1. Introduction
According to the 2021 Global Burden of Disease (GBD), stroke incidence rose by 70% while stroke deaths rose 44% between the years of 1990 and 2021, respectively [
1]. Due to the high prevalence of risk factors such as high Body Mass Index (BMI), hypertension, diabetes, smoking rates, and increasing global temperatures, stroke presents as a leading medical emergency globally. Not only does this prevalence cause a strain on global healthcare systems from a quantity standpoint, but it also demonstrates a financial significance, with an estimated global cost of
$890 billion U.S. dollars accounting for 0.66% of the global Gross Domestic Product (GDP) [
1]. In analyzing where these costs specifically lie, the cost models within the United States and Europe can be referenced. In Europe, for example, over a single year, it is estimated that informal care attributed 1.3 billion euros (approximately
$1.5 billion US dollars) to the total cost, which directly translates to the care directly provided by families and friends as opposed to paid healthcare workers. Direct healthcare costs contributed 27 billion euros (approximately
$31.8 billion US dollars), while losses due to a lack of productivity contributed 12 billion euros (approximately
$14.1 billion US dollars). In contrast, indirect costs amounted to a higher contribution within the United States models, amounting to
$103.5 billion dollars making up 66% of total costs. Further contributions were made with productivity loss at
$38.1 billion and premature death-associated costs at
$30.4 billion [
2]. The significant presence of informal costs can perhaps be explained by the current inefficiency of rehabilitative systems or rehabilitative practices.
Despite the fact that there is a significant financial contribution relating to stroke and stroke recovery, studies show that patients often do not receive sufficient rehabilitation therapy following a stroke, especially if they present with mild to moderate deficits [
3]. Recent studies highlight access limitations as a global issue, especially when considering factors such as socioeconomic status and geographical location [
4]. This could be attributed to a multitude of reasons, including the significant cost burden seen with rehabilitation therapy, considerable time investment and transportation required to transport patients to rehabilitation sessions, and the unengaging nature of current rehabilitation therapy [
5]. These reasons all contribute to the overarching phenomenon where there is a lack of current therapies with an ease of access.
The effects of stroke are multisystemic, and the most often overlooked, especially when considering rehabilitation therapy, is the cognitive impact. About 60% of stroke survivors present with cognitive impairment during their first year following a stroke [
2]. These impairments can vary, including, but not limited to, memory loss, attention difficulty, language deficits, and even progress to stroke associated dementia, commonly seen as “vascular dementia [
6].” Additional reviews have demonstrated that these cognitive impairments can persist long term, which further emphasizes a need for appropriate cognitive rehabilitation [
7].
Cognitive dysfunction is typically identified through cognitive domains such as attention, memory, language, and executive function. Cognitive therapies thus employ systems that target these cognitive domains. Current therapies are commonly accessed through computer systems, psychosocial education, pen and paper assessments, as well as behavioral therapies [
8,
9]. These therapies present similar limitations to the ones previously mentioned, especially when considering from an engagement standpoint and generalizability to the real world [
9]. Engagement is commonly associated with compliance when it comes to rehabilitation methods. Inevitably, employing methods such as standardized computer programs and pen and paper techniques provides a relatively mundane experience. When considering the other rehabilitative obligations stroke patients commonly have, it can be easy to see how compliance would falter in the cognitive training. These methods are also not direct translations to how patients use cognitive domains in their everyday lives. They present a one-dimensional representation of a much more complex use of cognitive function in the real world. These limitations further restrict patients from receiving and completing appropriate therapeutic interventions, especially from a cognitive standpoint. The modest benefits seen with limited applicability not only explain the restriction in patients receiving therapy, but also why they do not maintain compliance with it [
10].
The concept at the heart of these rehabilitation therapies is neuroplasticity. Neuroplasticity describes organizational changes in the neuronal components within the central nervous system [
11]. Much of this process occurs naturally throughout someone’s lifespan, but it can also be seen through adaptive necessity, such as the tissue death seen in strokes [
11]. In order for rehabilitative practices to achieve proper neuroplasticity, certain parameters must be met. For example, not only must a specific function be trained directly, but it must also be trained with sufficient repetition. This increases the likelihood of lasting neuronal changes. Additionally, important in the initiation of neuroplasticity is the intensity of the therapy, with higher intensity more often inducing neuroplastic changes. The rehabilitative practice must also have the capacity to be implemented early as an intervention; this is especially critical following stroke-related brain damage. An important feature that seems to be lacking in current therapies is the promotion of engagement and motivation in the patient with an understanding as to why the therapy is relevant and meaningful. Lastly, the therapy must be transferable across multiple domains. For example, therapy targeting working memory must also be able to improve executive function [
12].
