Pediatric Burns: Biological and Tissue Engineered Skin Substitutes—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
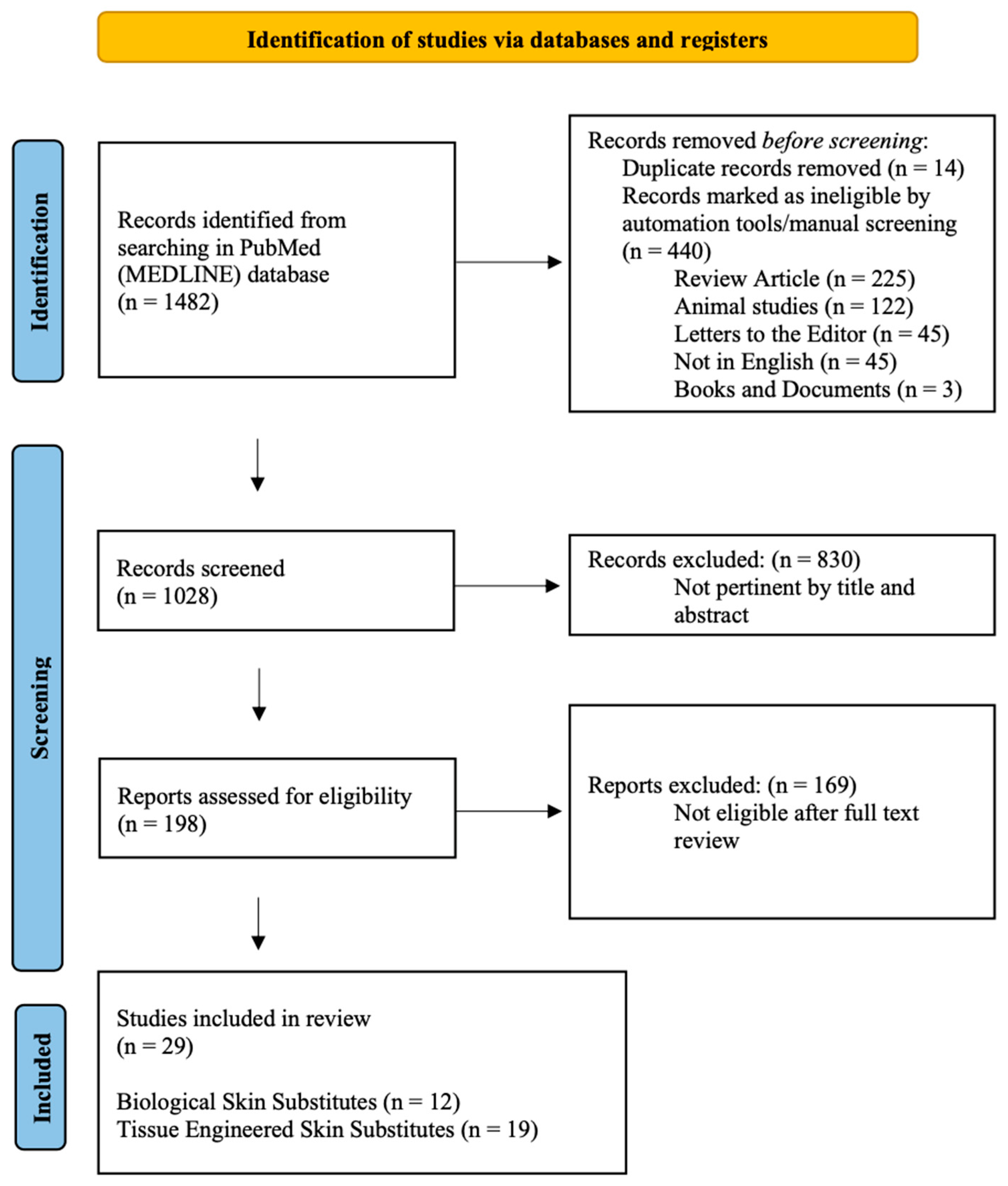
2.1. The Data Sources and Search Strategy
2.2. Study Selection
2.3. Risk of Bias and Quality Assessment
2.4. Data Extraction and Analysis
3. Results
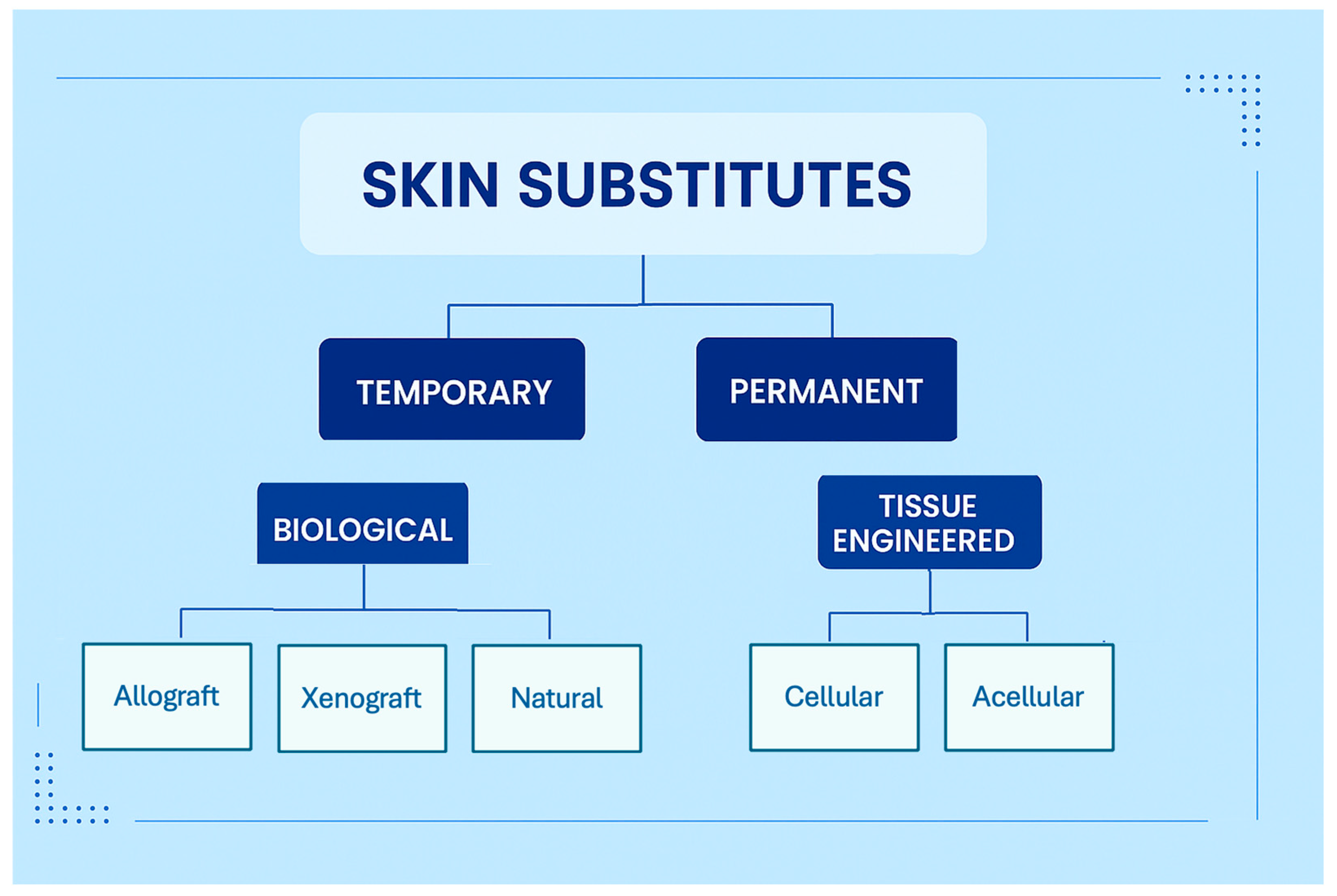
3.1. Outcomes for Biological Skin Substitutes
3.2. Outcomes for Tissue Engineered Skin Substitutes
4. Discussion
4.1. Biological Skin Substitutes
4.1.1. Allograft
4.1.2. Amniotic Membranes
4.1.3. Xenograft
4.1.4. Porcine Xenograft
4.1.5. Tilapia Fish Skin
4.2. Tissue Engineered Skin Substitutes
4.3. Acellular Tissue Engineered Skin Substitutes
4.3.1. Integra®
4.3.2. Biobrane®
4.3.3. AlloDerm®
4.3.4. Matriderm®
4.3.5. Kerecis®
4.3.6. NovoSorb®
4.3.7. Cytal® Burn Matrix
4.4. Cellular Tissue Engineered Skin Substitutes
4.4.1. Autologous/Allogeneic Cultured Skin
4.4.2. OrCelTM®
4.4.3. TransCyte®
4.4.4. Dermagraft®
4.4.5. Apligraf®
4.5. Comparative Interpretation and Evidence Ranking
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSS | Biological Skin Substitutes |
| BTM | Biodegradable Temporizing Matrix |
| CEA | Cultured Epithelial Autograft |
| dHACM | Dehydrated Human Amniotic/Chorionic Membrane |
| FDA | Food and Drug Administration |
| FGF | Fibroblast Growth Factor |
| HIV | Human Immunodeficiency Virus |
| HTLV | Human T-cell Lymphotropic Virus |
| IL-4 | Interleukin 4 |
| NA | Not Available |
| PICO | Population, Intervention, Comparator, Outcomes |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SS | Skin Substitutes |
| TBSA | Total Body Surface Area |
| TESS | Tissue Engineered Skin Substitutes |
References
- Armstrong, M.; Wheeler, K.K.; Shi, J.; Thakkar, R.K.; Fabia, R.B.; Groner, J.I.; Noffsinger, D.; Giles, S.A.; Xiang, H. Epidemiology and trend of US pediatric burn hospitalizations, 2003–2016. Burns 2021, 47, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Strobel, A.M.; Fey, R. Emergency Care of Pediatric Burns. Emerg. Med. Clin. N. Am. 2018, 36, 441–458. [Google Scholar] [CrossRef]
- World Health Organization. Burns. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 7 December 2024).
