliferation in culture. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 235, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.I. Differential inhibition of cutaneous T-cell-mediated reactions and epidermal cell proliferation by cyclosporin A, FK-506, and rapamycin. J. Investig. Dermatol. 1994, 102, 84–88. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shim, M.S.; Kim, K.Y.; Weinreb, R.N.; Wheeler, L.A.; Ju, W.K. Inhibition of cyclophilin D by cyclosporin A promotes retinal ganglion cell survival by preventing mitochondrial alteration in ischemic injury. Cell Death Dis. 2014, 5, e1105. [Google Scholar] [CrossRef]
- Ríos, J.D.; Shatos, M.; Urashima, H.; Tran, H.; Dartt, D.A. OPC-12759 increases proliferation of cultured rat conjunctival goblet cells. Cornea 2006, 25, 573–581. [Google Scholar] [CrossRef]
- Suzuki, M.; Miura, S.; Mori, M.; Kai, A.; Suzuki, H.; Fukumura, D.; Suematsu, M.; Tsuchiya, M. Rebamipide, a novel antiulcer agent, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut 1994, 35, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.D.; Shatos, M.A.; Urashima, H.; Dartt, D.A. Effect of OPC-12759 on EGF receptor activation, p44/p42 MAPK activity, and secretion in conjunctival goblet cells. Exp. Eye Res. 2008, 86, 629–636. [Google Scholar] [CrossRef]
- Kim, C.D.; Kim, H.H.; Hong, K.W. Inhibitory effect of rebamipide on the neutrophil adherence stimulated by conditioned media from Helicobacter pylori-infected gastric epithelial cells. J. Pharmacol. Exp. Ther. 1999, 288, 133–138. [Google Scholar] [CrossRef]
- Kwon, S.G.; Roh, D.H.; Yoon, S.Y.; Moon, J.Y.; Choi, S.R.; Choi, H.S.; Kang, S.Y.; Han, H.J.; Beitz, A.J.; Oh, S.B.; et al. Acid evoked thermal hyperalgesia involves peripheral P2Y1 receptor mediated TRPV1 phosphorylation in a rodent model of thrombus induced ischemic pain. Mol. Pain 2014, 10, 2. [Google Scholar] [CrossRef]
- Huang, R.; Wang, F.; Yang, Y.; Ma, W.; Lin, Z.; Cheng, N.; Long, Y.; Deng, S.; Li, Z. Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. FEBS Open Bio 2019, 9, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Jian, T.; Yang, N.; Yang, Y.; Zhu, C.; Yuan, X.; Yu, G.; Wang, C.; Wang, Z.; Shi, H.; Tang, M.; et al. TRPV1 and PLC participate in histamine H4 receptor-induced itch. Neural Plast. 2016, 2016, 1682972. [Google Scholar] [CrossRef] [PubMed]
- Saloman, J.L.; Chung, M.K.; Ro, J.Y. P2X3 and TRPV1 functionally interact and mediate sensitization of trigeminal sensory neurons. Neuroscience 2013, 232, 226–238. [Google Scholar] [CrossRef]
- Wang, S.; Brigoli, B.; Lim, J.; Karley, A.; Chung, M.K. Roles of TRPV1 and TRPA1 in spontaneous pain from inflamed masseter Muscle. Neuroscience 2018, 384, 290–299. [Google Scholar] [CrossRef]
- Obi, S.; Nakajima, T.; Hasegawa, T.; Kikuchi, H.; Oguri, G.; Takahashi, M.; Nakamura, F.; Yamasoba, T.; Sakuma, M.; Toyoda, S.; et al. Heat induces interleukin-6 in skeletal muscle cells via TRPV1/PKC/CREB pathways. J. Appl. Physiol. 2017, 122, 683–694. [Google Scholar] [CrossRef]
- Takayama, Y.; Uta, D.; Furue, H.; Tominaga, M. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 5213–5218. [Google Scholar] [CrossRef]
