Anticoagulation Strategies for Left Ventricular Thrombus After Myocardial Infarction: A Review
Abstract
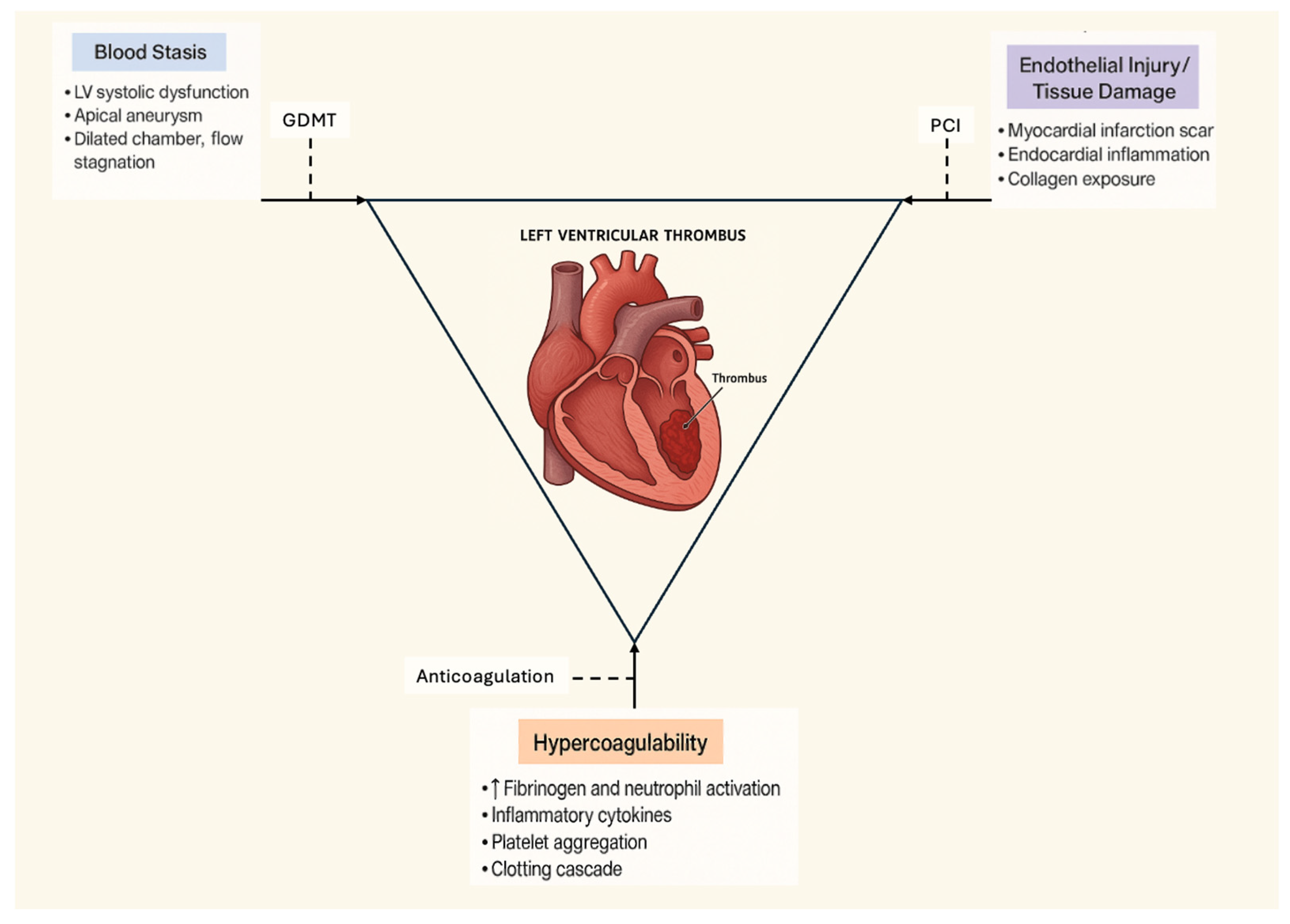
1. Introduction
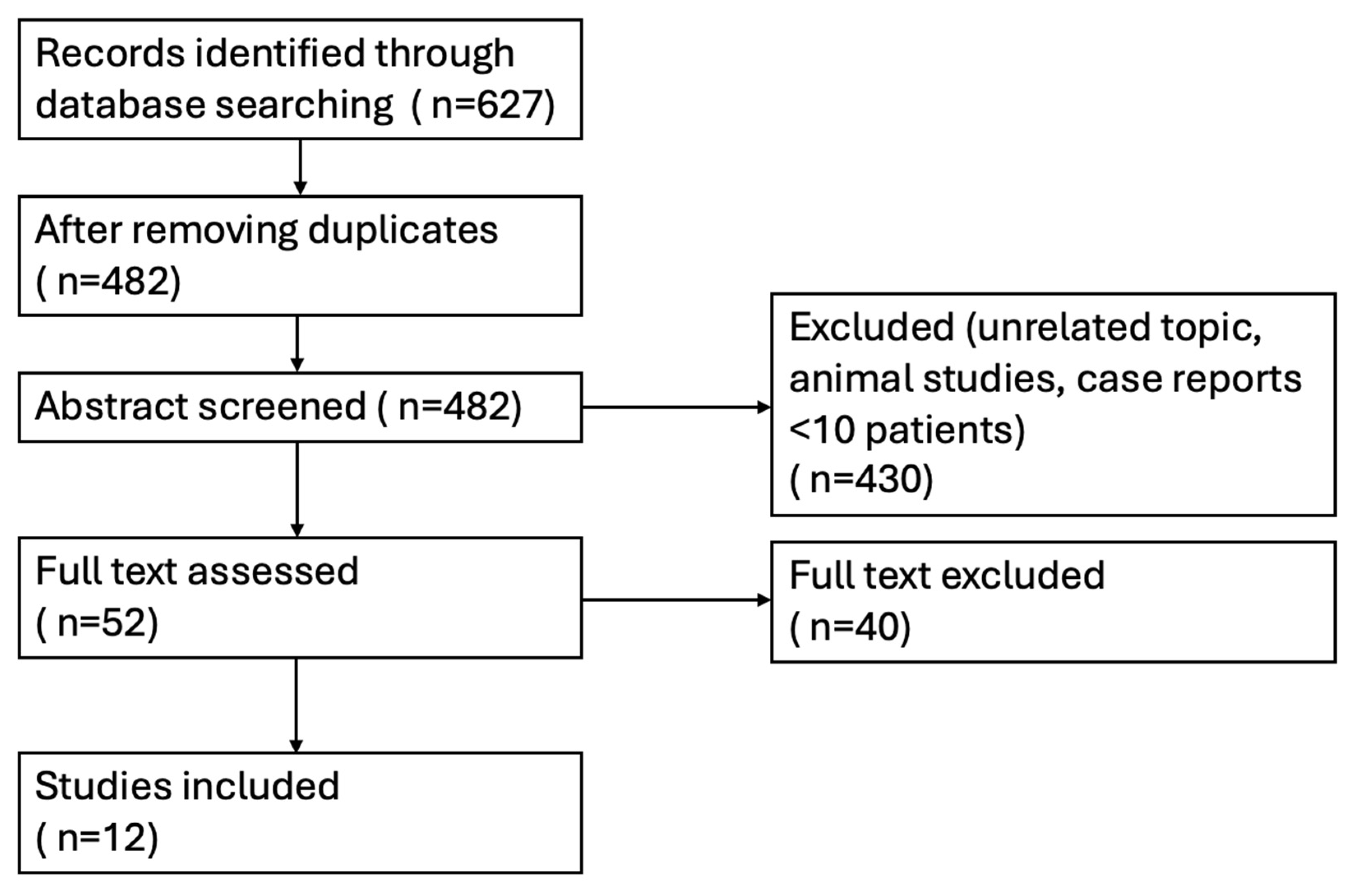
2. Methods
2.1. Search Strategy and Study Selection
- Enrolled adult patients (≥18 years) with documented LVT following MI, confirmed by echocardiography, contrast echocardiography, or cardiac magnetic resonance (CMR);
- Included a comparison between a direct oral anticoagulant (DOAC: rivaroxaban, apixaban, dabigatran, or edoxaban) and a vitamin K antagonist (VKA: warfarin);
- Reported at least one relevant outcome, including thrombus resolution, systemic embolism or ischemic stroke, major bleeding, or all-cause mortality.
2.2. Data Extraction and Outcomes of Interest
2.3. Quality Assessment
2.4. Synthesis of Evidence
2.5. Used of GenAI
3. Results
3.1. Thrombus Resolution
3.2. Stroke and Systemic Embolism
3.3. Major Bleeding
3.4. All-Cause Mortality
4. Discussion
- [1]
- Persistent LV dysfunction: Patients with ongoing severe systolic dysfunction remain at high risk for recurrent thrombi, with recurrence rates up to 15% [4]. Extended anticoagulation beyond 6 months may be appropriate, but the optimal duration is unknown.