Novel therapies aimed at improving ease of access and providing engagement incentives have shown significant promise within the neurorehabilitation space. One example is the use of extended reality (XR). XR is an all-encompassing term that includes technologies that combine both the virtual and the physical world [
13]. A subset of XR is virtual reality (VR), which is further subdivided into standard VR vs. immersive VR. Standard VR presents with a completely virtual platform; however, presented within a 2D space, similar to playing a standard video game [
14]. Immersive virtual reality is applied using a VR headset, where patients are completely visually (and sometimes auditorily) immersed in the virtual environment with the creation of engaging and stimulating tasks [
14]. VR has shown effectiveness in cognitive rehabilitation, specifically, without the limitations of conventional rehabilitation across multiple studies [
15]. Additionally, studies suggest validity with improvement of cognition with the use of VR while increasing adherence and involvement [
9]. There is even the possibility of improved outcomes when compared to traditional rehabilitative methods [
9]. Other facets also appear to be improved with VR. For one, outside of the cognitive analysis, it has allowed for versatility in motor deficit improvement due to its ability to display varying environments. Additionally, it has allowed for the personalization of rehabilitation, again supported by its adaptability and its ability to cater to the patient’s needs [
6]. While initial integration of the systems within rehabilitation spaces would present higher up-front costs, longer-term care would prove cost-effective, especially when considering home integration and reduced lengths of hospitalization [
15].
A platform that can potentially provide further improvement is a similar system known as mixed reality (MR). MR functions similarly to VR, but instead of a completely virtual environment, virtual elements are superimposed over a real-world viewpoint. This potentially addresses some of the limitations of VR. What is inevitably seen with patients who have suffered a stroke is additional incapacities, such as motor deficits. Considering this, providing therapy in a completely virtual environment may present safety concerns and mobility challenges. MR has the ability to address this while also integrating real-world applications that could improve the rehabilitation process. In theory, the combination of repetitive practice, real-time feedback, and increased safety measures provides a space ripe for rehabilitative benefit [
16]. Therefore, the purpose of this case series is to explore the potential cognitive benefits of MR game training for individuals who have suffered a stroke.
2. Materials and Methods
This was a small-scale feasibility, interventional study where participants were recruited to complete 12, 1 h MR intervention sessions across a 4-week time period to determine the effects of MR on cognitive performance following stroke.
2.1. Institutional Review Board and Informed Consent Statements
All experimental protocols were approved by the institutional review board at the University of Kentucky (protocol # 43449, approved 5 January 2019). All procedures were carried out following the rules of the Declaration of Helsinki of 1975 (revised 2013). Informed consent was obtained from all participants prior to enrollment, and this case series has not been previously reported in the literature.
2.2. Participants
A total of 3 participants (Participant A-C) were included in the case series (see
Table 1 for details). Participants were considered for inclusion in this study if they had experienced a stroke no less than 4 months prior to enrolling in the study, no history of seizures or behavioral impairments that would prevent the safe and consistent participation in the study, not currently taking opioids for pain management (as this could affect balance while using the MR headset), being able to consent for participation (having a diagnosis of mild cognitive impairment or no diagnosis of cognitive impairment), and not having a change in spasticity medication within 3 months of enrolling (this was included because motor function was assessed for some participants, but will not be presented here).
Participant A was a 31-year-old right-handed, Caucasian, non-Hispanic male with residual left hemiparesis 15 years post right MCA stroke. Participant B was a 61-year-old, Caucasian, non-Hispanic female with right spastic hemiparesis 10 years after a hemorrhagic stroke of basal ganglia. Other deficits included aphasia with word-finding difficulty and foot drop. As a result of the foot drop, Participant B completed the intervention while seated in an office chair with wheels for safety. Participant C was a 50-year-old, Caucasian, non-Hispanic female 5 months post-inferior medulla ischemic stroke, causing left hemisensory deficits, reduced temperature perception, and dysphagia. Participant A had prior experience with MR, as he had participated in a small pilot trial two years prior to enrolling in this study. Participants B and C had no prior experience with MR.
