Optimal Segment Selection on Gadoxetic Acid-Enhanced MRI to Improve Diagnostic Accuracy in the Histological Grading of Liver Inflammation and Fibrosis in Patients with Chronic Hepatitis B
Abstract
1. Introduction
2. Materials and Methods
2.1. MRI
2.2. Image Analysis and Biopsy Procedure
2.3. Statistical Analysis
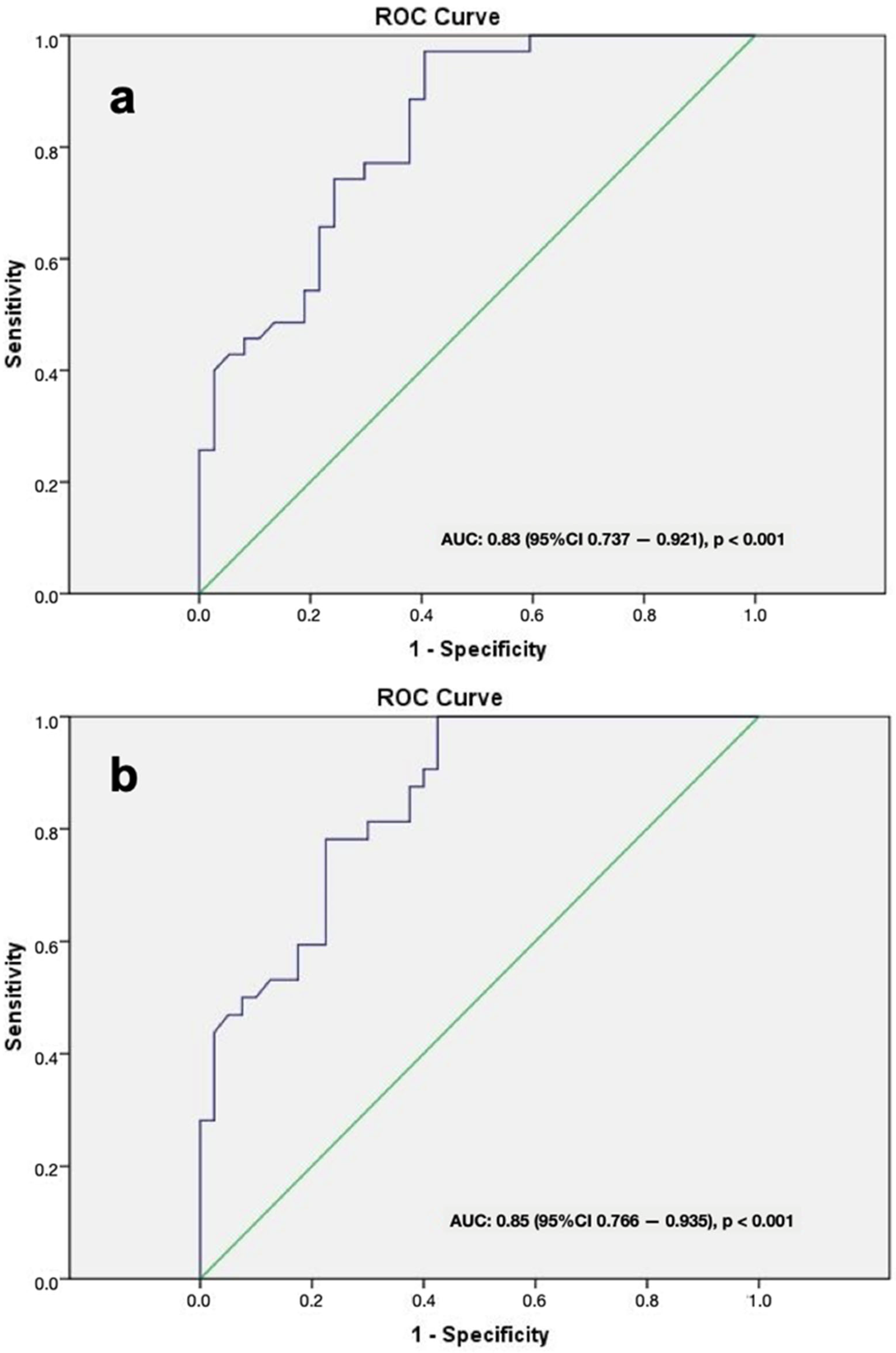
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHB | Chronic hepatitis B |
| HCC | Hepatocellular carcinoma |
| US | Ultrasound |
| MRE | Magnetic resonance elastography |
| ADC | Apparent diffusion coefficient |
| DWI | Diffusion-weighted imaging |
| GA | Gadoxetic acid |
| MRI | Magnetic resonance imaging |
| CEI | Contrasted enhancement index |
| ROI | Region of interest |
| SI | Signal intensity |
| HBP | Hepatobiliary phase |
| IR | Interventional radiology |
| mHAI | Modified histologic activity index |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| PT | Prothrombin time |
| APTT | Activated partial thromboplastin time |
| APRI | Aspartate aminotransferase (AST)-to-platelet ratio index |
| FIB-4 | Fibrosis-4 |
| SWE | Shear wave elastography |
| OATP1B1 and OATP1B3 | Organic anion transporting polypeptide 1 and 3 |
| MRP2 | Multidrug resistance protein 2 |
| RLE | Relative liver enhancement |
References
- Yang, L.; Zhou, G.; Liu, L.; Rao, S.; Wang, W.; Jin, K.; Wu, F.; Fu, C.; Zeng, M.; Ding, Y. Staging Chronic Hepatitis B Related Liver Fibrosis with Diffusion-weighted MRI-based Virtual Elastography: Comparisons with Serum Fibrosis Indexes. Clin. Radiol. 2025, 80, 106750. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Huang, D.Q.; Nguyen, M.H. Global burden of hepatitis B virus: Current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 524–537. [Google Scholar] [CrossRef]