- [2]
- Elderly patients: Older age increases both thrombotic and bleeding risks. DOACs may be preferable due to reduced intracranial bleeding [9], but renal clearance must be carefully monitored.
- [3]
- Chronic kidney disease (CKD): VKAs can be used across the full spectrum of renal functions, while DOAC dosing must be adjusted or avoided in advanced CKD. Observational studies of DOACs in CKD populations show reassuring efficacy and safety, though data in LVT are sparse [27].
- [4]
- Non-ischemic cardiomyopathy: LVT also occurs in dilated or hypertrophic cardiomyopathy. Small case series suggest DOACs may be effective, but evidence is limited, and further study is required [33].
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Delewi, R.; Zijlstra, F.; Piek, J.J. Left ventricular thrombus formation after acute myocardial infarction. Heart 2012, 98, 1743–1749. [Google Scholar] [CrossRef]
- Robinson, A.A.; Jain, A.; Gentry, M.; McNamara, R.L. Left ventricular thrombi after STEMI in the contemporary era: Systematic review and meta-analysis of prevalence and predictors. J. Am. Coll. Cardiol. 2016, 68, 236–246. [Google Scholar]
- Meurin, P.; Carreira, V.B.; Dumaine, R.; Shqueir, A.; Milleron, O.; Safar, B.; Perna, S.; Smadja, C.; Genest, M.; Garot, J.; et al. Incidence, diagnostic methods, and evolution of left ventricular thrombus in patients with anterior myocardial infarction and low left ventricular ejection fraction: A prospective multicenter study. Am. Heart J. 2015, 170, 256–262. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.P.; Murphy, S.; Venkateswaran, R.V.; Singh, A.; Chang, L.L.; Joice, M.G.; Rivero, J.M.; Vaduganathan, M.; Januzzi, J.L.; Bhatt, D.L. Left ventricular thrombus: Contemporary etiologies, treatment strategies, and outcomes. J. Am. Coll. Cardiol. 2019, 73, 2007–2009. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, N.; Fujino, M.; Nakai, M.; Nishimura, K.; Miyamoto, Y.; Kataoka, Y.; Asaumi, Y.; Tahara, Y.; Nakanishi, M.; Anzai, T.; et al. Anticoagulation combined with antiplatelet therapy in patients with left ventricular thrombus after acute myocardial infarction. J. Am. Coll. Cardiol. 2019, 73, 1733–1740. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- O’gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, e78–e140. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar]
- Robinson, A.A.; Trankle, C.R.; Eubanks, G.; Schumann, C.; Thompson, P.; Wallace, R.L.; Gottiparthi, S.; Ruth, B.; Kramer, C.M.; Salerno, M.; et al. Off-label use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol. 2020, 5, 685–692. [Google Scholar] [CrossRef]
- Alcalai, R.; Butnaru, A.; Moravsky, G.; Yagel, O.; Rashad, R.; Ibrahimli, M.; Planer, D.; Amir, O.; Elbaz-Greener, G.; Leibowitz, D. Apixaban vs. warfarin in patients with left ventricular thrombus: A prospective multicentre randomized clinical trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 660–667. [Google Scholar] [CrossRef]
- Youssef, A.A.; Alrefae, M.A.; Khalil, H.H.; Abdullah, H.I.; Khalifa, Z.S.; Al Shaban, A.A.; Wali, H.A.; AlRajab, M.R.; Saleh, O.M.; Nashy, B.N. Apixaban in Patients with Post-Myocardial Infarction Left Ventricular Thrombus: A Randomized Clinical Trial. CJC Open 2022, 5, 191–199. [Google Scholar] [CrossRef]