2.3. Mixed Reality (MR) Platform
For this study, the MR gaming program was delivered via the Microsoft HoloLens 2 (1 Microsoft Way, Redmond, WA, USA) [
17]. As previously detailed in Glueck and Han [
18], the HoloLens is a self-contained holographic computer that superimposes high-definition, virtual holograms across the user’s visual field through a series of clear screens via a head-mounted display. This allows the user to maintain their real-world reference while interacting with three-dimensional virtual stimuli. This is a unique feature that distinguishes this technology from other extended reality headsets and potentially offers a safer and better-tolerated delivery for programs.
The program used for the intervention in this study was RoboRaid version 1 (1 Microsoft Way, Redmond, WA, USA). RoboRaid is a first-person shooter action game that was developed by Microsoft. During gameplay, the user must eliminate or “blast” virtual opponents while avoiding “enemy fire,” while the levels of play become progressively more difficult. Blasting required participants to bring their pointer finger and thumb together while targeting the opponent. For a more detailed description of the game, see Glueck and Han [
18]. To successfully navigate the game and move through the various levels of play, participants needed to be able to discriminate between virtual opponents and recall the number of “strikes or hits” needed to destroy the various types of robots that they encounter. Furthermore, the participants must also move through the 3-dimensional playing field of the real-world, which makes the game unique to other extended reality platforms.
2.4. Outcome Assessments
Cognitive performance was determined using the NIH ToolBox, version 2 (V2). This study used a selection of the cognitive assessments for memory, attention, working memory, executive function, and processing speed (see
Table 2 for a description of each assessment).
2.5. Procedure
After consenting, participants completed a baseline neurocognitive assessment using the tablet-based NIH Toolbox. The assessment was conducted in a quiet, well-lit room, with the participant and an experimenter seated next to each other at a table. The tablet (and iPad) was placed within a comfortable reaching distance from the participant, and the experimenter guided the participant through the five cognitive domain assessments (see
Table 2). The cognitive assessment took roughly 30–40 min to complete, and after which the experimenter scheduled the intervention sessions and the immediate post-assessment with the participant.
The intervention consisted of 12 1 h intervention sessions distributed across 4 weeks, with 3 intervention sessions scheduled each week. This interval was selected as it mimics the typical outpatient rehabilitation sessions used at the experimenter’s home facility, without posing too much burden on the participant. Participants selected 3 days of the week as well as the time of the sessions between 8:00 am and 6:00 pm. Furthermore, the total duration of the intervention of 12, 1 h intervention sessions was selected based on two factors. First, in a previous study from our lab, we used a total of an 8 h intervention distributed across an average of 35 days for a sample of 14 nonclinical participants [
18]. Further examination of this study’s in-game performance data (i.e., the number of games played per session, end game scores, and each session’s highest score achieved), demonstrated that participants failed to reach asymptotic performance. Author A.C.G. interpreted this data to suggest that a longer total duration for the intervention may be beneficial for future clinical studies. Second, a review of the 38 published extended reality rehabilitation literature from 2002 to 2021 revealed a wide range of total intervention durations ranging from 15 min to 40 h, with an average intervention time of 12.06 h (SD = 8.68). Based on this information, the total training duration of 12 h was selected.
Each of the 12 intervention sessions took place in a dimly lit, quiet, relatively empty room. We have found through repeated pilot testing that lower light levels help to accentuate the 3-dimensional holographic images from the MR headset and improve the game training experience for participants. Even though the HoloLens allows the participant to maintain their visual awareness of the real-world environment, we have found that participants may still bump into objects during the intervention session; therefore, we elected to utilize a relatively open area to mitigate the risk of falling. In instances where participants may have a balance impairment or lower extremity mobility impairments, we allow participants to remain seated during game training in a wheeled office chair with armrests. We have elected to employ this approach out of an abundance of caution and because unpublished pilot testing has revealed similar motor, balance, and cognitive benefits in both nonclinical and clinical participants. Prior to the start of each intervention session, participants were reminded that they could rest whenever they needed to and were provided with water during breaks.
Upon the completion of 12 intervention sessions, participants returned for the immediate post-intervention assessment between 12 and 36 h. The immediate post-intervention involved a repeat of the baseline assessment. We employed this post-intervention assessment window to ensure any transient effects of game play were eliminated as potential confounds for the cognitive assessments rather than the effects of MR intervention itself [
16,
19]. Prior to leaving the immediate post-intervention assessment, participants scheduled their 90-day follow-up assessment, which was a repeat of the immediate post-intervention assessment. We employed this wash-out period because we were interested in learning whether any cognitive performance gains observed during the immediate post-intervention assessment would be stable for our participants or whether performance would revert to baseline following a 90-day washout period (
Figure 1).
