Submucosal Mitomycin C Injection in the Endoscopic Treatment of Laryngotracheal Stenosis: Experience of a Tertiary Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Outcome Measures
2.3. Demographic Data
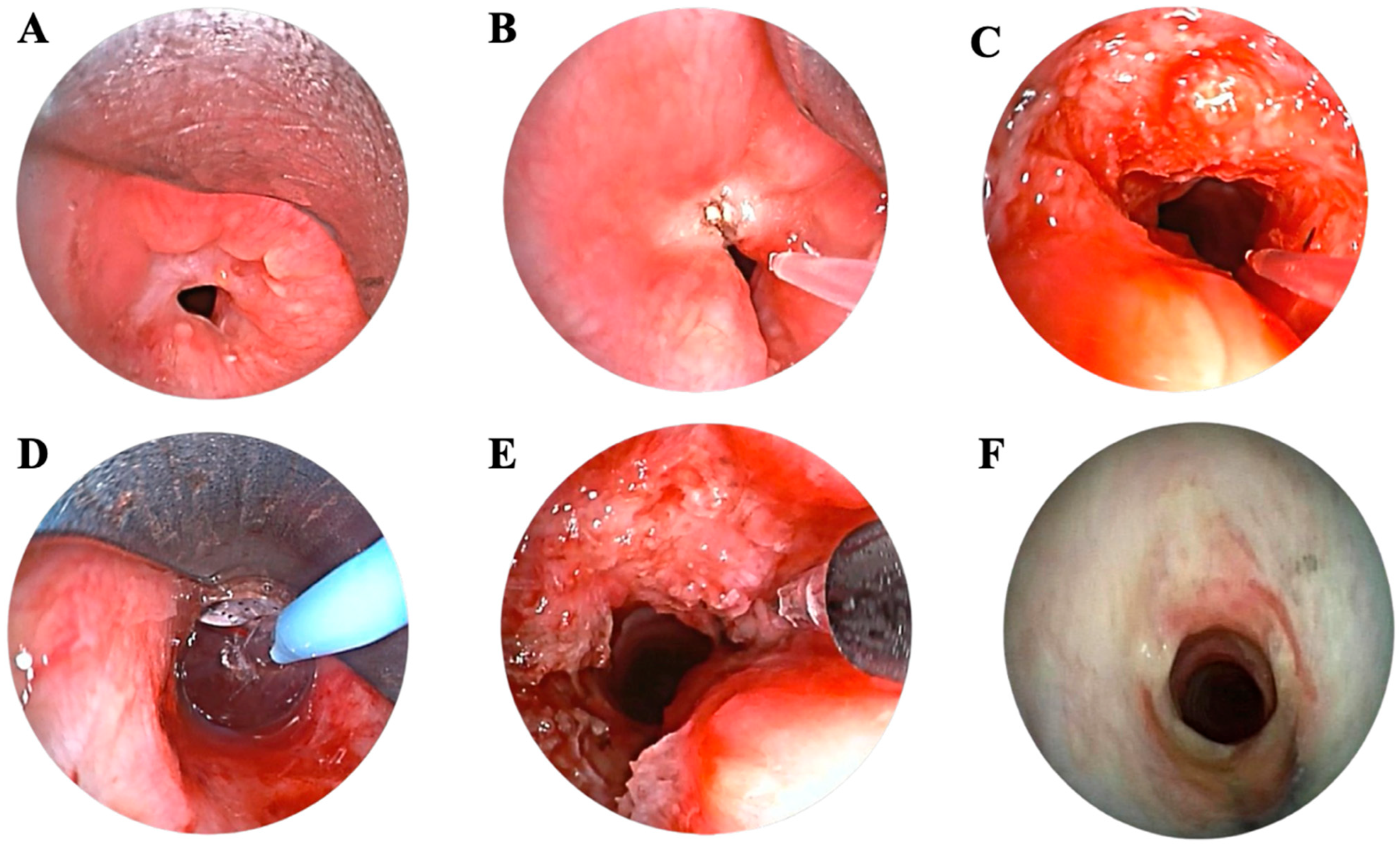
2.4. Flexible Bronchoscopy
2.5. Radiological Data
2.6. Surgical Treatment
2.7. Post-Treatment Follow-Up
2.8. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Bronchoscopic and Radiological Findings
3.3. Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spector, G. Developmental Anatomy of the Larynx. In Diseases of the Ear, Nose, and Throat; Ballenger, J., Ed.; Elsevier: Philadelphia, PA, USA, 1984. [Google Scholar]
- Szmuk, P.; Ezri, T.; Evron, S.; Roth, Y.; Katz, J. A brief history of tracheostomy and tracheal intubation, from the Bronze Age to the Space Age. Intensive Care Med. 2008, 34, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.R. Adult laryngotracheal stenosis: Etiology and surgical management. Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Nikolovski, N.; Kopacheva-Barsova, G.; Pejkovska, A. Laryngotracheal Stenosis: A Retrospective Analysis of Their Aetiology, Diagnose and Treatment. Open Access Maced. J. Med. Sci. 2019, 7, 1649–1656. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarper, A.; Ayten, A.; Eser, I.; Ozbudak, O.; Demircan, A. Tracheal stenosis aftertracheostomy or intubation: Review with special regard to cause and management. Tex. Heart Inst. J. 2005, 32, 154–158. [Google Scholar] [PubMed] [PubMed Central]
- Rosow, D.E.; Barbarite, E. Review of adult laryngotracheal stenosis: Pathogenesis, management, and outcomes. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, K.O.; Houlton, J.J.; Compton, W.; Ying, J.; Khosla, S.M. Laryngotracheal reconstruction: A ten-year review of risk factors for decannulation failure. Laryngoscope 2015, 125, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.T.; Strong, M.S.; Healy, G.B.; Shapshay, S.M.; Vaughan, C.W. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann. Otol. Rhinol. Laryngol. 1982, 91 Pt 1, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Rutter, M.J. Role of balloon dilation in the management of adult idiopathic subglottic stenosis. Ann. Otol. Rhinol. Laryngol. 2008, 117, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Frazer-Green, L.; Gonzalez, A.V.; Shofer, S.L.; Argento, A.C.; Welsby, I.; Hales, R.; Shojaee, S.; Gardner, D.D.; Chang, J.Y.; et al. Management of Central Airway Obstruction: An American College of Chest Physicians Clinical Practice Guideline. Chest 2025, 167, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, T.L.O.; Cataneo, D.C.; Martins, R.H.G.; Reis, T.A.; Cataneo, A.J.M. Mitomycin C in the Endoscopic Treatment of Laryngotracheal Stenosis: Systematic Review and Proportional Meta-Analysis. Int. Arch. Otorhinolaryngol. 2020, 24, e112–e124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sen, S.; Meteoglu, I.; Ogurlu, M.; Sen, S.; Derincegoz, O.O.; Barutca, S. Topical heparin: A promising agent for the prevention of tracheal stenosis in airway surgery. J. Surg. Res. 2009, 157, e23–e29, Erratum in J. Surg. Res. 2010, 162, 168. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Mao, X.; Li, C.; Ao, H.; Yang, X. A Novel Therapy for Laryngotracheal Stenosis: Treatment With Ethosomes Containing 5-Fluorouracil: Treatment With Ethosomes Containing 5-Fluorouracil. Ann. Otol. Rhinol. Laryngol. 2015, 124, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Sano, Y.; Sugawara, R.; Matsumae, A.; Kanamori, K.; Shima, T.; Hoshi, T. Mitomycin, a new antibiotic from streptomyces. Int. J. Antibiot. 1956, 9, 141–146. [Google Scholar]