- Shah, J.A.; Hussain, J.; Ahmed, B.; Batra, M.K.; Ali, G.; Naz, M.; Khan, W.; Bhatti, K.I.; Karim, M.; Hakeem, A. Rivaroxaban vs Warfarin in Acute Left Ventricular Thrombus Following Myocardial Infarction: RIVAWAR, An Open-Label RCT. JACC Adv. 2025, 4, 101978. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Wright, P.; Alizadeh, M.A.; Fhadil, S.; Rathod, K.S.; Guttmann, O.; Knight, C.; Timmis, A.; Baumbach, A.; Wragg, A.; et al. The use of novel oral anticoagulants compared to vitamin K antagonists (warfarin) in patients with left ventricular thrombus after acute myocardial infarction. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ott. Hosp. Res. Inst. 2014, 2, 1–12. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Attachaipanich, T.; Thanyaratsarun, T.; Attachaipanich, S.; Danpanichkul, P.; Kaewboot, K. Efficacy of direct oral anticoagulants vs. warfarin in left ventricular thrombus in myocardial infarction: Systematic review and meta-analysis. J. Cardiovasc. Med. 2025, 26, 40–49. [Google Scholar] [CrossRef]
- Albabtain, M.A.; Alhebaishi, Y.; Al-Yafi, O.; Kheirallah, H.; Othman, A.; Alghosoon, H.; Arafat, A.A.; Alfagih, A. Rivaroxaban versus warfarin for the management of left ventricle thrombus. Egypt. Heart J. 2021, 73, 41. [Google Scholar] [CrossRef]
- Jaidka, A.K.Z.T.; Lavi, S.; Johri, A. Treatment of left ventricular thrombus using warfarin versus direct oral anticoagulants following anterior myocardial infarction. Can. J. Cardiol. 2018, 34, S143. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Zhou, Y.; Shen, H.; Chai, M.; Ma, X.; Han, H.; Shao, Q.; Li, Q. Efficacy and Safety of Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus After Acute Anterior Myocardial Infarction in Patients Who Underwent Percutaneous Coronary Intervention. Curr. Vasc. Pharmacol. 2022, 20, 517–526. [Google Scholar] [CrossRef]
- Mansouri, P.; Jazi, Z.A.; Mansouri, M.H.; Dehghan, H.; Zavar, R.; Hashemi, S.M.; Sattar, F.; Sadeghi, M.; Amirpour, A.; Abdar, M. Evaluation of the efficacy and safety of rivaroxaban compared to warfarin in patients with left ventricular apical thrombus: A randomized clinical trial. Thromb. J. 2024, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Gue, Y.; Lip, G.Y.H. Comparison of Direct Oral Anticoagulants and Vitamin K Antagonists for Left Ventricular Thrombus: A Global Retrospective Study. Am. J. Med. 2025, 138, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Si, D.; Zhang, Q.; Qu, M.; Yu, M.; Jiang, Z.; Li, D.; Yang, P.; Zhang, W. Rivaroxaban versus Vitamin K Antagonists (warfarin) based on the triple therapy for left ventricular thrombus after ST-Elevation myocardial infarction. Heart Vessels. 2022, 37, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Brueckmann, M.; Granger, C.B.; Kappetein, A.P.; Mack, M.J.; Blatchford, J.; Devenny, K.; Friedman, J.; Guiver, K.; et al. Dabigatran versus warfarin in patients with mechanical heart valves. N. Engl. J. Med. 2013, 369, 1206–1214. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Weitz, J.I. Warfarin faring better: Vitamin K antagonists beat rivaroxaban and apixaban in the INVICTUS and PROACT Xa trials. J. Thromb. Haemost. 2023, 21, 3067–3071. [Google Scholar] [CrossRef]
- Sgarra, L.; Desantis, V.; Matteucci, A.; Caccavo, V.P.; Troisi, F.; Di Monaco, A.; Mangini, F.; Katsouras, G.; Guaricci, A.I.; Dadamo, M.L.; et al. Non-Anticoagulation Strategies Aimed at Primary Stroke Prevention in Nascent Atrial Fibrillation. Biomedicines 2025, 13, 660. [Google Scholar] [CrossRef]
- Gibson, M.C.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; van Eickels, M.; et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI (PIONEER AF-PCI). N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.I.H.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation (RE-DUAL PCI). N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Tyler Massaro, M.P.H.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic therapy after ACS or PCI in atrial fibrillation (AUGUSTUS). N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef]
