Four-Year Outcomes of Immediately Loaded Full-ArchMaxillary Dental Implants with Hybrid Versus Roughened Surfaces: A Split-Mouth Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
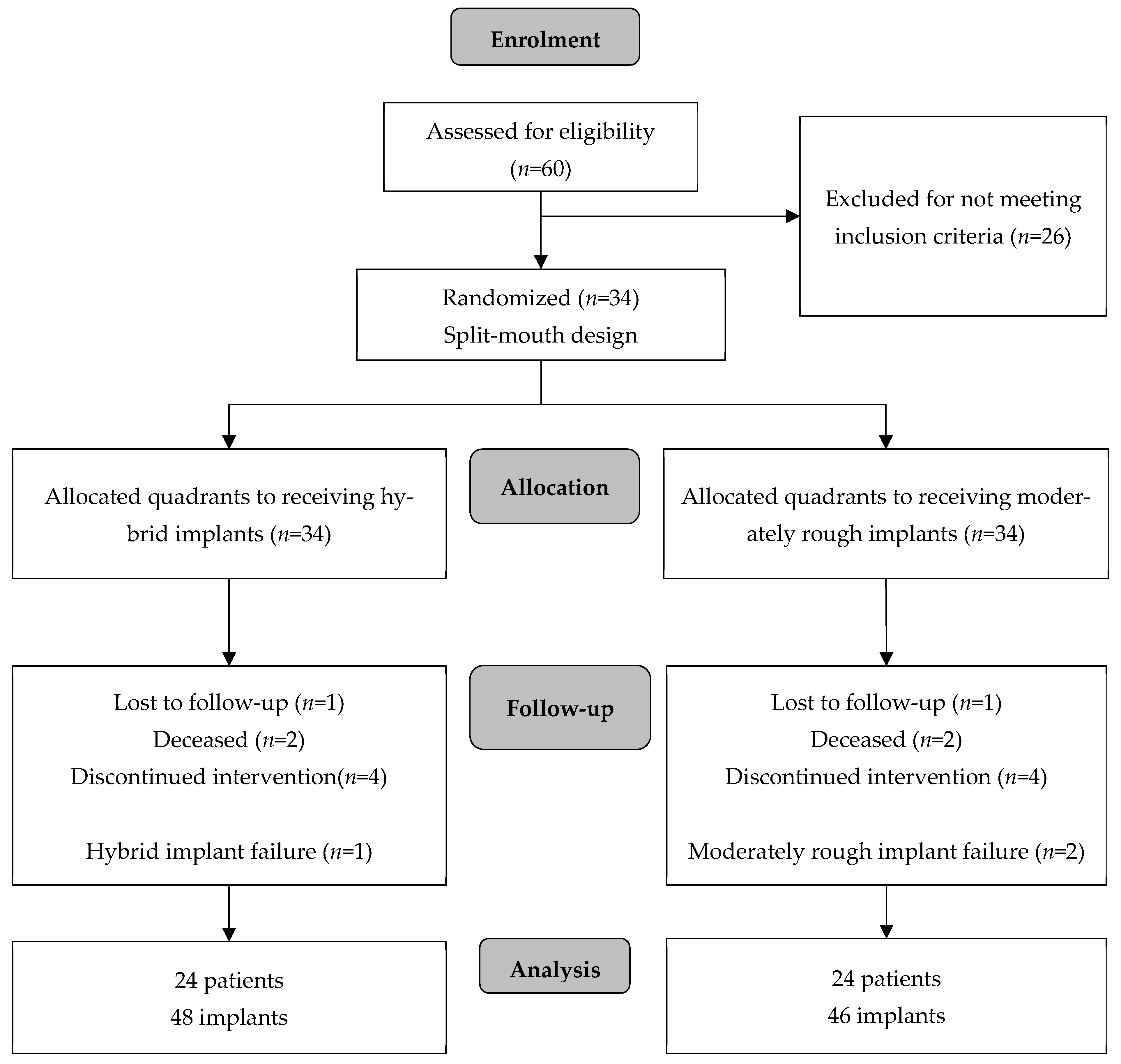
2.1. Study Design and Patient Selection
- Partially and fully edentulous patients older than 21 years requiring full arch rehabilitation of the maxilla;
- Sufficient bone volume to place implants at least 4 mm in diameter and 10 mm long, respectively;
- Willingness to participate and complete the study and provide consent.