- Tomasz, M.; Palom, Y. The mitomycin bioreductive antitumor agents: Cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol. Ther. 1997, 76, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, N.; Mori, S. Studies on pterygium. Part 4. A treatment of the pterygium by mitomycin C instillation. Acta Soc. Ophthalmol. Jpn. 1963, 67, 601–607. [Google Scholar]
- Ingrams, D.R.; Volk, M.S.; Biesman, B.S.; Pankratov, M.M.; Shapshay, S.M. Sinus surgery: Does mitomycin C reduce stenosis? Laryngoscope 1998, 108, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Eliashar, R.; Gross, M.; Maly, B.; Sichel, J.Y. Mitomycin does not prevent laryngotracheal repeat stenosis after endoscopic dilation surgery: An animal study. Laryngoscope 2004, 114, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Shvidler, J.; Bothwell, N.E.; Cable, B. Refining indications for the use of mitomycin C using a randomized controlled trial with an animal model. Otolaryngol. Head Neck Surg. 2007, 136, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Perepelitsyn, I.; Shapshay, S.M. Endoscopic treatment of laryngeal and tracheal stenosis-has mitomycin C improved the outcome? Otolaryngol. Head Neck Surg. 2004, 131, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Reichert, L.K.; Zhao, A.S.; Galati, L.T.; Shapshay, S.M. The Efficacy of Mitomycin C in the Treatment of Laryngotracheal Stenosis: Results and Experiences with a Difficult Disease Entity. ORL J. Otorhinolaryngol. Relat. Spec. 2015, 77, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.B.; James, J.C. The efficacy of mitomycin-C in the treatment of laryngotracheal stenosis. Laryngoscope 2006, 116, 1923–1925. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, R.; Shapshay, S.M.; Healy, G.B. Mitomycin: Effects on laryngeal and tracheal stenosis, benefits, and complications. Ann. Otol. Rhinol. Laryngol. 2001, 110, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Elstad, M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: Are two applications better than one? Laryngoscope 2009, 119, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Yung, K.C.; Chang, J.; Courey, M.S. A randomized controlled trial of adjuvant mitomycin-c in endoscopic surgery for laryngotracheal stenosis. Laryngoscope 2020, 130, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Myer III, D.M.; Cotton, R.T. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann. Otol. Rhinol. Laryngol. 