4. Discussion
As technological advances begin to integrate within rehabilitation spaces, questions arise as to their applicability towards rehabilitative efforts, such as within cognitive rehabilitation. While studies exist showing promising results using VR as a cognitive rehabilitative platform, mixed reality (MR) has yet to be fully explored within the same space. This case series was an early feasibility examination into the efficacy of using MR as a cognitive rehabilitation aid for subacute and chronic stroke patients.
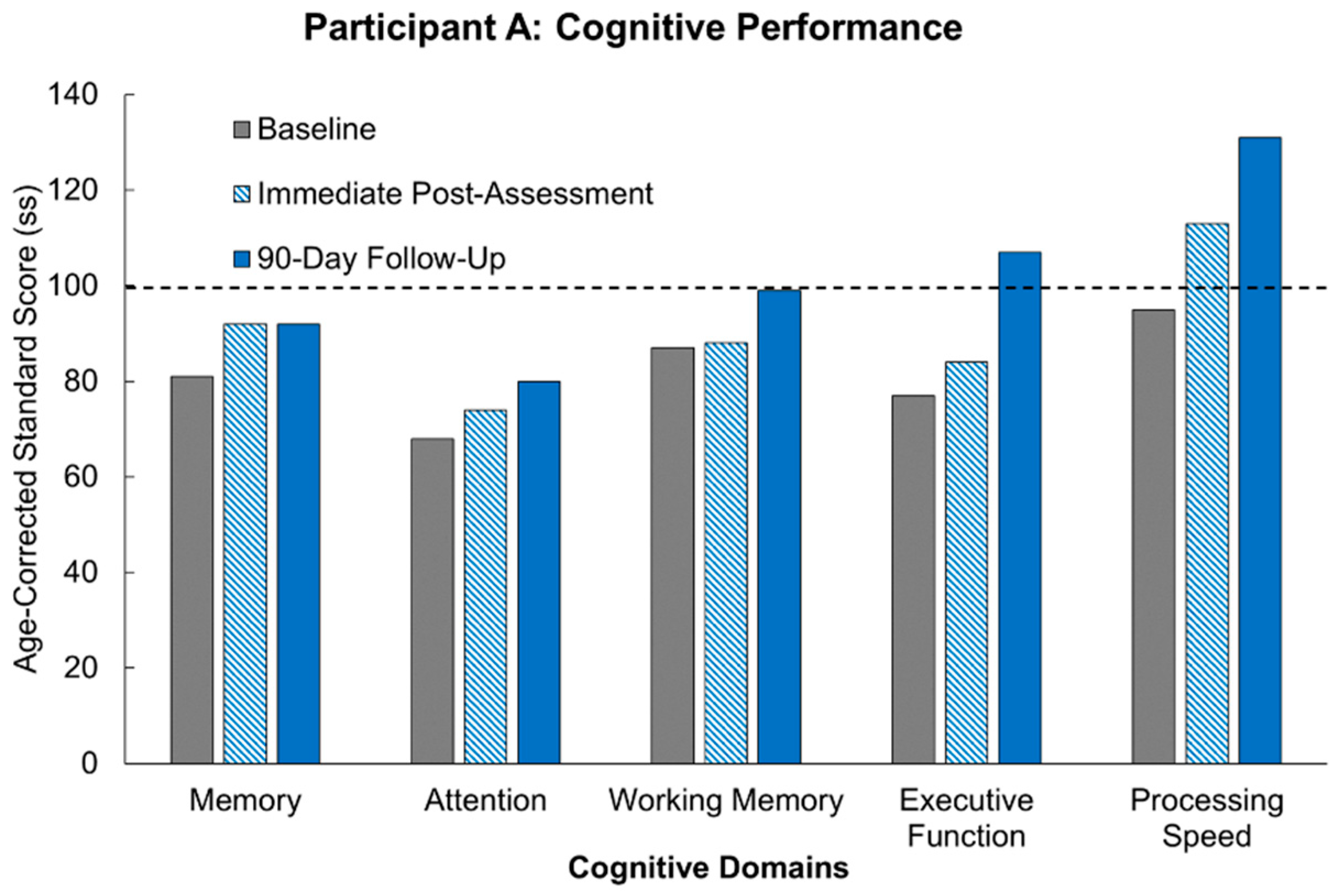
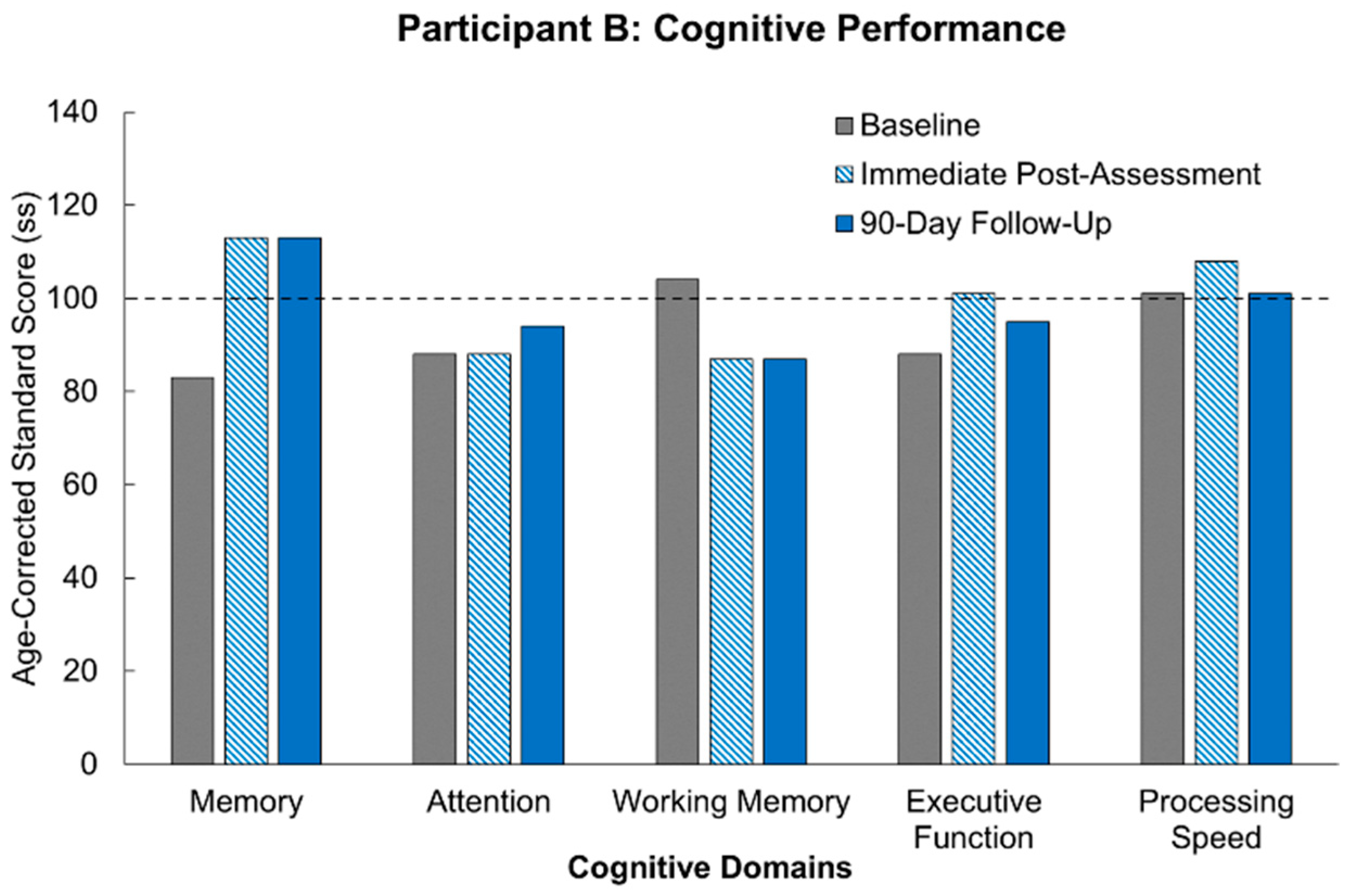
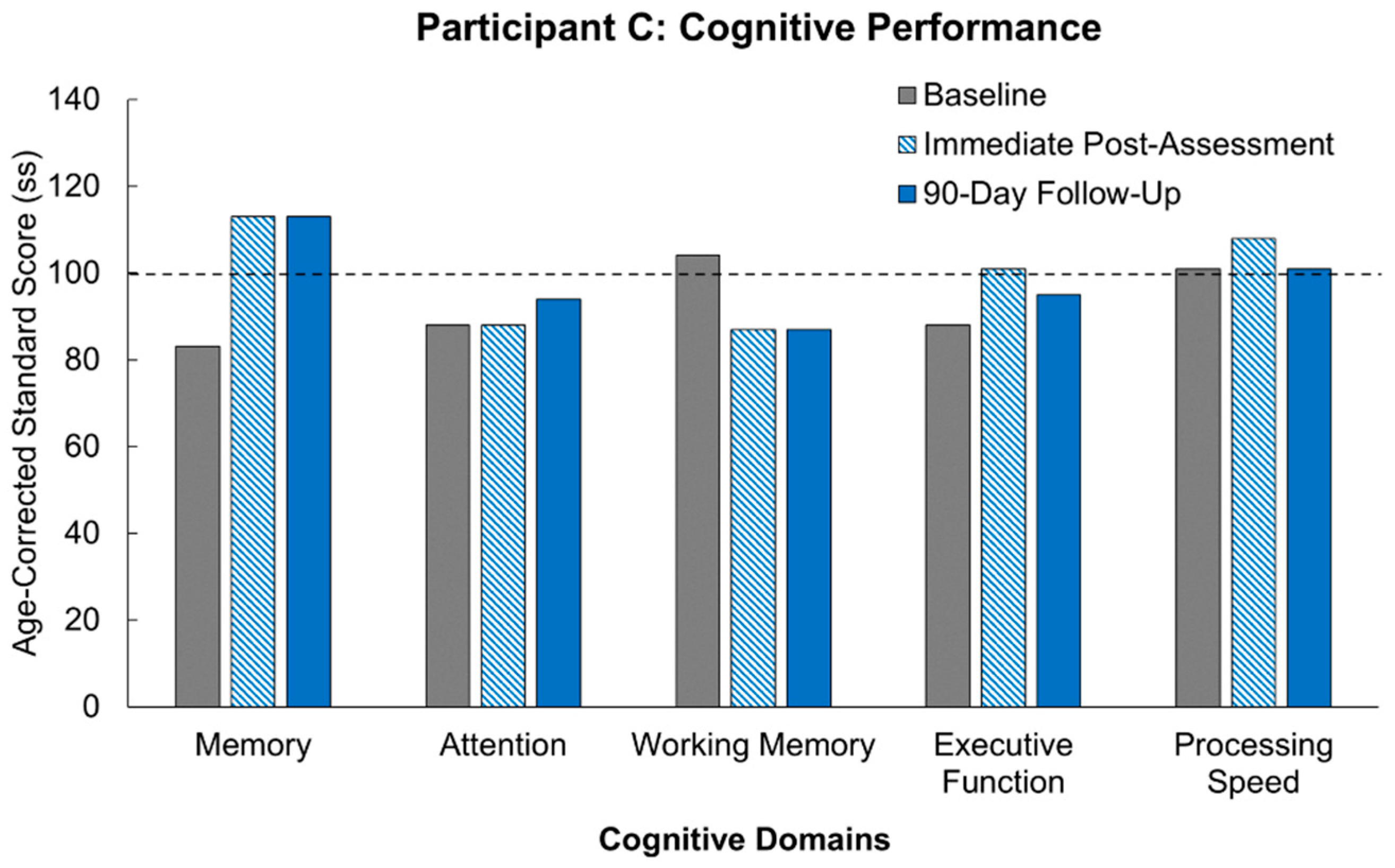
Our results following MR intervention amongst our participants demonstrate the promise of the true potential of MR usage. Of significant note is the fact that all three participants demonstrated improvement across multiple domains following the MR intervention. Not only was there improvement, but for most of the cognitive domains, the participants demonstrated improvements above their own starting baselines. These results are congruent with supporting studies aligning immersive technology with subsequent enhanced neuroplastic changes [
10]. At the start of the study, two out of the three participants were performing at least a standard deviation below others of their age-group who had not suffered from a stroke. What is truly remarkable for our participants is that following 12 h of our MR intervention, the participants showed cognitive recovery to the level of performance of healthy participants, and in a few domains, we actually saw the participants’ performance surpass that of healthy controls. Furthermore, these improvements were stable across 90 days of no intervention, and in some cases, the participants continued to show improvement. This sustained effect is in line with the parameters supporting neuroplasticity including task relevance, repetition, and intensity [
12]. Another important aspect of our study to note is that two of our three participants were more than 10 years out from their strokes. Demonstrating continued recovery is possible for chronic patients.