- Kaido, M.; Kawashima, M.; Ishida, R.; Tsubota, K. Relationship of corneal pain sensitivity with dry eye symptoms in dry eye with short tear break-up time. Investig. Ophthalmol. Vis. Sci. 2016, 57, 914–919. [Google Scholar] [CrossRef]
- Yamane, M.; Ogawa, Y.; Fukui, M.; Kamoi, M.; Saijo-Ban, Y.; Yaguchi, S.; Mukai, S.; Kawakita, T.; Simmura, S.; Tsubota, K. Long-term rebamipide and diquafosol in two cases of immune-mediated dry eye. Optom. Vis. Sci. 2015, 92 (Suppl. S1), S25–S32. [Google Scholar] [CrossRef]
- Byun, Y.S.; Yoo, Y.S.; Kwon, J.Y.; Joo, J.S.; Lim, S.A.; Whang, W.J.; Mok, J.W.; Choi, J.S.; Joo, C.K. Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp. Eye Res. 2016, 143, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, T.; Ohnishi, T.; Ishimori, A. Significance of ectodomain shedding of TNF receptor 1 in ocular surface. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2419–2423. [Google Scholar] [CrossRef] [PubMed]
- Camlar, S.A.; Soylu, A.; Kavukçu, S. Cyclosporine in pediatric nephrology. Iran J. Kidney Dis. 2018, 12, 319–330. [Google Scholar]
- Fakih, D.; Migeon, T.; Moreau, N.; Baudouin, C.; Goazigo, A.R.-L.; Parsadaniantz, S.M. Transient receptor potential channels: Important players in ocular pain and dry eye disease. Pharmaceutics 2022, 14, 1859. [Google Scholar] [CrossRef]
- Ma, L.; Yang, L.; Wang, X.; Zhao, L.; Bai, X.; Qi, X.; Chen, Q.; Li, Y.; Zhou, Q. CGRP released by corneal sensory nerve maintains tear secretion of the lacrimal gland. Investig. Ophthalmol. Vis. Sci. 2024, 65, 30. [Google Scholar] [CrossRef]
- Shinomiya, K.; Ueta, M.; Kinoshita, S. A new dry eye mouse model produced by exorbital and intraorbital lacrimal gland excision. Sci. Rep. 2018, 8, 1483. [Google Scholar] [CrossRef] [PubMed]
- Kaido, M.; Inoue, S.; Kawashima, M.; Ishida, R.; Nakamura, S.; Tsubota, K. Capsaicin-induced pain sensitivity in short tear break-up time dry eye. Ocul. Surf. 2020, 18, 620–626. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunouchi, C.; Hayashi, T.; Yamagami, S.; Sakimoto, T. Evaluation of the Effects of Eye Drops for Dry Eyes on Neuronal Pain Receptors in a Primary Culture Model of Trigeminal Ganglion Cells. J. Clin. Med. 2025, 14, 8038. https://doi.org/10.3390/jcm14228038
Sunouchi C, Hayashi T, Yamagami S, Sakimoto T. Evaluation of the Effects of Eye Drops for Dry Eyes on Neuronal Pain Receptors in a Primary Culture Model of Trigeminal Ganglion Cells. Journal of Clinical Medicine. 2025; 14(22):8038. https://doi.org/10.3390/jcm14228038
Chicago/Turabian StyleSunouchi, Chihiro, Takahiko Hayashi, Satoru Yamagami, and Tohru Sakimoto. 2025. "Evaluation of the Effects of Eye Drops for Dry Eyes on Neuronal Pain Receptors in a Primary Culture Model of Trigeminal Ganglion Cells" Journal of Clinical Medicine 14, no. 22: 8038. https://doi.org/10.3390/jcm14228038
APA StyleSunouchi, C., Hayashi, T., Yamagami, S., & Sakimoto, T. (2025). Evaluation of the Effects of Eye Drops for Dry Eyes on Neuronal Pain Receptors in a Primary Culture Model of Trigeminal Ganglion Cells. Journal of Clinical Medicine, 14(22), 8038. https://doi.org/10.3390/jcm14228038