- Watanabe, H.; Kanematsu, M.; Goshima, S.; Kondo, H.; Onozuka, M.; Moriyama, N.; Bae, K.T. Staging hepatic fibrosis: Comparison of gadoxetate disodium–enhanced and diffusion-weighted MR imaging—preliminary observations. Radiology 2011, 259, 142–150. [Google Scholar] [CrossRef]
- Lee, G.M.; Kim, Y.R.; Ryu, J.H.; Kim, T.H.; Cho, E.Y.; Lee, Y.H.; Yoon, K.H. Quantitative Measurement of Hepatic Fibrosis with Gadoxetic Acid-Enhanced Magnetic Resonance Imaging in Patients with Chronic Hepatitis B Infection: A Comparative Study on Aspartate Aminotransferase to Platelet Ratio Index and Fibrosis-4 Index. Korean J. Radiol. 2017, 18, 444–451. [Google Scholar] [CrossRef]
- Poetter-Lang, S.; Bastati, N.; Messner, A.; Kristic, A.; Herold, A.; Hodge, J.C.; Ba-Ssalamah, A. Quantification of liver function using gadoxetic acid-enhanced MRI. Abdom. Radiol. 2020, 45, 3532–3544. [Google Scholar] [CrossRef]
- Smith, A.D.; Porter, K.K.; Elkassem, A.A.; Sanyal, R.; Lockhart, M.E. Current Imaging Techniques for Noninvasive Staging of Hepatic Fibrosis. Am. J. Roentgenol. 2019, 213, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Schaapman, J.J.; Tushuizen, M.E.; Coenraad, M.J.; Lamb, H.J. Multiparametric MRI in Patients With Nonalcoholic Fatty Liver Disease. J. Magn. Reson. Imaging 2021, 53, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Suyama, Y.; Tomita, K.; Soga, S.; Kuwamura, H.; Murakami, W.; Hokari, R.; Shinmoto, H. T1ρ magnetic resonance imaging value as a potential marker to assess the severity of liver fibrosis: A pilot study. Eur. J. Radiol. Open 2021, 8, 100321. [Google Scholar] [CrossRef]