What can further be observed is that the consistency of improvement across participants persists despite their varying sites of injury. In Participant B, for example, her stroke affected the basal ganglia, which is most associated with cognitive dysfunction across the three participants, yet multiple cognitive domains demonstrated improvement following intervention. In contrast, Participant C suffered injury to her brainstem following stroke, which is the location across all three participants least associated with cognitive dysfunction relatively. This explains why she tested at age-controlled baseline levels across multiple domains initially. The apparent improvement across multiple cognitive domains despite the initial baseline results further suggests efficacy when using MR gaming as an intervention.
Participant B presents notable results considering she completed the study while seated. It was mentioned prior that MR could improve upon the success of VR as a rehabilitative method as its real-world integration could present a safer modality for stroke patients who would inevitably have additional motor dysfunction. This participant experienced foot drop following her stroke and was able to complete the intervention without any complications and with demonstrated improvement across multiple domains. The success of this could be used as corroboration of the previous claim. One set of results for Participant B raised some concern. Her working memory regressed from her initial baseline at both post-intervention assessments. Up to this point it has been difficult to identify an explanation for this anomaly. However, there is no reason to assume that this result is anything more than an isolated irregularity. Despite this likelihood, there is demonstrative importance in monitoring individualized variability in response to cognitive interventions [
10]. As the researchers expand the sample size, it will be a point of monitoring to see if a trend arises.
What presents as further promise amongst the results is participant scoring following the 90-day washout period. An imperative to post-stroke rehabilitation is the ability for intervention to maintain rehabilitative improvements after therapy has ceased. The characteristics driving this maintenance is highlighted by the integral parameters promoting neuroplasticity, as mentioned earlier. While not all participants maintained continued cognitive improvement following the 90-day washout period, general trends displayed maintenance of post-intervention levels. Memory displayed the most promise as it was the cognitive domain that was either improved or maintained at baseline across all participants. In the individual domains that did display decreases following washout, scores did not reach below age-corrected baseline levels, especially when considering results that helped achieve immediate post-intervention scores above standard age-corrected levels. Furthermore, as with other measures, there are minor fluctuations in performance across days within the same individual, and it is generally believed that performance fluctuations that are less than half a standard deviation are generally considered to be normal performance [
21].
One benefit previously mentioned in regard to immersive technology within rehabilitation is the increased engagement factor it can provide. This factor can help maintain the parameters required for neuroplastic changes. However, it is important to note that there is inevitable variability across demographics such as age and sex, that would affect engagement levels in an MR system. For example, the elderly, who do not have advanced technological experience, would have a larger learning curve, limiting their engagement. However, author A.C.G. has conducted several extended reality studies in aging populations (with our oldest participant being 94 years of age), and the novelty of the technology has, to date, been an asset rather than a deterrent. Furthermore, in our experience, the novelty of the MR platform has been a draw to our older and younger participants alike. Specifically related to the current study, we have promising pilot feedback from participants as old as 75, to support that MR is accessible and engaging to a wide age range. Moreover, as the population continues to age, more and more individuals will have had experience with more advanced and immersive technologies. Therefore, the previously mentioned challenge of unfamiliarity will eventually become obsolete. Secondly, due to video games being more popular amongst males, there may be more initial engagement amongst males than females. This is an important consideration to have as it does present potential challenges in the full integration of an MR system. However, two factors around MR could address these challenges. For one, it has potential as a standard within the rehabilitative space. Most medical innovations begin with some unfamiliarity, especially when being first introduced to its respective sector. However, becoming standard practice inevitably creates familiarity, especially as there are proven outcomes. Secondly, MR has customizability, which would ease the transition of learning a new technology and cater to individual experience.
While the limited scope of this review cannot draw a causal relationship, it opens the door for more extensive evaluation. Thus, studies with larger samples sizes and varying periods of intervention are needed before widespread integration of MR into cognitive rehabilitation. With further experimentation, the future applicability of MR within post-stroke rehabilitation can be considered. For example, it is potential to act as an integrated telemedicine tool, considering it is a platform accessible to patients outside of a healthcare setting. Additionally, because there is versatility in the types of programs displayed by MR consoles, there is potential for rehabilitative personalization that caters to each patient’s needs.