1994, 103 Pt 1, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Monnier, P.; Dikkers, F.G.; Eckel, H.; Sittel, C.; Piazza, C.; Campos, G.; Remacle, M.; Peretti, G. Preoperative assessment and classification of benign laryngotracheal stenosis: A consensus paper of the European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2015, 272, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Morshed, K.; Trojanowska, A.; Szymański, M.; Trojanowski, P.; Szymańska, A.; Smoleń, A.; Drop, A. Evaluation of tracheal stenosis: Comparison between computed tomography virtual tracheobronchoscopy with multiplanar reformatting, flexible tracheofiberoscopy and intra-operative findings. Eur. Arch. Otorhinolaryngol. 2011, 268, 591–597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puyo, C.A.; Dahms, T.E. Innate immunity mediating inflammation secondary to endotracheal intubation. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 854–858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lawrence, D.A.; Branson, B.; Oliva, I.; Rubinowitz, A. The wonderful world of the windpipe: A review of central airway anatomy and pathology. Can. Assoc. Radiol. J. 2015, 66, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Caron, R.M.; Hamilton, J.W. Preferential effects of the chemotherapeutic DNA crosslinking agent mitomycin C on inducible gene expression in vivo. Environ. Mol. Mutagen. 1995, 25, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Caron, R.M.; Hamilton, J.W. Developmentally specific effects of the DNA cross-linking agent mitomycin C on phosphoenolpyruvate carboxykinase gene expression in vivo: Correlation with changes in chromatin structure within the promoter region of the gene. J. Biochem. Mol. Toxicol. 1998, 12, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ihnat, M.A.; Lariviere, J.P.; Warren, A.J.; La Ronde, N.; Blaxall, J.R.; Pierre, K.M.; Turpie, B.W.; Hamilton, J.W. Suppression of P-glycoprotein expression and multidrug resistance by DNA cross-linking agents. Clin. Cancer Res. 1997, 3, 1339–1346. [Google Scholar] [PubMed]
- Gray, S.D.; Tritle, N.; Li, W. The effect of mitomycin on extracellular matrix proteins in a rat wound model. Laryngoscope 2003, 113, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kunnavatana, S.S.; Koch, R.J. Effects of mitomycin-C on normal dermal fibroblasts. Laryngoscope 2006, 116, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Simman, R.; Alani, H.; Williams, F. Effect of mitomycin C on keloid fibroblasts: An in vitro study. Ann. Plast. Surg. 2003, 50, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.; Gray, S.D.; Thibeault, S. Time and dose effects of mitomycin C on extracellular matrix fibroblasts and proteins. Laryngoscope 2005, 115, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sewall, G.K.; Robertson, K.M.; Connor, N.P.; Heisey, D.M.; Hartig, G.K. Effect of topical mitomycin on skin wound contraction. Arch. Facial Plast. Surg. 2003, 5, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Fde, A.; Guaraldo, L.; Borges Jde, P.; Zacchi, F.F.; Eckley, C.A. Clinical and histological healing of surgical wounds treated with mitomycin C. Laryngoscope 2004, 114, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Eliashar, R.; Eliachar, I.; Esclamado, R.; Gramlich, T.; Strome, M. Can topical mitomycin prevent laryngotracheal stenosis? Laryngoscope 1999, 109, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Spector, J.E.; Werkhaven, J.A.; Spector, N.C.; Huang, S.; Sanders, D.; Reinisch, L. Prevention of anterior glottic restenosis in a canine model with topical mitomycin-C. Ann. Otol. Rhinol. Laryngol. 