Global Variations in Surgical Techniques and Postoperative Care for Radial Forearm Free Flap (RFFF) in Head & Neck Surgery: A Cross-Sectional International Survey
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Participants
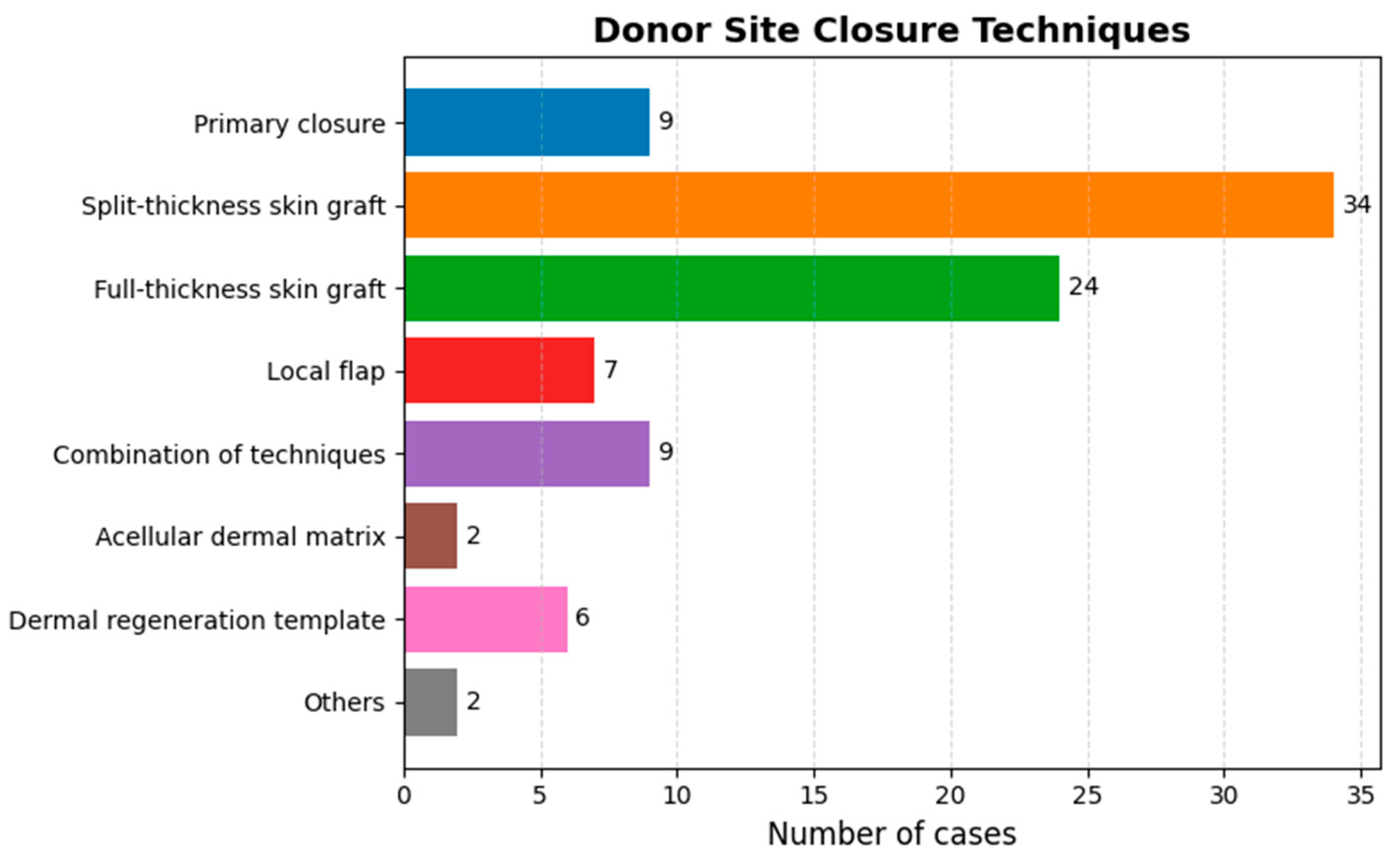
3.2. Harvesting Technique
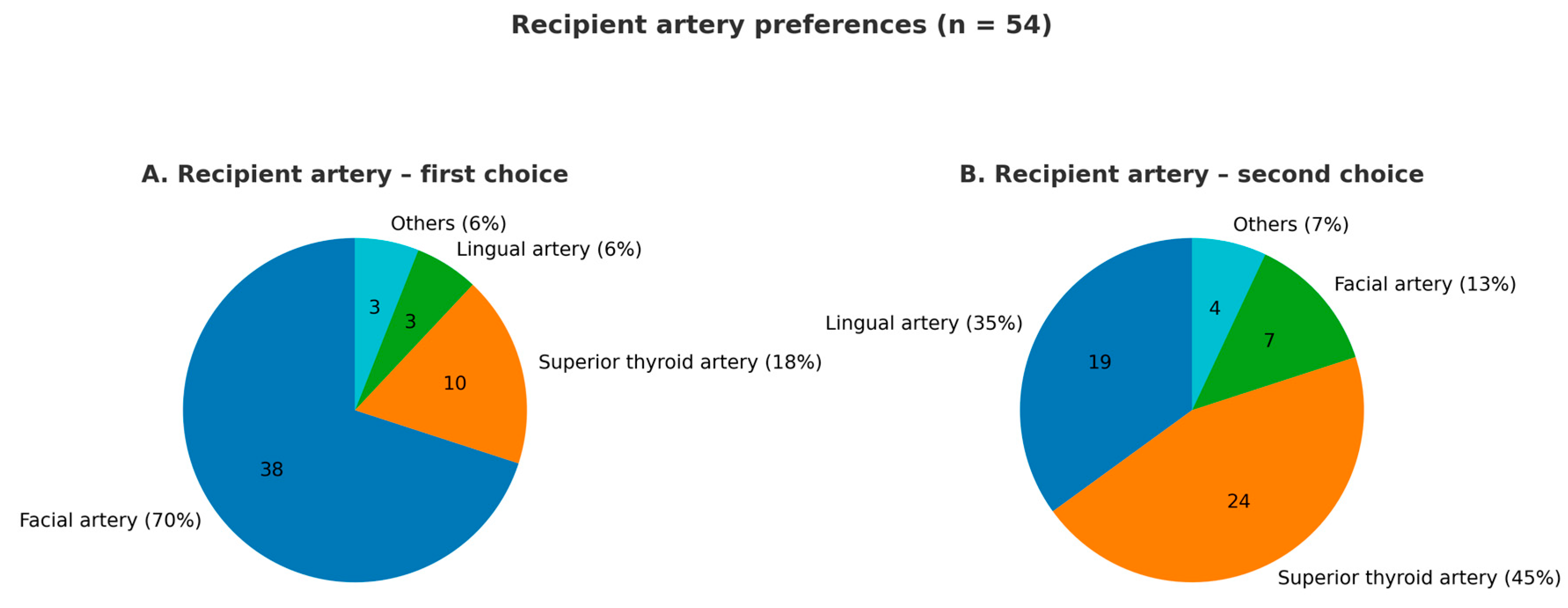
3.3. Microsurgery
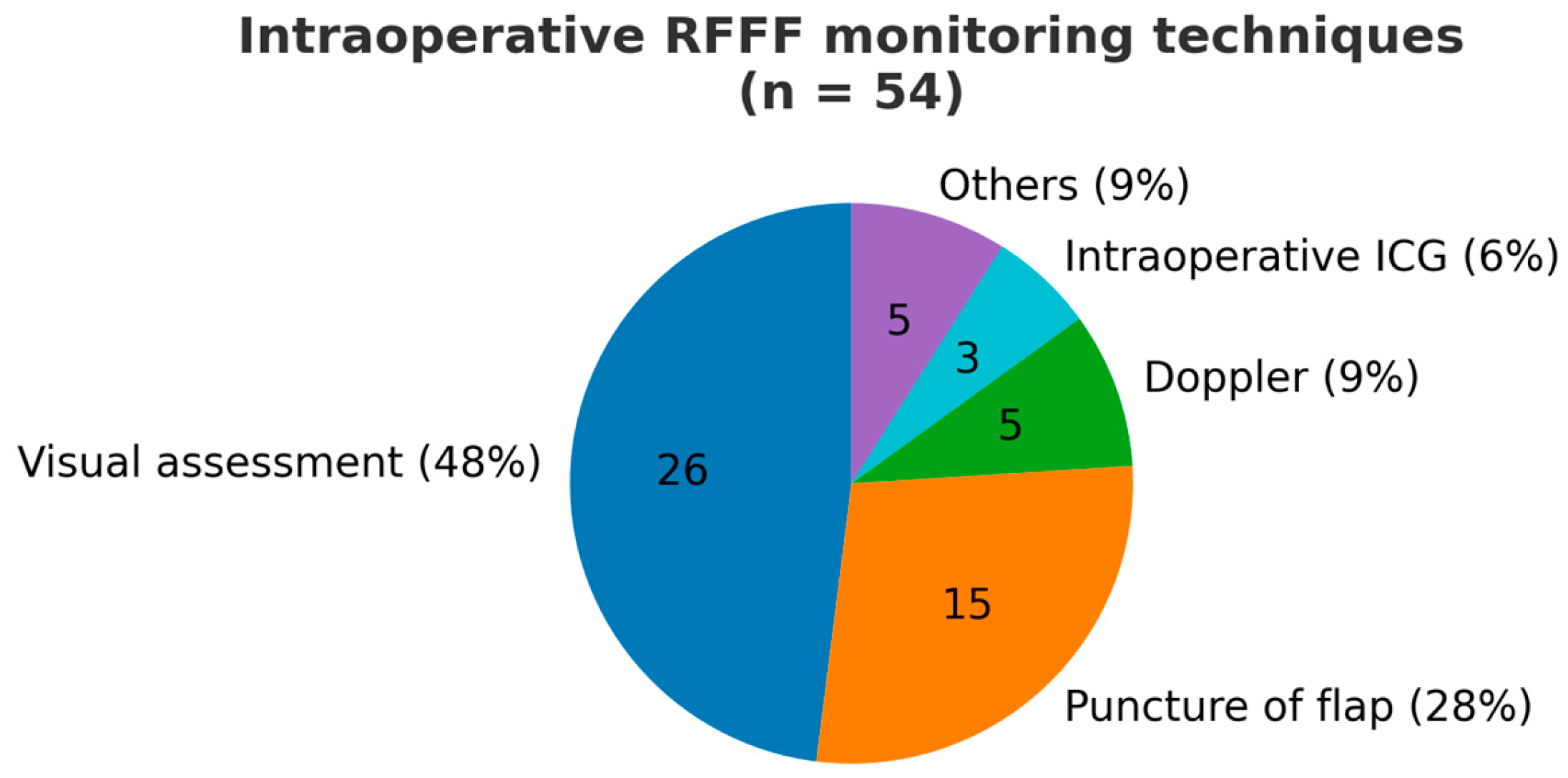
3.4. Postoperative Care and Flap Monitoring Modalities
3.5. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, G.F.; Chen, P.J.; Gao, Y.Z.; Liu, X.Y.; Li, J.; Jiang, S.X.; He, S.P. Forearm free skin flap transplantation: A report of 56 cases. Br. J. Plast. Surg. 1997, 50, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, L.J.; Tachmes, L.; Pielet, R.W. Improved venous drainage of the radial artery forearm free flap: Use of the profundus cubitalis vein. J. Reconstr. Microsurg. 1993, 9, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Razzano, S.; Esposito, L.; Schonauer, F. The venae comitantes clipping test for the evaluation of the venous drainage of the radial forearm free flap. Microsurgery 2016, 36, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Dziegielewski, P.T.; Rieger, J.; Shama, M.A.; O’Connell, D.A.; Harris, J.R.; Seikaly, H. Beavertail modification of the radial forearm free flap in total oral glossectomy reconstruction: Technique and functional outcomes. Oral Oncol. 2019, 96, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.H.; Polat, J.K. Microvascular flap reconstruction by otolaryngologists: Prevalence, postoperative care, and monitoring techniques. Laryngoscope 2007, 117, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Fisher, S.E.; Vaughan, E.D.; Brown, J.S. Radial forearm flap donor-site complications and morbidity: A prospective study. Plast. Reconstr. Surg. 1997, 99, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Couch, M.E.; Huchton, D.M. Assessment of donor-site functional morbidity from radial forearm fasciocutaneous free flap harvest. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Liu, J. Prospective Comparison of Donor-Site Morbidity following Radial Forearm and Ulnar Artery Perforator Flap Harvest. Plast. Reconstr. Surg. 2020, 145, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.G.; DiMercurio, S.; Angel, M. Tissue-expanded radial forearm free flap in neck burn contracture. J. Burn. Care Rehabil. 1990, 11, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.M.; Hou, C.L.; Zhang, F.; Lineaweaver, W.C.; Chen, Z.W.; Gu, Y.D. Distally based radial forearm flap with preservation of the radial artery: Anatomic, experimental, and clinical studies. Microsurgery 2003, 23, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.C.; Choi, M.S.S.; Hwang, W.J.; Sung, K.Y. The transverse radial artery forearm flap. Plast. Reconstr. Surg. 2007, 119, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Mateev, M.A.; Ogawa, R.; Trunov, L.; Moldobaeva, N.; Hyakusoku, H. Shape-modified radial artery perforator flap method: Analysis of 112 cases. Plast. Reconstr. Surg. 2009, 123, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Xi, W.; Lazzeri, D.; Zhou, X.; Li, Z.; Nicoli, F.; Zenn, M.R.; Torresetti, M.; Grassetti, L.; Spinelli, G. Bipaddle radial forearm flap for head and neck reconstruction. J. Craniofacial Surg. 2015, 26, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Melissinos, E.G.; Marques, E.S. “Racing-stripe” Modification of Radial Forearm Free Flap: Technique and Experience (704 Consecutive Cases). Plast. Reconstr. Surg. Glob. Open 2022, 10, e4682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gessert, T.G.; Pflum, Z.E.; Thompson, J.D.; Hoffman, M.R.; Sanchez, R.; Glazer, T.A.; Wieland, A.M.; McCulloch, T.M.; Hartig, G.K. The radial forearm snake flap: An underutilized technique for fasciocutaneous and osteocutaneous forearm flaps with primary closure. Head Neck 2022, 44, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Wirthmann, A.; Finke, J.C.; Giovanoli, P.; Lindenblatt, N. Long-term follow-up of donor site morbidity after defect coverage with Integra