- Poor oral health;
- Active periodontal disease;
- Previous irradiation treatment to the head and neck;
- Immunosuppressed or immunocompromised;
- Bisphosphonate medication;
- Smoking > 10 cigarettes per day;
- Uncontrolled diabetes.
2.2. Surgical Protocol
2.3. Radiographic Analysis
2.4. Clinical Evaluation
2.5. Statistical Analysis
3. Results
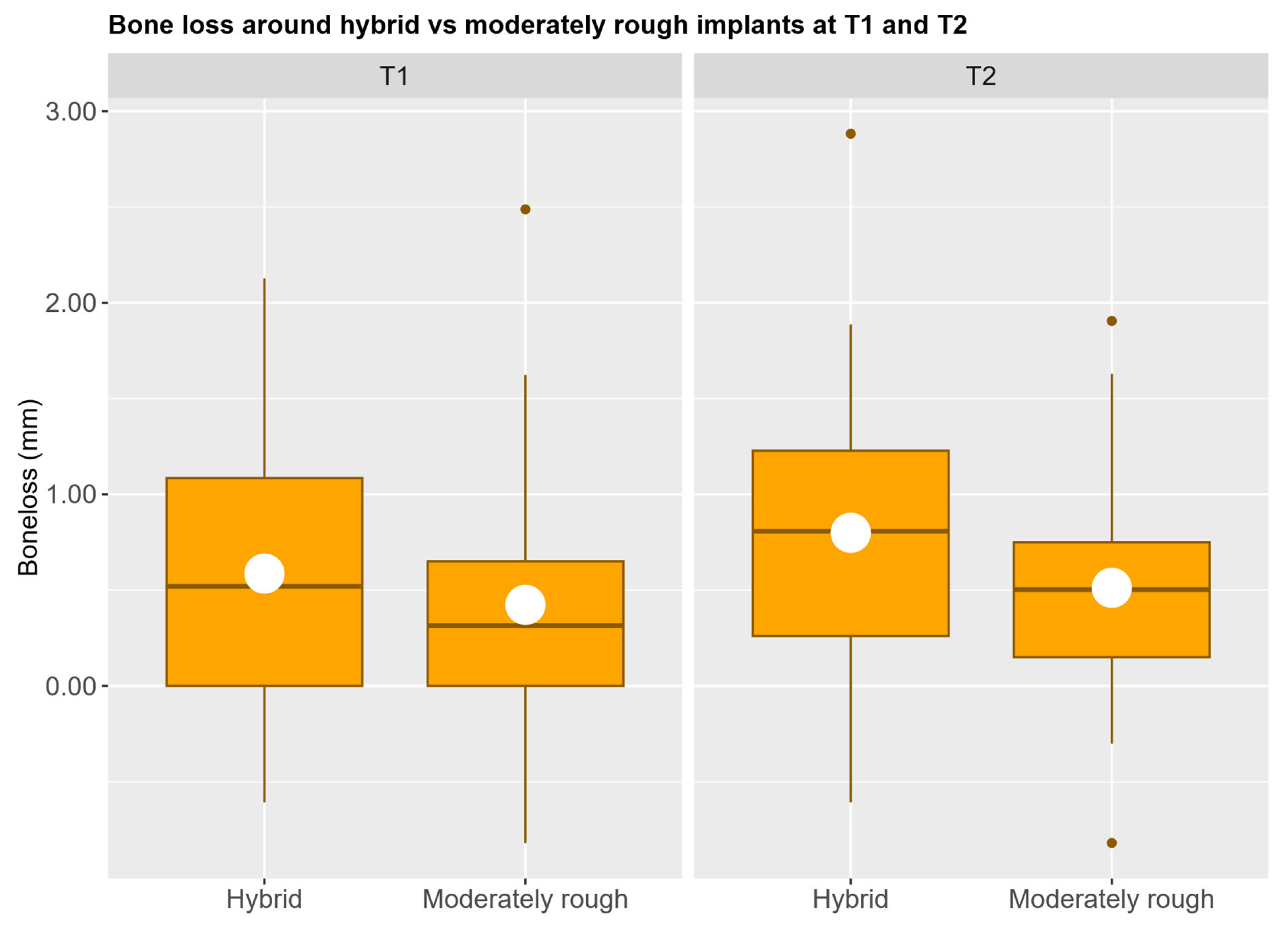
3.1. Crestal Bone Loss
3.2. Implant Survival
3.3. Pocket Probing Depth (PPD), Plaque Index (PI), and Bleeding on Probing (BOP)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Sa | Arithmetical mean height of surface area |
| Sdr | Developed interfacial area ratio |
| mm | Millimeters |
| Ncm | Newton centimeters |
| PPD | Pocket Probing Depth |
| PI | Plaque Index |
| BOP | Bleeding on Probing |
References
- Slot, W.; Raghoebar, G.M.; Cune, M.S.; Vissink, A.; Meijer, H.J.A. Maxillary overdentures supported by four or six implants in the anterior region: 10-year randomized controlled trial results. J. Clin. Periodontol. 2023, 50, 36–44. [Google Scholar] [CrossRef]
- Schimmel, M.; Janner, S.F.M.; Joda, T.; Wittneben, J.G.; McKenna, G.; Brägger, U. Mandibular implant-supported fixed complete dental prostheses on implants with ultrashort and standard length: A pilot treatment. J. Prosthet. Dent. 2021, 126, 137–143. [Google Scholar] [CrossRef]
- Zygogiannis, K.; Aartman, I.H.; Parsa, A.; Tahmaseb, A.; Wismeijer, D. Implant Mandibular Overdentures Retained by Immediately Loaded Implants: A 1-Year Randomized Trial Comparing the Clinical and Radiographic Outcomes Between Mini Dental Implants and Standard-Sized Implants. Int. J. Oral Maxillofac. Implant. 2017, 32, 1377–1388. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Gonçalves, A.; Gabrielli, M.A.C.; de Andrade, C.R.; Vieira, E.H.; Pereira-Filho, V.A. Evaluation of Alveolar Bone Quality: Correlation Between Histomorphometric Analysis and Lekholm and Zarb Classification. J. Craniofacial Surg. 2021, 32, 2114–2118. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, J.M.; Oliveira, Z.F.; Mansini, R.; Cabral, A.M. Correlation between placement torque and survival of single-tooth implants. Int. J. Oral Maxillofac. Implant. 2005, 20, 769–776. [Google Scholar]
- De Bruyn, H.; Raes, S.; Ostman, P.O.; Cosyn, J. Immediate loading in partially and completely edentulous jaws: A review of the literature with clinical guidelines. Periodontology 2014, 66, 153–187. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, S.J.; Zitzmann, N.U. Protocols for the Maxillary Implant Overdenture: A Systematic Review. Int. J. Oral Maxillofac. Implants 2016, 31, 182–191. [Google Scholar] [CrossRef]