- Shazly, A.; Afifi, A. RE-ALIGN: First trial of novel oral anticoagulant in patients with mechanical heart valves—The search continues. Glob. Cardiol. Sci. Pract. 2014, 2014, 88–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, J.; Wang, L.; Yin, N.; Zhang, Z. Embolic stroke complicating left ventricular thrombus in Takotsubo syndrome: A case report. J. Int. Med. Res. 2025, 53, 3000605251326764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pieszko, K.; Hiczkiewicz, J.; Łojewska, K.; Uziębło-Życzkowska, B.; Krzesiński, P.; Gawałko, M.; Budnik, M.; Starzyk, K.; Wożakowska-Kapłon, B.; Daniłowicz-Szymanowicz, L.; et al. Artificial intelligence in detecting left atrial appendage thrombus by transthoracic echocardiography and clinical features: The Left Atrial Thrombus on Transoesophageal Echocardiography (LATTEE) registry. Eur. Heart J. 2024, 45, 32–41. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Goodman, S.G.; Bhatt, D.L.; Eikelboom, J.W.; Price, M.J.; Moliterno, D.J.; Cannon, C.P.; Tanguay, J.F.; Granger, C.B.; Mauri, L.; et al. Antithrombotic Therapy in Patients with Atrial Fibrillation Treated With Oral Anticoagulation Undergoing Percutaneous Coronary Intervention: A North American Perspective-2018 Update. Circulation 2018, 138, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kimura, K.; Kimura, T.; Ishihara, M.; Otsuka, F.; Kozuma, K.; Kosuge, M.; Shinke, T.; Nakagawa, Y.; Natsuaki, M.; et al. JCS 2020 Guideline Focused Update on Antithrombotic Therapy in Patients with Coronary Artery Disease. Circ. J. 2020, 84, 831–865. [Google Scholar] [CrossRef] [PubMed]
- Osherov, A.B.; Borovik-Raz, M.; Aronson, D.; Agmon, Y.; Kapeliovich, M.; Kerner, A.; Grenadier, E.; Hammerman, H.; Nikolsky, E.; Roguin, A. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am. Heart J. 2009, 157, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, W.; Kim, Y.T.; Cho, J.; Shin, S.Y.; Kim, C.; Kim, J.B. Cost-effectiveness of Direct Oral Anticoagulant vs. Warfarin Among Atrial Fibrillation Patients with Intermediate Stroke Risk. Front. Cardiovasc. Med. 2022, 9, 849474. [Google Scholar] [CrossRef]
- Afzal, S.K.; Hasan, S.S.; Babar, Z.U. A systematic review of patient-reported outcomes associated with the use of direct-acting oral anticoagulants. Br. J. Clin. Pharmacol. 2019, 85, 2652–2667. [Google Scholar] [CrossRef]
- Ng, D.L.-C.; Gan, G.-G.; Chai, C.-S.; Chee, K.-H.; Tan, K.-L.; Tan, S.-B.; Bee, P.-C. Comparing quality of life and treatment satisfaction between patients on warfarin and direct oral anticoagulants: A cross-sectional study. Patient Prefer. Adherence 2019, 13, 1363–1373. [Google Scholar] [CrossRef]
- Lavalle, C.; Pierucci, N.; Mariani, M.V.; Piro, A.; Borrelli, A.; Grimaldi, M.; Rossillo, A.; Notarstefano, P.; Compagnucci, P.; Russo, A.D.; et al. Italian Registry in the Setting of Atrial Fibrillation Ablation with Rivaroxaban—IRIS. Minerva Cardiol. Angiol. 2024, 72, 625–637. [Google Scholar] [CrossRef]
| Study, Year (Country) | Design/ Population | LV Thrombus Detection | DOAC Regimen (n) | Warfarin Regimen (n/INR) | Antiplatelet/ Duration | Follow-Up |
|---|---|---|---|---|---|---|
| Albabtain, 2021 [19] (Saudi Arabia) | Retrospective; LVT post-MI | TTE | Rivaroxaban (n = 6) | n = 7, INR 2–3 | Anticoag median 9.5 mo (6–32.5) | Median 36 mo |