- Huang, J.; Leporq, B.; Hervieu, V.; Dumortier, J.; Beuf, O.; Ratiney, H. Diffusion-Weighted MRI of the Liver in Patients With Chronic Liver Disease: A Comparative Study Between Different Fitting Approaches and Diffusion Models. J. Magn. Reson. Imaging 2024, 59, 894–906. [Google Scholar] [CrossRef]
- Huang, P.; Wu, F.; Hou, K.; Zhou, C.; Xiao, Y.; Wang, C.; Miao, G.; Yang, C.; Zeng, M. Diagnostic algorithm for subcentimeter hepatocellular carcinoma using alpha-fetoprotein and imaging features on gadoxetic acid–enhanced MRI. Eur. Radiol. 2024, 34, 2271–2282. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Prince, M.; Wang, F.; Sun, J.; Yang, X.; Wang, W.; Ye, J.; Chen, L.; Luo, X. Gd-EOB-DTPA enhanced MRI based radiomics combined with clinical variables in stratifying hepatic functional reserve in HBV infected patients. Abdom. Radiol. 2024, 49, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Verloh, N.; Probst, U.; Utpatel, K.; Zeman, F.; Brennfleck, F.; Werner, J.M.; Fellner, C.; Stroszczynski, C.; Evert, M.; Wiggermann, P.; et al. Influence of hepatic fibrosis and inflammation: Correlation between histopathological changes and Gd-EOB-DTPA-enhanced MR imaging. PLoS ONE 2019, 14, e0215752. [Google Scholar] [CrossRef] [PubMed]
- Verloh, N.; Utpatel, K.; Haimerl, M.; Zeman, F.; Beyer, L.; Fellner, C.; Brennfleck, F.; Dahlke, M.H.; Stroszczynski, C.; Evert, M.; et al. Detecting liver fibrosis with Gd-EOB-DTPA-enhanced MRI: A confirmatory study. Sci. Rep. 2018, 8, 6207. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Adams, L.A.; Bulsara, M.; Jeffrey, G.P. Assessing liver fibrosis with serum marker models. Clin. Biochem. Rev. 2007, 28, 3–10. [Google Scholar] [PubMed] [PubMed Central]