- De Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontology 2017, 73, 218–227. [Google Scholar] [CrossRef]
- Doornewaard, R.; Christiaens, V.; De Bruyn, H.; Jacobsson, M.; Cosyn, J.; Vervaeke, S.; Jacquet, W. Long-Term Effect of Surface Roughness and Patients’ Factors on Crestal Bone Loss at Dental Implants. A Systematic Review and Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2017, 19, 372–399. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef]
- Nagay, B.E.; Dini, C.; Borges, G.A.; Mesquita, M.F.; Cavalcanti, Y.W.; Magno, M.B.; Maia, L.C.; Barão, V.A.R. Clinical efficacy of anodized dental implants for implant-supported prostheses after different loading protocols: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2021, 32, 1021–1040. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.; Ohe, J.Y.; Song, S.J.; Paek, J. Correlation between implant angulation and crestal bone changes: A 5-year retrospective study. J. Prosthet. Dent. 2025, 133, e1–e162. [Google Scholar] [CrossRef]
- Howes, D. Angled Implant Design to Accommodate Screw-retained Implant-supported Prostheses. Compend. Contin. Educ. Dent. 2017, 38, 458–463. [Google Scholar] [PubMed]
- Galve-Huertas, A.; Zilleruelo-Pozo, M.J.; García-González, S.; Ortíz-Puigpelat, O.; Hernández-Alfaro, F.; Aboul-Hosn Centenero, S. Clinical Evidence on a Novel Macrohybrid Design Dental Implant with 12° Angled Platform: A Systematic Review. Materials 2022, 15, 5011. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Berglundh, T.; Gotfredsen, K.; Zitzmann, N.U.; Lang, N.P.; Lindhe, J. Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: An experimental study in dogs. Clin. Oral Implant. Res. 2007, 18, 655–661. [Google Scholar] [CrossRef]
- Glibert, M.; Vervaeke, S.; Ibrahim, W.; Doornewaard, R.; De Bruyn, H. A Split-Mouth Study to Assess the Effect of Implant Surface Roughness on Implant Treatment Outcome After 5 Years. Int. J. Periodontics Restor. Dent. 2023, 43, 113–119. [Google Scholar] [CrossRef]
- Raes, M.; D’hondt, R.; Teughels, W.; Coucke, W.; Quirynen, M. A 5-year randomized clinical trial comparing minimally with moderately rough implants in patients with severe periodontitis. J. Clin. Periodontol. 2018, 45, 711–720. [Google Scholar] [CrossRef]