following radial forearm flap elevation. Eur. J. Plast. Surg. 2014, 37, 159–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitano, D.; Morimatsu, Y.; Murai, N.; Osaki, T.; Sakakibara, S. The superficial branch of the radial nerve and sensory disturbance in the radial forearm flap donor-site. Regen. Ther. 2023, 24, 174–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poublon, A.R.; Walbeehm, E.T.; Duraku, L.S.; Eilers, P.H.; Kerver, A.L.; Kleinrensink, G.J.; Coert, J.H. The anatomical relationship of the superficial radial nerve and the lateral antebrachial cutaneous nerve: A possible factor in persistent neuropathic pain. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Suominen, S.; Ahovuo, J.; Asko-Seljavaara, S. Donor site morbidity of radial forearm flaps. A clinical and ultrasonographic evaluation. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1996, 30, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Angel, M.F.; Mellow, C.G.; Knight, K.R.; O’Brien, B.M. Secondary ischemia time in rodents: Contrasting complete pedicle interruption with venous obstruction. Plast. Reconstr. Surg. 1990, 85, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Marzella, L.; Jesudass, R.R.; Manson, P.N.; Myers, R.A.; Bulkley, G.B. Functional and structural evaluation of the vasculature of skin flaps after ischemia and reperfusion. Plast. Reconstr. Surg. 1988, 81, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Harashina, T.; Sawada, Y.; Watanabe, S. The relationship between venous occlusion time in island flaps and flap survivals. Plast. Reconstr. Surg. 1977, 60, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Hjortdal, V.E.; Hansen, E.S.; Hauge, E. Myocutaneous flap ischemia: Flow dynamics following venous and arterial obstruction. Plast. Reconstr. Surg. 1992, 89, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Hjortdal, V.E.; Hauge, E.; Hansen, E.S. Differential effects of venous stasis and arterial insufficiency on tissue oxygenation in myocutaneous island flaps: An experimental study in pigs. Plast. Reconstr. Surg. 1992, 89, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pang, Y.; Buntic, R.; Jones, M.; Cai, Z.; Buncke, H.J.; Lineaweaver, W.C. Effect of sequence, timing of vascular anastomosis, and clamp removal on survival of microsurgical flaps. J. Reconstr. Microsurg. 