- Guido, M.; Rugge, M. Liver biopsy sampling in chronic viral hepatitis. Semin. Liver Dis. 2004, 24, 89–97. [Google Scholar] [CrossRef]
- Chi, H.; Hansen, B.E.; Tang, W.Y.; Schouten, J.N.; Sprengers, D.; Taimr, P.; Janssen, H.L.; de Knegt, R.J. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur. J. Gastroenterol. Hepatol. 2017, 29, 36–41. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Hako, R.; Kristian, P.; Jarčuška, P.; Haková, I.; Hockicková, I.; Schréter, I.; Janičko, M. Noninvasive Assessment of Liver Fibrosis in Patients with Chronic Hepatitis B or C by Contrast-Enhanced Magnetic Resonance Imaging. Can. J. Gastroenterol. Hepatol. 2019, 2019, 3024630. [Google Scholar] [CrossRef]
- Verloh, N.; Utpatel, K.; Haimerl, M.; Zeman, F.; Fellner, C.; Fichtner-Feigl, S.; Teufel, A.; Stroszczynski, C.; Evert, M.; Wiggermann, P. Liver fibrosis and Gd-EOB-DTPA-enhanced MRI: A histopathologic correlation. Sci. Rep. 2015, 5, 15408. [Google Scholar] [CrossRef]
- Hayashi, T.; Saitoh, S.; Fukuzawa, K.; Tsuji, Y.; Takahashi, J.; Kawamura, Y.; Akuta, N.; Kobayashi, M.; Ikeda, K.; Fujii, T.; et al. Noninvasive Assessment of Advanced Fibrosis Based on Hepatic Volume in Patients with Nonalcoholic Fatty Liver Disease. Gut Liver 2017, 11, 674–683. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Huang, W. A noninvasive model to predict liver histology for antiviral therapy decision in chronic hepatitis B with alanine aminotransferase < 2 upper limit of normal. BMC Gastroenterol. 2021, 21, 4. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Ding, H.; Fu, T.T.; Peng, S.Y.; Chen, S.Y.; Luo, J.J.; Wang, W.P. Portal hypertension in hepatitis B-related cirrhosis: Diagnostic accuracy of liver and spleen stiffness by 2-D shear-wave elastography. Hepatol. Res. 2019, 49, 540–549. [Google Scholar] [CrossRef]