| Alcalai, 2022 [12] (Israel) | RCT; LVT 1–14 days post-MI | TTE | Apixaban (n = 17) | n = 15, INR 2–3 | A + C (1 mo) → C; 3 mo | 3 mo |
| Jones, 2021 [15] (UK) | Prospective; post-MI with PCI | Echo or CMR | Riva 57.9%, Apix 36.8%, Edox 5.3% (n = 38) | n = 60 (INR NR) | NA | Median 1.8 y |
| Byrne, 2023 [6] (UK) | Retrospective; post-MI | NA | Riva 62.5%, Apix 31.3%, Edox 6.3% (n = 16) | n = 41 (INR NR) | SAPT 12.5%/DAPT 88% vs. SAPT 7.5%/DAPT 90% | 1 y |
| Jaidka, 2018 [20] (UK) | Retrospective; anterior-wall STEMI | TTE; contrast echo (83.3%) | n = 9 | n = 21 (INR NR) | 6 mo | 6 mo |
| Jones, 2021 [15] (UK) | Prospective; post-STEMI | TTE; contrast 22.8%; CMR 70.3% | Riva 58.5%, Apix 36.5%, Edox 5% (n = 41) | n = 60, INR 2–3 | DAPT 68.3%/SAPT 24.4%; triple therapy median 3 mo | Median 2.2 y |
| Liang, 2022 [21] (China) | Retrospective; anterior-wall STEMI | TTE | n = 56 (Riva 48, Dabig 8) | n = 72, INR 2–3 | Mostly triple (A + C/T); imaging at 3, 6, 12 mo | 12 mo |
| Mansouri, 2024 [22] (Iran) | RCT; LVT in ACS | TTE | Rivaroxaban (n = 26) | n = 26 (INR NR) | NA | 3 mo |
| Yao, 2025 [23] (International) | Retrospective; LVT in ACS | NA | n = 7151 | n = 7151 | NA | 90 days |
| Youssef, 2022 [13] (Saudi Arabia) | RCT; post-anterior MI | TTE | Apixaban (n = 25) | n = 25, INR 2–3 | DAPT 1 mo 80%; A 80%; C 96–100%; ≥3 mo | 6 mo |
| Zhang, 2022 [24] (China) | Retrospective; post-STEMI | TTE | n = 33 | n = 31, INR 2–2.5 | A + C median 8.5 mo; triple median 8.5 mo (5–17) | Median 25 mo |
| RIVAWAR, 2025 [14] (International) | RCT; post-MI LVT | TTE | Rivaroxaban (n ≈ 130) | Warfarin (n ≈ 131), INR 2–3 | DAPT permitted; 3 mo | 3 mo |
| Outcome | Studies/N | Pooled Effect (DOACs vs. Warfarin) |
|---|---|---|
| Thrombus resolution | 10 studies/625 pts | RR 1.02 (0.98–1.06) |
| Stroke/systemic embolism | 8 studies/14,720 pts | RR 0.84 (0.78–0.90) |
| Major bleeding | 9 studies/14,862 pts | RR 0.87 (0.76–1.01) |
| Any bleeding | 7 studies/443 pts | RR 0.57 (0.33–1.00) |
| All-cause mortality | 6 studies/287 pts | RR 1.08 (0.98–1.19) |
| Study | Major Bleeding Definition Used |
|---|---|
| Albabtain, 2021 [19] | NA |
| Alcalai, 2022 [12] | ISTH |
| Jones, 2021 [15] | ICH, major GI bleeding, hospitalization |
| Byrne, 2023 [6] | NA |
| Jaidka, 2018 [20] | NA |
| Jones, 2021 [15] | BARC > 2 |
| Liang, 2022 [21] | TIMI |
| Mansouri, 2024 [22] | NA |
| Yao, 2025 [23] | ICH and GI bleeding |
| Youssef, 2022 [13] | BARC ≥ 2 |
| Zhang, 2022 [24] | ISTH |
| RIVAWAR, 2025 [14] | ISTH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folman, A.; Toukan, N.; Kobo, O.M.; Roguin, A.; Barel, M.S. Anticoagulation Strategies for Left Ventricular Thrombus After Myocardial Infarction: A Review. J. Clin. Med. 2025, 14, 7982. https://doi.org/10.3390/jcm14227982
Folman A, Toukan N, Kobo OM, Roguin A, Barel MS. Anticoagulation Strategies for Left Ventricular Thrombus After Myocardial Infarction: A Review. Journal of Clinical Medicine. 2025; 14(22):7982. https://doi.org/10.3390/jcm14227982
Chicago/Turabian StyleFolman, Adam, Nicola Toukan, Ofer M. Kobo, Ariel Roguin, and Maguli S. Barel. 2025. "Anticoagulation Strategies for Left Ventricular Thrombus After Myocardial Infarction: A Review" Journal of Clinical Medicine 14, no. 22: 7982. https://doi.org/10.3390/jcm14227982
APA StyleFolman, A., Toukan, N., Kobo, O. M., Roguin, A., & Barel, M. S. (2025). Anticoagulation Strategies for Left Ventricular Thrombus After Myocardial Infarction: A Review. Journal of Clinical Medicine, 14(22), 7982. https://doi.org/10.3390/jcm14227982