- Serrano, B.; Sanz-Sánchez, I.; Serrano, K.; Montero, E.; Sanz, M. One-year outcomes of dental implants with a hybrid surface macro-design placed in patients with history of periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2022, 49, 90–100. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 statement: Updated guideline for reporting randomised trials. BMJ 2025, 389, e081123. [Google Scholar] [CrossRef] [PubMed]
- Vervaeke, S.; Dierens, M.; Besseler, J.; De Bruyn, H. The influence of initial soft tissue thickness on peri-implant bone remodeling. Clin. Implant. Dent. Relat. Res. 2014, 16, 238–247. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Svanborg, L.M.; Andersson, M.; Wennerberg, A. Surface characterization of commercial oral implants on the nanometer level. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 462–469. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tirone, F.; Salzano, S.; Rodi, D.; Pozzatti, L. Three-Year Evaluation of the Influence of Implant Surfaces on Implant Failure and Peri-implantitis: A Double-Blind Randomized Controlled Trial with Split-Mouth Design. Int. J. Oral Maxillofac. Implant. 2021, 36, e23–e30. [Google Scholar] [CrossRef]
- Lee, S.Y.; Piao, C.M.; Koak, J.Y.; Kim, S.K.; Kim, Y.S.; Ku, Y.; Rhyu, I.C.; Han, C.H.; Heo, S.J. A 3-year prospective radiographic evaluation of marginal bone level around different implant systems. J. Oral Rehabil. 2010, 37, 538–544. [Google Scholar] [CrossRef]
- Zetterqvist, L.; Feldman, S.; Rotter, B.; Vincenzi, G.; Wennström, J.L.; Chierico, A.; Stach, R.M.; Kenealy, J.N. A prospective, multicenter, randomized-controlled 5-year study of hybrid and fully etched implants for the incidence of peri-implantitis. J. Periodontol. 2010, 81, 493–501. [Google Scholar] [CrossRef]


| Time Point | Parameter | Implant Group | Estimated Marginal Means | Standard Error | 95% Confidence Level | p-Value | |
|---|---|---|---|---|---|---|---|
| Upper Bound | Lower Bound | ||||||
| T1 | Implant type | Hybrid | 0.57 | 0.17 | 0.24 | 0.91 | 0.716 |
| Moderately rough | 0.54 | 0.16 | 0.22 | 0.85 | |||
| Implant angulation | Hybrid | 0.53 | 0.17 | 0.18 | 0.87 | 0.685 | |
| Moderately rough | 0.16 | 0.16 | 0.26 | 0.90 | |||
| Surgery type | Hybrid | 0.55 | 0.16 | 0.24 | 0.87 | 0.999 | |
| Moderately rough | 0.55 | 0.18 | 0.19 | 0.92 | |||
| Graft | Hybrid | 0.50 | 0.25 | 0.00 | 1.00 | 0.644 | |
| Moderately rough | 0.61 | 0.11 | 0.38 | 0.84 | |||
| Restoration type | Hybrid | 0.51 | 0.20 | 0.11 | 0.92 | 0.710 | |