- Jianping, D.; Xi, C.; Guangwen, C.; Fankun, M.; Ying, Z.; Bulin, Z.; Wei, Z.; Yao, Z.; Zhiyong, Y.; Hong, Y.; et al. Dual elastography to discriminate adjacent stages of fibrosis and inflammation in chronic hepatitis B: A prospective multicenter study. Hepatology 2024, 79, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Peng, J.Y.; Long, H.Y.; Zhong, X.; Xie, Y.H.; Yao, L.; Xie, X.Y.; Lin, M.X. Liver stiffness in hepatocellular carcinoma and chronic hepatitis patients: Hepatitis B virus infection and transaminases should be considered. World J. Hepatol. 2024, 16, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, N.; Okada, M.; Murakami, T. New proposal for the staging of nonalcoholic steatohepatitis: Evaluation of liver fibrosis on Gd-EOB-DTPA-enhanced MRI. Eur. J. Radiol. 2010, 73, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Choi, J.Y.; Park, C.H.; Song, M.J.; Song, D.S.; Kim, C.W.; Bae, S.H.; Yoon, S.K.; Lee, Y.J.; Rha, S.E. Clinical factors predictive of insufficient liver enhancement on the hepatocyte-phase of Gd-EOB-DTPA-enhanced magnetic resonance imaging in patients with liver cirrhosis. J. Gastroenterol. 2013, 48, 1180–1187. [Google Scholar] [CrossRef]
- Kanki, A.; Tamada, T.; Higaki, A.; Noda, Y.; Tanimoto, D.; Sato, T.; Higashi, H.; Ito, K. Hepatic parenchymal enhancement at Gd-EOB-DTPA-enhanced MR imaging: Correlation with morphological grading of severity in cirrhosis and chronic hepatitis. Magn. Reson. Imaging 2012, 30, 356–360. [Google Scholar] [CrossRef]