2002, 18, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, X.; Huang, J.; Wu, Y.; Wang, G.; Jiang, L.; Li, W.; Zhao, Y. Reliability of the superficial venous drainage of the radial forearm free flaps in oral and maxillofacial reconstruction. Microsurgery 2008, 28, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, E.D. The radial forearm free flap in orofacial reconstruction. Personal experience in 120 consecutive cases. J. Craniomaxillofac Surg. 1990, 18, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Beckingham, I.J.; Wilson, G.R.; McLean, N.R.; Soames, J.V. Free flap failure due to venous occlusion secondary to previous intravenous cannulation: A case report. Microsurgery 1992, 13, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Xu, Z.F.; Duan, W.Y.; Liu, F.Y.; Huang, D.H.; Sun, C.F. Single Superficial versus Dual Systems Venous Anastomoses in Radial Forearm Free Flap: A Meta-Analysis. PLoS ONE 2015, 10, e0134805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Feng, T.; Ou, Y.; Lin, Y.; Gong, W.; Wang, Y. Superficial versus deep system single venous anastomosis in the radial forearm free flap: A meta-analysis. Int. J. Oral. Maxillofac. Surg. 2021, 50, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Selber, J.C.; Sanders, E.; Lin, H.; Yu, P. Venous drainage of the radial forearm flap: Comparison of the deep and superficial systems. Ann. Plast. Surg. 2011, 66, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Han, Z. Single versus dual venous anastomosis in radial forearm free flaps in head and neck reconstruction. J. Plast. Surg. Hand Surg. 2023, 57, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, A.; Tahara, S.; Terashi, H.; Yokoo, S.; Nakahara, M.; Hashikawa, K.; Kenmoku, K. Importance of the deep vein in the drainage of a radial forearm flap: A haemodynamic study. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2003, 37, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Khariwala, S.S.; Le, B.; Pierce, B.H.; Vogel, R.I.; Chipman, J.G. Antibiotic Use after Free Tissue Reconstruction of Head and Neck Defects: Short Course vs. Long Course. Surg. Infect. 2016, 17, 100–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mücke, T.; Rohleder, N.H.; Rau, A.; Ritschl, L.M.; Kesting, M.; Wolff, K.D.; Mitchell, D.A.; Loeffelbein, D.J. The value of perioperative antibiotics on the success of oral free flap reconstructions. Microsurgery 2015, 35, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.M. A world survey of anticoagulation practice in clinical microvascular surgery. Br. J. Plast. Surg. 1982, 35, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Ashjian, P.; Chen, C.M.; Pusic, A.; Disa, J.J.; Cordeiro, P.G.; Mehrara, B.J. The effect of postoperative anticoagulation on microvascular thrombosis. Ann. Plast. Surg. 2007, 59, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.M.; Yousaf, N.; Khan, F.A. The outcome of microvascular free flap surgery with or without the use of postoperative heparin. J. Coll. Physicians Surg. Pak. 2014, 24, 412–415. [Google Scholar] [PubMed]
- Chien, W.; Varvares, M.A.; Hadlock, T.; Cheney, M.; Deschler, D.G. Effects of aspirin and low-dose heparin in head and neck reconstruction using microvascular free flaps. Laryngoscope 2005, 115, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.L.; Chen, G.X.; Shao, H.W.; Han, C.M.; Zhang, L.P.; Zhi, L.Z. Effect of heparin on prevention of flap loss in microsurgical free flap transfer: A meta-analysis. PLoS ONE 2014, 9, e95111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, K.T.; Mun, G.H. The efficacy of postoperative antithrombotics in free flap surgery: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2015, 135, 1124–1139. [Google Scholar] [CrossRef] [PubMed]