2001, 110, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Spector, J.E.; Werkhaven, J.A.; Spector, N.C.; Huang, S.; Page, R.N.; Baranowski, B.; Luther, M.; McGehee, B.; Reinisch, L. Preservation of function and histologic appearance in the injured glottis with topical mitomycin-C. Laryngoscope 1999, 109 Pt 1, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C.G.; Soto, J.; Riddick, J.; Billante, C.R.; Reinisch, L. Effect of mitomycin-C on vocal fold healing in a canine model. Ann. Otol. Rhinol. Laryngol. 2001, 110, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.J.; Reinisch, L.; Sanders, D.L.; Huang, S.; Deriso, W.; Duncavage, J.A.; Garrett, C.G. Inhibition of subglottic stenosis with mitomycin-C in the canine model. Ann. Otol. Rhinol. Laryngol. 1999, 108 Pt 1, 1053–1060. [Google Scholar] [CrossRef]
- Roh, J.L.; Yoon, Y.H. Prevention of anterior glottic stenosis after bilateral vocal fold stripping with mitomycin C. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, D.H.; Rha, K.S.; Sung, M.W.; Kim, K.H.; Park, C.I. Benefits and risks of mitomycin use in the traumatized tracheal mucosa. Otolaryngol. Head Neck Surg. 2007, 136, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L. Prevention of posterior glottic stenosis by mitomycin C. Ann. Otol. Rhinol. Laryngol. 2005, 114, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Lee, Y.W.; Park, C.I. Can mitomycin C really prevent airway stenosis? Laryngoscope 2006, 116, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Hardillo, J.; Vanclooster, C.; Delaere, P.R. An investigation of airway wound healing using a novel in vivo model. Laryngoscope 2001, 111, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Coppit, G.; Perkins, J.; Munaretto, J.; Nielsen, R.; McKinney, L.; Ulnick, K. The effects of mitomycin-C and stenting on airway wound healing after laryngotracheal reconstruction in a pig model. Int. J. Pediatr. Otorhinolaryngol. 2000, 53, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.F.; April, M.M. Mitomycin-C in the treatment of tracheal cicatrix after tracheal reconstruction. Int. J. Pediatr. Otorhinolaryngol. 1998, 44, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hueman, E.M.; Simpson, C.B. Airway complications from topical mitomycin C. Otolaryngol. Head Neck Surg. 2005, 133, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Varani, J.; Soong, H.K.; Lichter, P.R. Effects of 5-fluorouracil and mitomycin C on cultured rabbit subconjunctival fibroblasts. Ophthalmology 1990, 97, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Crowston, J.G.; Chang, L.H.; Constable, P.H.; Daniels, J.T.; Akbar, A.N.; Khaw, P.T. Apoptosis gene expression and death receptor signaling in mitomycin-C-treated human tenon capsule fibroblasts. Investig. Ophthalmol. Vis. Sci. 2002, 43, 692–699. [Google Scholar] [PubMed]
- Tiran, B.; Parluk, T.; Kleinhendler, E.; Man, A.; Fomin, I.; Schwarz, Y. Fiberoptic Bronchoscopic Submucosal Injection of Mitomycin C for Recurrent Bening Tracheal Stenosis: A Case Series. Isr. Med. Assoc. J. 2020, 22, 757–760. [Google Scholar] [PubMed]