- Verloh, N.; Haimerl, M.; Rennert, J.; Müller-Wille, R.; Nießen, C.; Kirchner, G.; Scherer, M.N.; Schreyer, A.G.; Stroszczynski, C.; Fellner, C.; et al. Impact of liver cirrhosis on liver enhancement at Gd-EOB-DTPA enhanced MRI at 3 Tesla. Eur. J. Radiol. 2013, 82, 1710–1715. [Google Scholar] [CrossRef]
- Yamada, T.; Obata, A.; Kashiwagi, Y.; Rokugawa, T.; Matsushima, S.; Hamada, T.; Watabe, H.; Abe, K. Gd-EOB-DTPA-enhanced-MR imaging in the inflammation stage of nonalcoholic steatohepatitis (NASH) in mice. Magn. Reson. Imaging 2016, 34, 724–729. [Google Scholar] [CrossRef]
- Bruce, R.; Wentland, A.L.; Haemel, A.K.; Garrett, R.W.; Sadowski, D.R.; Djamali, A.; Sadowski, E.A. Incidence of Nephrogenic Systemic Fibrosis Using Gadobenate Dimeglumine in 1423 Patients With Renal Insufficiency Compared With Gadodiamide. Investig. Radiol. 2016, 51, 701–705. [Google Scholar] [CrossRef]
- Yamada, A.; Hara, T.; Li, F.; Fujinaga, Y.; Ueda, K.; Kadoya, M.; Doi, K. Quantitative evaluation of liver function with use of gadoxetate disodium-enhanced MR imaging. Radiology 2011, 260, 727–733. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, J.M.; Kang, H.J.; Ahn, S.J.; Yang, H.; Kim, E.; Okuaki, T.; Han, J.K. Quantitative Assessment of Liver Function by Using Gadoxetic Acid–enhanced MRI: Hepatocyte Uptake Ratio. Radiology 2019, 290, 125–133. [Google Scholar] [CrossRef]
- Wang, Q.; Kesen, S.; Liljeroth, M.; Nilsson, H.; Zhao, Y.; Sparrelid, E.; Brismar, T.B. Quantitative evaluation of liver function with gadoxetic acid enhanced MRI: Comparison among signal intensity-, T1-relaxometry-, and dynamic-hepatocyte-specific-contrast-enhanced MRI- derived parameters. Scand. J. Gastroenterol. 