- Lohman, R.F.; Langevin, C.J.; Bozkurt, M.; Kundu, N.; Djohan, R. A prospective analysis of free flap monitoring techniques: Physical examination, external Doppler, implantable Doppler, and tissue oximetry. J. Reconstr. Microsurg. 2013, 29, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Carrière, M.E.; Mokkink, L.B.; Tyack, Z.; Westerman, M.J.; Pijpe, A.; Pleat, J.; van de Kar, A.L.; Brown, J.; de Vet, H.C.W.; van Zuijlen, P.P.M. Development of the Patient Scale of the Patient and Observer Scar Assessment Scale (POSAS) 3.0: A qualitative study. Qual. Life Res. 2023, 32, 583–592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aarstad, H.J.; Østhus, A.A.; Aarstad, H.H.; Lybak, S.; Aarstad, A.K.H. EORTC Quality of Life Questionnaire Head and Neck (H&N)-35 scores from H&N squamous cell carcinoma patients obtained at diagnosis and at 6, 9 and 12 months following diagnosis predict 10-year overall survival. Eur. Arch. Otorhinolaryngol. 2019, 276, 3495–3505. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | |
|---|---|
| Gender | |
| Male | 48 (88.8%) |
| Female | 4 (7.5%) |
| Not reported | 2 (3.7%) |
| Age (mean ± SD) | 46 ± 9.97 |
| Country | |
| Australia | 1 (1.8%) |
| Brazil | 4 (7.5%) |
| Canada | 2 (3.7%) |
| China | 1 (1.8%) |
| Colombia | 2 (3.7%) |
| Germany | 4 (7.5%) |
| India | 2 (3.7%) |
| Italy | 19 (35.2%) |
| Japan | 1 (1.8%) |
| Netherlands | 3 (5.5%) |
| Spain | 4 (7.5%) |
| Switzerland | 1 (1.8%) |
| Thailand | 2 (3.7%) |
| UK | 2 (3.7%) |
| USA | 6 (11.1%) |
| Type of medical center | |
| University | 37 (68.5%) |
| Non-university | 17 (31.5%) |
| Specialization | |
| ENT | 39 (64%) |
| Plastic Surgery | 2 (3%) |
| Maxillofacial Surgery | 11 (18%) |
| Other | 9 (15%) |
| Type of training | |
| Residency training | 21 (38.9%) |
| Fellowship training | 34 (62.9%) |
| Attending dedicated workshops and courses | 25 (46.3%) |
| Simulation training | 6 (11.1%) |
| Mentorship | 13 (24.1%) |
| Other | 2 (3.7%) |
| Experience in H&N reconstructive surgery (years) | |
| <1 | 3 (5.5%) |
| 1–2 | 1 (1.8%) |
| 2–5 | 7 (13.0%) |
| 5–10 | 11 (20.4%) |