- Sahin, M.F.; Beyoglu, M.A.; Turkkan SYazicioglu, A.; Yekeler, E. Submucosal Injection of Mitomycin-C Due to Development of Tracheal Restenosis After Resection. India. J. Surg. 2021, 83 (Suppl. 1), 198–200. [Google Scholar] [CrossRef]
- Sahin, M.F.; Turkkan, S.; Beyoglu, M.A.; Yazicioglu, A.; Yekeler, E. Submucosal Injection of Mitomycin C in a Case of Vanishing Bronchus After Lung Transplant. Exp. Clin. Transplant. 2022, 20, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Morrison, G.A. Laryngeal cancer after topical mitomycin C application. J. Laryngol. Otol. 2006, 120, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
| Age (y)/Sex | Comorbidities | BMI | Smoker | Etiology | Intubation (d) | Trach. | Re-trach. | COVID-19 (Y = yes, N = no) | Previous Treatment | Site | Grade | ELS Classification | L (mm) | D (mm) | Pattern | Complications | Time to Recur. (d) | Further Treatments | Status | FU (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 78/F | AF, HTN | 36.1 | No | Idiopathic | N/A | None | None | N | endoscopic procedures (n = 2) | Subglottic | N/A | N/A | N/A | N/A | CONC | None | 129 | stent (n = 1), endoscopic procedure (n = 2) | Dyspnea during exercise | 924 |
| 69/M | HTN, diabetes, HCE | 29.4 | Ex | Idiopathic | N/A | None | None | N | None | Subglottic | III | IIIa | 24 | 9 | ECC | None | 143 | endoscopic procedure (n = 1), tracheal resection (n = 1) | No sympt. | 731 |
| 48/M | HTN, HCE | 29.8 | No | Post-tracheostomy | 42 | None | None | Y | tracheal resection with end-to-end anastomosis (n = 1) | Subglottic | III | IIIa | 22 | 23 | CONC | None | N/A | None | No sympt. | 461 |
| 48/M | None | 19.6 | Ex | Prolonged intubation | N/A | None | None | N | endoscopic procedures (n = 2) | Cervical trachea | II | IIa | 19 | 32 | CONC | None | 207 | endoscopic procedure (n = 1) | No sympt. | 455 |
| 39/M | None | 25.8 | No | Prolonged intubation | 15 | None | None | Y | endoscopic procedures (n = 2) | Subglottic | N/A | N/A | N/A | N/A | CONC | None | N/A | None | Dyspnea during exercise | 392 |
| 43/F | Ankylosing spondylitis | 22.1 | No | Idiopathic | N/A | None | None | N | endoscopic procedure (n = 1) | Subglottic | N/A | N/A | N/A | N/A | CONC | None | 174 | endoscopic procedure (n = 1) | No sympt. | 430 |
| 62/M | HTN | 27.2 | No | Prolonged intubation | 6 | None | None | N | endoscopic procedure (n = 1) | Subglottic | II | IIa | 44 | 0 | CONC | None | N/A | None | No sympt. | 742 |
| 50/F | None | 32.8 | No | Post-tracheostomy | N/A | Percutaneous | Surgical | Y | tracheal resection with end-to-end anastomosis (n = 1), endoscopic procedure (n = 7) | Subglottic | III | IIIa | 25 | 19 | CONC | None | 78 | endoscopic procedures (n = 2) | No sympt. | 452 |
| 27/F | Asthma | 17.4 | No | Idiopathic | N/A | None | None | N | None | Subglottic | II | IIa | 23 | 13 | CONC | None | N/A | endoscopic procedures (n = 1) | No sympt. | 496 |
| 28/M | None | 30.2 | Ex | Prolonged intubation | 28 | None | None | N | endoscopic procedure (n = 1) | Subglottic | II | IIa | 16 | 38 | CONC | None | N/A | None | No sympt. | 342 |
| 65/M | COPD | 34.3 | Ex | Post-tracheostomy | 35 | None | None | Y | endoscopic procedure (n = 1) | Subglottic | III | IIIa | 26 | 20 | ECC | None | N/A | None | No sympt. | 614 |