| Moderately rough | 0.60 | 0.17 | 0.25 | 0.95 | |||
| T2 | Implant type | Hybrid | 0.77 | 0.14 | 0.49 | 1.06 | 0.074 |
| Moderately rough | 0.57 | 0.14 | 0.30 | 0.84 | |||
| Implant angulation | Hybrid | 0.68 | 0.15 | 0.37 | 0.98 | 0.892 | |
| Moderately rough | 0.66 | 0.14 | 0.39 | 0.93 | |||
| Surgery type | Hybrid | 0.75 | 0.15 | 0.44 | 1.05 | 0.282 | |
| Moderately rough | 0.60 | 0.14 | 0.32 | 0.87 | |||
| Graft | Hybrid | 0.53 | 0.23 | 0.07 | 0.98 | 0.217 | |
| Moderately rough | 0.81 | 0.08 | 0.64 | 0.99 | |||
| Restoration type | Hybrid | 0.67 | 0.14 | 0.40 | 0.95 | 0.979 | |
| Moderately rough | 0.67 | 0.17 | 0.34 | 1.00 | |||
| Parameter | Implant Group | T1 | T2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | p-Value | Mean | SD | Range | p-Value | ||
| PPD (mm) | Hybrid | 2.38 | 1.02 | 0–3.55 | 0.564 | 2.04 | 0.57 | 1.15–3.40 | 0.295 |
| Moderately rough | 2.34 | 1.00 | 0–3.50 | 2.18 | 0.61 | 1.20–3.50 | |||
| PI (%) | Hybrid | 22 | 23 | 0–83 | 0.830 | 9 | 13 | 0–42 | 0.076 |
| Moderately rough | 22 | 23 | 0–75 | 13 | 13 | 0–42 | |||
| BOP (%) | Hybrid | 20 | 19 | 0–75 | 0.214 | 9 | 12 | 0–42 | 0.637 |
| Moderately rough | 16 | 18 | 0–67 | 20 | 19 | 0–75 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Offord, D.; Kingsford, N.; Glibert, M.; Pitman, J.; Christiaens, V. Four-Year Outcomes of Immediately Loaded Full-ArchMaxillary Dental Implants with Hybrid Versus Roughened Surfaces: A Split-Mouth Randomized Controlled Trial. J. Clin. Med. 2025, 14, 7996. https://doi.org/10.3390/jcm14227996
Offord D, Kingsford N, Glibert M, Pitman J, Christiaens V. Four-Year Outcomes of Immediately Loaded Full-ArchMaxillary Dental Implants with Hybrid Versus Roughened Surfaces: A Split-Mouth Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(22):7996. https://doi.org/10.3390/jcm14227996
Chicago/Turabian StyleOfford, David, Nicola Kingsford, Maarten Glibert, Jeremy Pitman, and Véronique Christiaens. 2025. "Four-Year Outcomes of Immediately Loaded Full-ArchMaxillary Dental Implants with Hybrid Versus Roughened Surfaces: A Split-Mouth Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 22: 7996. https://doi.org/10.3390/jcm14227996
APA StyleOfford, D., Kingsford, N., Glibert, M., Pitman, J., & Christiaens, V. (2025). Four-Year Outcomes of Immediately Loaded Full-ArchMaxillary Dental Implants with Hybrid Versus Roughened Surfaces: A Split-Mouth Randomized Controlled Trial. Journal of Clinical Medicine, 14(22), 7996. https://doi.org/10.3390/jcm14227996