2022, 57, 705–712. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Nishie, A.; Asayama, Y.; Ishigami, K.; Tajima, T.; Kakihara, D.; Nakayama, T.; Takayama, Y.; Okamoto, D.; Taketomi, A.; Shirabe, K.; et al. MR prediction of liver fibrosis using a liver-specific contrast agent: Superparamagnetic iron oxide versus Gd-EOB-DTPA. J. Magn. Reson. Imaging 2012, 36, 664–671. [Google Scholar] [CrossRef]
- Pandey, N.; Hoilat, G.J.; John, S. Liver Biopsy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025; Bookshelf ID: NBK470567. [Google Scholar] [PubMed]
- Theilig, D.; Elkilany, A.; Schmelzle, M.; Müller, T.; Hamm, B.; Denecke, T.; Geisel, D. Consistency of hepatocellular gadoxetic acid uptake in serial MRI examinations for evaluation of liver function. Abdom. Radiol. 2019, 44, 2759–2768. [Google Scholar] [CrossRef]
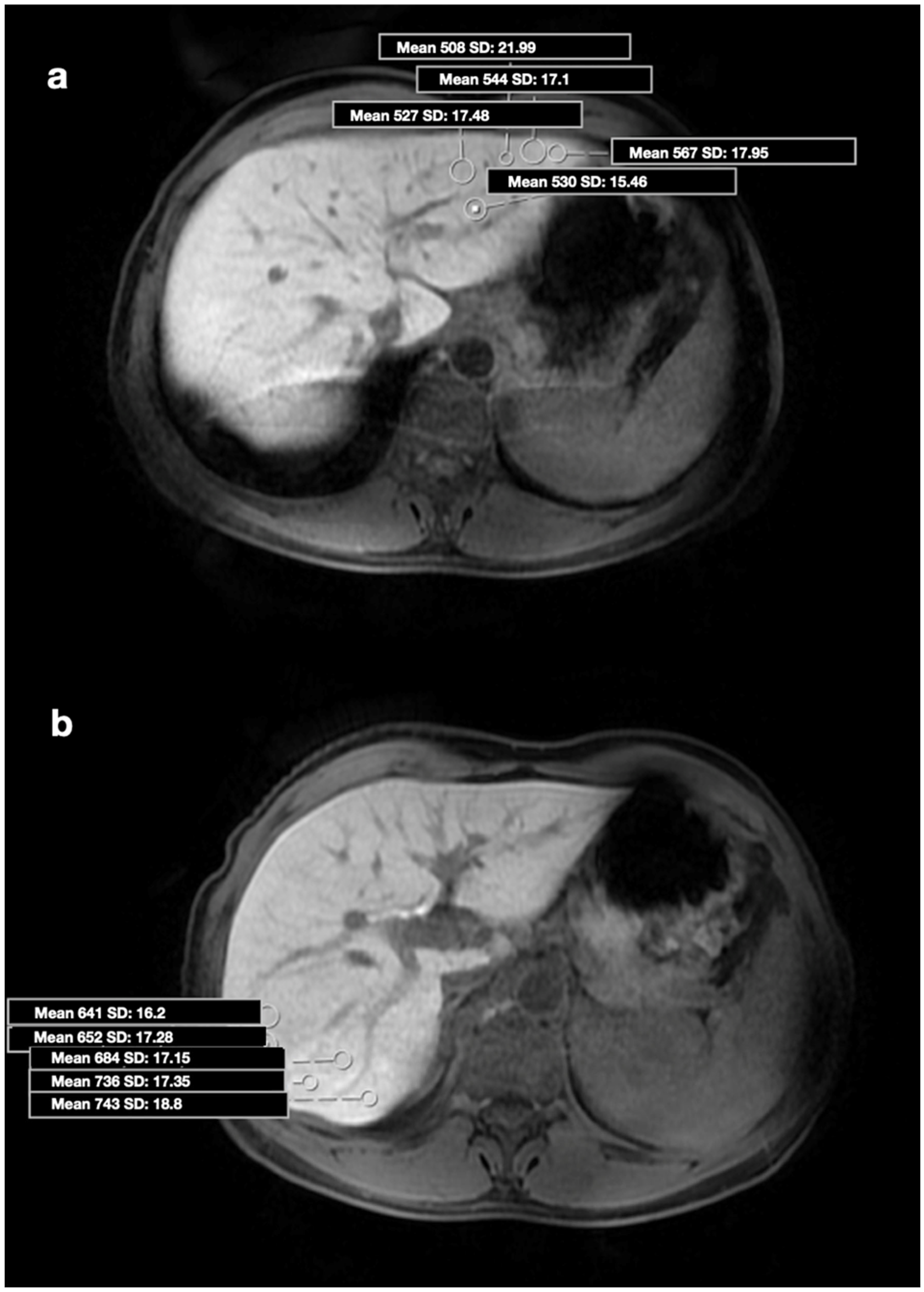
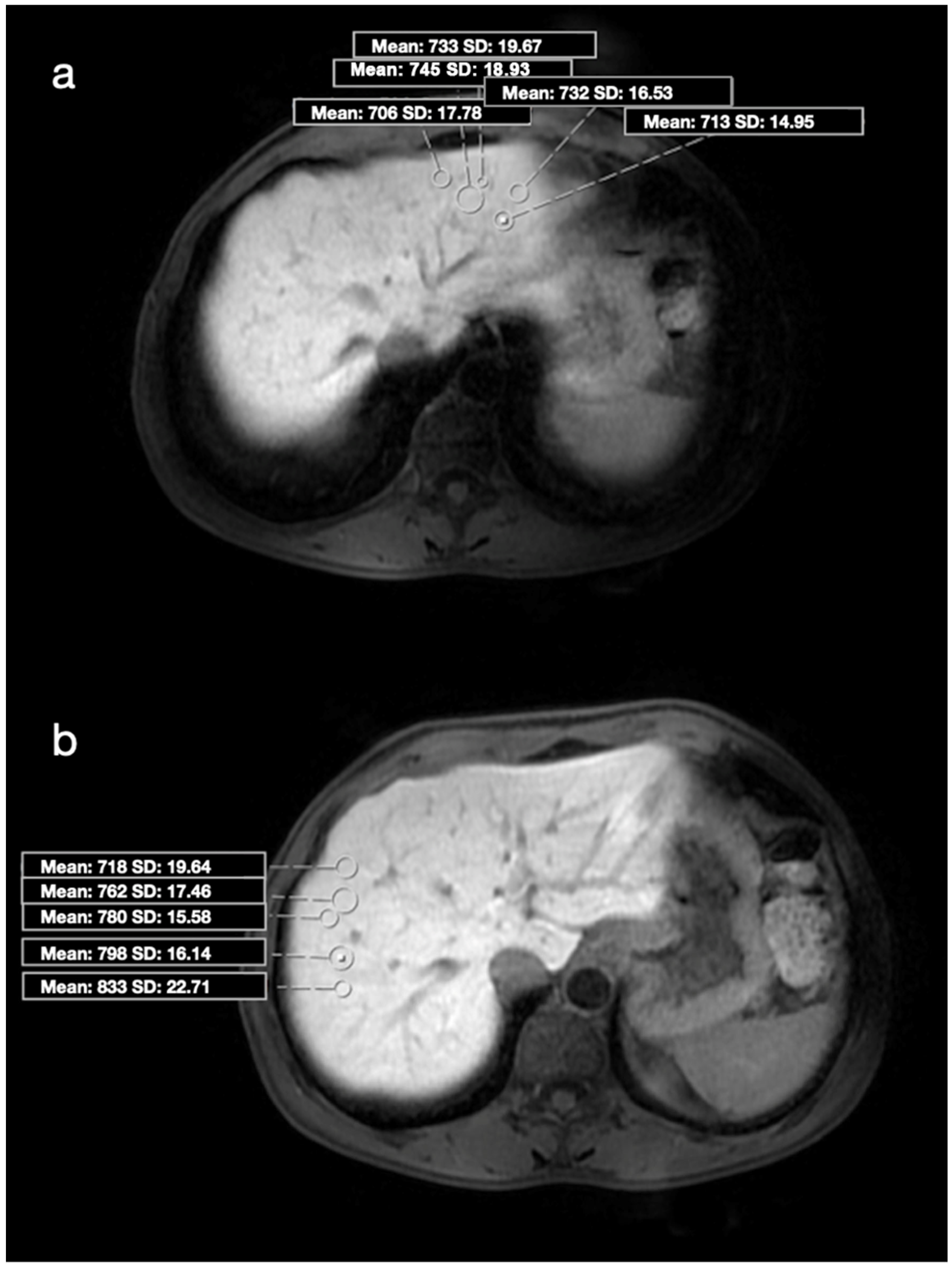
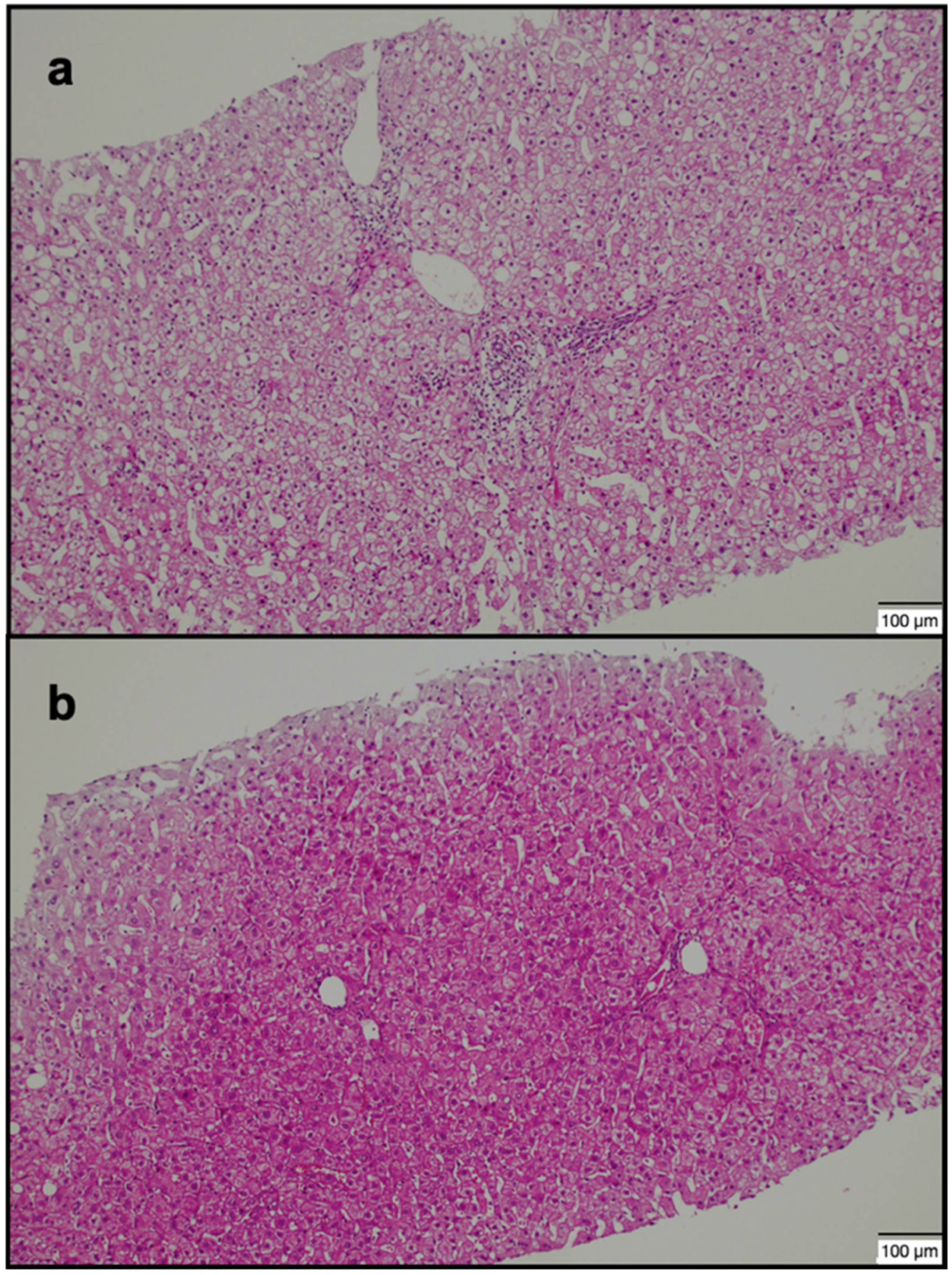
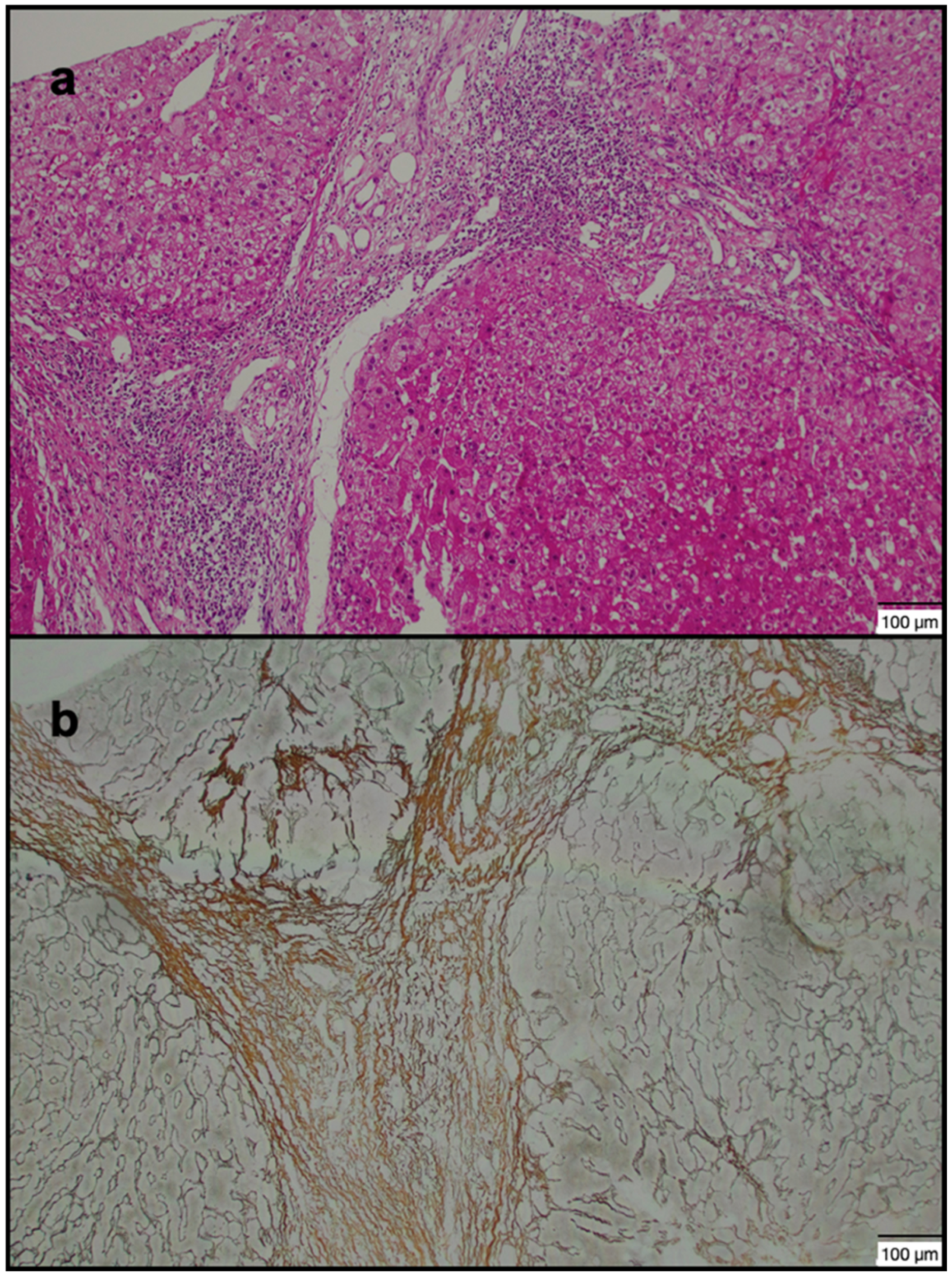
| Group 1 (n = 40) | Group 2 (n = 40) | p-Value | |
|---|---|---|---|
| Liver segment (right) | 31/40 | 22/40 | 0.033 |
| SI, mean (range) | 710.1 ± 231.9 (323–1592) | 573.2 ± 175.4 (161–1095) | 0.004 |
| mHAI grade (≥5) | 20/40 | 22/40 | 0.654 |
| Fibrosis grade (≥2) | 17/40 | 20/40 | 0.501 |
| mHAI grade, mean (range) | 5.25 ± 2.63 (2–13) | 5.28 ± 2.63 (2–13) | 0.966 |
| Fibrosis grade, mean (range) | 1.70 ± 1.04 (0–5) | 1.75 ± 1.06 (0–5) | 0.832 |
| SI < 617 (n = 40) | SI > 617 (n = 40) | p-Value | |
|---|---|---|---|
| Liver segment (right) | 24/40 | 29/40 | 0.237 |
| SI, mean | 489.5 ± 100.6 | 793.8 ± 191.1 | <0.001 |
| mHAI grade (≥5) | 30/40 | 12/40 | <0.001 |
| Fibrosis grade (≥2) | 28/40 | 9/40 | <0.001 |