| 46/F | GERD, hypothyroidism | 23.7 | No | Idiopathic | N/A | None | None | N | endoscopic procedure (n = 1) | Subglottic | N/A | N/A | N/A | N/A | CONC | None | N/A | None | No sympt. | 217 |
| 57/F | Asthma | 19.9 | No | Idiopathic | N/A | None | None | N | None | Subglottic | II | IIa | 21 | 13 | ECC | None | N/A | None | No sympt. | 409 |
| 44/M | HTN | 25.1 | No | Prolonged intubation | Unknown | None | None | Y | tracheal resection with end-to-end anastomosis (n = 1), endoscopic procedure (n = 2) | Subglottic | N/A | N/A | N/A | N/A | CONC | None | 130 | endoscopic procedure (n = 1) | No sympt. | 308 |
| 56/F | HTN, GERD | 39.3 | Ex | Post-tracheostomy | Unknown | Surgical | Surgical | N | T tube (n = 1), endoscopic procedures (n = 1) | Subglottic | N/A | N/A | N/A | N/A | ECC | None | N/A | endoscopic procedure (n = 1) | No sympt. | 181 |
| 60/F | HTN, asthma, diabetes | 36.7 | No | Post-tracheostomy | Unknown | None | None | Y | tracheal resection with end-to-end anastomosis (n = 1) | Cervical trachea | N/A | N/A | N/A | N/A | CONC | None | N/A | endoscopic procedure (n = 1) | No sympt. | 224 |
| Patient (Age/Gender) | Time to Recurrence (d) | Further Treatment |
|---|---|---|
| 78/F | 129 | MMC injection + tracheal stent placement (n = 1); Laser-assisted radial stenosis release + dilation + steroid injection (n = 1) |
| 69/M | 143 | Laser-assisted radial stenosis release + dilation + MMC injection (n = 1) |
| 48/M | 207 | Laser-assisted radial stenosis release + dilation + steroid injection (n = 1) |
| 43/F | 174 | Laser-assisted radial stenosis release + dilation + steroid injection (n = 1) |
| 50/F | 78 | Laser-assisted radial stenosis release + dilation + MMC injection (n = 2) |
| 44/M | 130 | Laser-assisted radial stenosis release + dilation + MMC injection (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, E.; Canali, L.; Cerri, L.; Mangiameli, G.; Cariboni, U.; Marulli, G.; Spriano, G.; Ferraroli, G.M.; De Virgilio, A. Submucosal Mitomycin C Injection in the Endoscopic Treatment of Laryngotracheal Stenosis: Experience of a Tertiary Center. J. Clin. Med. 2025, 14, 8022. https://doi.org/10.3390/jcm14228022
Russo E, Canali L, Cerri L, Mangiameli G, Cariboni U, Marulli G, Spriano G, Ferraroli GM, De Virgilio A. Submucosal Mitomycin C Injection in the Endoscopic Treatment of Laryngotracheal Stenosis: Experience of a Tertiary Center. Journal of Clinical Medicine. 2025; 14(22):8022. https://doi.org/10.3390/jcm14228022
Chicago/Turabian StyleRusso, Elena, Luca Canali, Luca Cerri, Giuseppe Mangiameli, Umberto Cariboni, Giuseppe Marulli, Giuseppe Spriano, Giorgio Maria Ferraroli, and Armando De Virgilio. 2025. "Submucosal Mitomycin C Injection in the Endoscopic Treatment of Laryngotracheal Stenosis: Experience of a Tertiary Center" Journal of Clinical Medicine 14, no. 22: 8022. https://doi.org/10.3390/jcm14228022
APA StyleRusso, E., Canali, L., Cerri, L., Mangiameli, G., Cariboni, U., Marulli, G., Spriano, G., Ferraroli, G. M., & De Virgilio, A. (2025). Submucosal Mitomycin C Injection in the Endoscopic Treatment of Laryngotracheal Stenosis: Experience of a Tertiary Center. Journal of Clinical Medicine, 14(22), 8022. https://doi.org/10.3390/jcm14228022