| >10 | 32 (59.3%) |
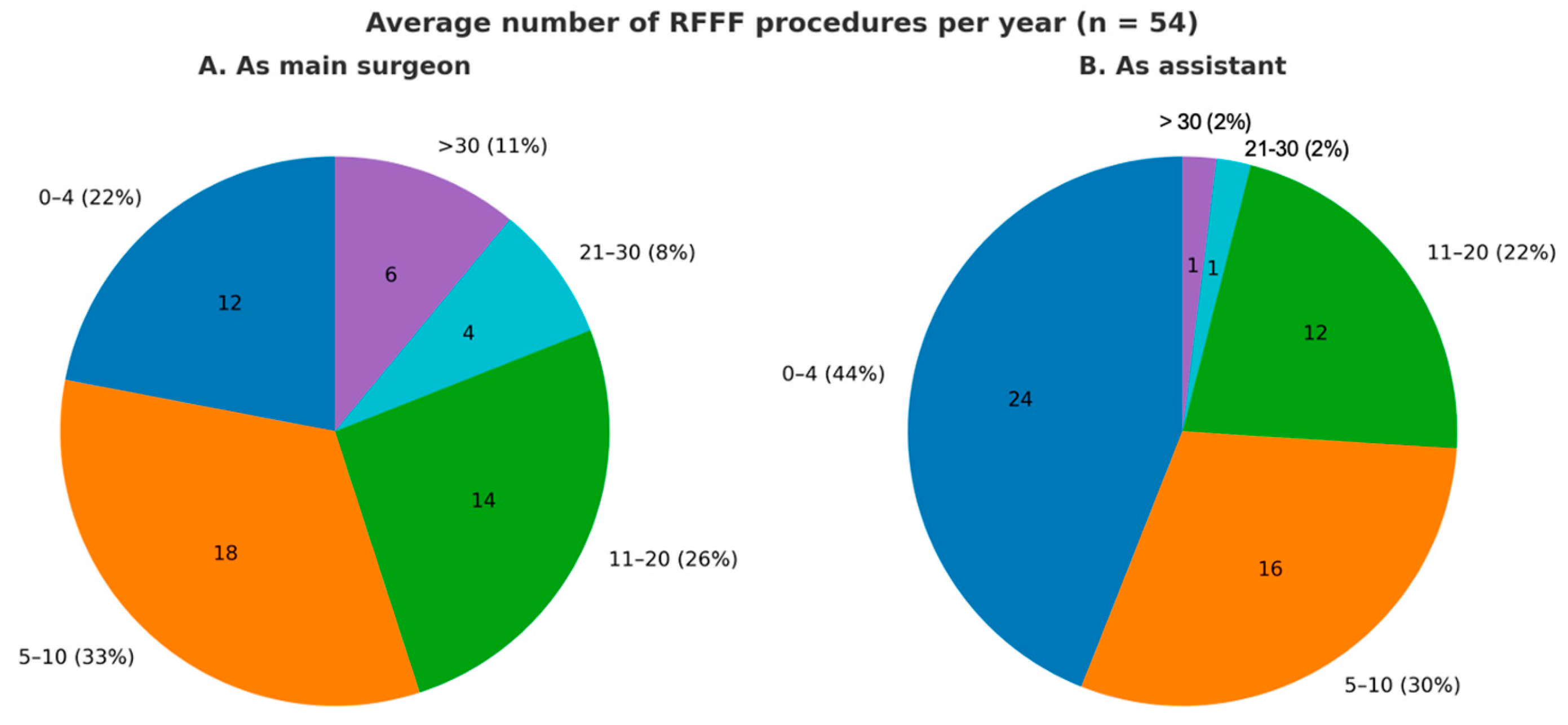
| Average number of RFFF as main surgeon | |
| 0–4 | 12 (22.2%) |
| 5–10 | 18 (33.3%) |
| 11–20 | 14 (26.0%) |
| 21–30 | 4 (7.4%) |
| >30 | 6 (11.1%) |
| Average number of RFFF as assistant | |
| 0–4 | 24 (44.5%) |
| 5–10 | 16 (29.6%) |
| 11–20 | 12 (22.3%) |
| 21–30 | 1 (1.8%) |
| >30 | 1 (1.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, E.; Costantino, A.; Iannella, G.; Marchi, F.; Greco, A.; Calabrese, L.; Polimeni, A.; Accorona, R.; De Virgilio, A.; RFFFSurv Collaborative. Global Variations in Surgical Techniques and Postoperative Care for Radial Forearm Free Flap (RFFF) in Head & Neck Surgery: A Cross-Sectional International Survey. J. Clin. Med. 2025, 14, 8023. https://doi.org/10.3390/jcm14228023
Russo E, Costantino A, Iannella G, Marchi F, Greco A, Calabrese L, Polimeni A, Accorona R, De Virgilio A, RFFFSurv Collaborative. Global Variations in Surgical Techniques and Postoperative Care for Radial Forearm Free Flap (RFFF) in Head & Neck Surgery: A Cross-Sectional International Survey. Journal of Clinical Medicine. 2025; 14(22):8023. https://doi.org/10.3390/jcm14228023
Chicago/Turabian StyleRusso, Elena, Andrea Costantino, Giannicola Iannella, Filippo Marchi, Antonio Greco, Luca Calabrese, Antonella Polimeni, Remo Accorona, Armando De Virgilio, and RFFFSurv Collaborative. 2025. "Global Variations in Surgical Techniques and Postoperative Care for Radial Forearm Free Flap (RFFF) in Head & Neck Surgery: A Cross-Sectional International Survey" Journal of Clinical Medicine 14, no. 22: 8023. https://doi.org/10.3390/jcm14228023
APA StyleRusso, E., Costantino, A., Iannella, G., Marchi, F., Greco, A., Calabrese, L., Polimeni, A., Accorona, R., De Virgilio, A., & RFFFSurv Collaborative. (2025). Global Variations in Surgical Techniques and Postoperative Care for Radial Forearm Free Flap (RFFF) in Head & Neck Surgery: A Cross-Sectional International Survey. Journal of Clinical Medicine, 14(22), 8023. https://doi.org/10.3390/jcm14228023