| mHAI grade, mean | 6.28 ± 2.47 | 4.25 ± 2.37 | <0.001 |
| Fibrosis grade, mean | 2.20 ± 1.09 | 1.25 ± 0.74 | <0.001 |
| Liver Segment | Left | Right |
|---|---|---|
| 2nd | 15 | |
| 3rd | 4 | |
| 4th | 9 | |
| 5th | 8 | |
| 6th | 10 | |
| 7th | 17 | |
| 8th | 17 | |
| Total | 28 | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonen, K.A.; Inecikli, M.F.; Mete, R.; Oznur, M. Optimal Segment Selection on Gadoxetic Acid-Enhanced MRI to Improve Diagnostic Accuracy in the Histological Grading of Liver Inflammation and Fibrosis in Patients with Chronic Hepatitis B. J. Clin. Med. 2025, 14, 8025. https://doi.org/10.3390/jcm14228025
Gonen KA, Inecikli MF, Mete R, Oznur M. Optimal Segment Selection on Gadoxetic Acid-Enhanced MRI to Improve Diagnostic Accuracy in the Histological Grading of Liver Inflammation and Fibrosis in Patients with Chronic Hepatitis B. Journal of Clinical Medicine. 2025; 14(22):8025. https://doi.org/10.3390/jcm14228025
Chicago/Turabian StyleGonen, Korcan Aysun, Mehmet Fatih Inecikli, Rafet Mete, and Meltem Oznur. 2025. "Optimal Segment Selection on Gadoxetic Acid-Enhanced MRI to Improve Diagnostic Accuracy in the Histological Grading of Liver Inflammation and Fibrosis in Patients with Chronic Hepatitis B" Journal of Clinical Medicine 14, no. 22: 8025. https://doi.org/10.3390/jcm14228025
APA StyleGonen, K. A., Inecikli, M. F., Mete, R., & Oznur, M. (2025). Optimal Segment Selection on Gadoxetic Acid-Enhanced MRI to Improve Diagnostic Accuracy in the Histological Grading of Liver Inflammation and Fibrosis in Patients with Chronic Hepatitis B. Journal of Clinical Medicine, 14(22), 8025. https://doi.org/10.3390/jcm14228025