- Delgado, J.; Ramirez-Cardich, M.E.; Gilman, R.H.; Lavarello, R.; Dahodwala, N.; Bazan, A.; Rodriguez, V.; Cama, R.I.; Tovar, M.; Lescano, A. Risk factors for burns in children: Crowding, poverty, and poor maternal education. Inj. Prev. 2002, 8, 38–41. [Google Scholar] [CrossRef]
- Fisher, M.D.; Norbury, W. Pediatric Burns: From Acute Care Through Reconstruction in 2024. Clin. Plast. Surg. 2024, 51, 379–390. [Google Scholar] [CrossRef]
- Samuel, J.; Gharde, P.; Surya, D.; Durge, S.; Gopalan, V. A Comparative Review of Meshed Versus Unmeshed Grafts in Split-Thickness Skin Grafting: Clinical Implications and Outcomes. Cureus 2024, 16, e69606. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, J.W.; Huh, G.Y.; Koh, J.H.; Seo, D.K.; Choi, J.K.; Jang, Y.C. Algorithm for Primary Full-thickness Skin Grafting in Pediatric Hand Burns. Arch. Plast. Surg. 2012, 39, 483–488. [Google Scholar] [CrossRef][Green Version]
- Quintero, E.C.; Machado, J.F.E.; Robles, R.A.D. Meek micrografting history, indications, technique, physiology and experience: A review article. J. Wound Care 2018, 27, S12–S18. [Google Scholar] [CrossRef]
- Kreis, R.W.; Mackie, D.P.; Hermans, R.R.; Vloemans, A.R. Expansion techniques for skin grafts: Comparison between mesh and Meek island (sandwich-) grafts. Burns 1994, 20 (Suppl. S1), S39–S42. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995, 21, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Rijpma, D.; Pijpe, A.; Claes, K.; Hoeksema, H.; de Decker, I.; Verbelen, J.; van Zuijlen, P.; Monstrey, S.; Meij-de Vries, A. Outcomes of Meek micrografting versus mesh grafting on deep dermal and full thickness (burn) wounds: Study protocol for an intra-patient randomized controlled trial. PLoS ONE 2023, 18, e0281347. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, P.; Chen, Y.; Yuan, Z.; Song, H. Efficacy of Two-Stage Meek Micrografting in Patients With Severe Burns. J. Burn. Care Res. 2022, 43, 1081–1085. [Google Scholar] [CrossRef]
- Medina, A.; Riegel, T.; Nystad, D.; Tredget, E.E. Modified Meek Micrografting Technique for Wound Coverage in Extensive Burn Injuries. J. Burn. Care Res. 2016, 37, 305–313. [Google Scholar] [CrossRef]
- Rijpma, D.; Claes, K.; Hoeksema, H.; de Decker, I.; Verbelen, J.; Monstrey, S.; Pijpe, A.; van Zuijlen, P.; Meij-de Vries, A. The Meek micrograft technique for burns; review on its outcomes: Searching for the superior skin grafting technique. Burns 2022, 48, 1287–1300. [Google Scholar] [CrossRef]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatol. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef]
- Kenny, E.M.; Lagziel, T.; Hultman, C.S.; Egro, F.M. Skin Substitutes and Autograft Techniques: Temporary and Permanent Coverage Solutions. Clin. Plast. Surg. 2024, 51, 241–254. [Google Scholar] [CrossRef]
- Diegidio, P.; Hermiz, S.J.; Ortiz-Pujols, S.; Jones, S.W.; van Duin, D.; Weber, D.J.; Cairns, B.A.; Hultman, C.S. Even Better Than the Real Thing? Xenografting in Pediatric Patients with Scald Injury. Clin. Plast. Surg. 2017, 44, 651–656. [Google Scholar] [CrossRef]
- Burkey, B.; Davis, W., 3rd; Glat, P.M. Porcine xenograft treatment of superficial partial-thickness burns in paediatric patients. J. Wound Care 2016, 25, S10–S15. [Google Scholar] [CrossRef]
- Coruh, A.; Tosun, Z.; Ozbebit, U. Close relative intermingled skin allograft and autograft use in the treatment of major burns in adults and children. J. Burn. Care Rehabil. 2005, 26, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Yanaga, H.; Udoh, Y.; Yamamoto, M.; Yoshii, S.; Mori, S.; Yamauchi, T.; Kiyokawa, K.; Koga, M.; Yanaga, K. Cryopreserved cultured epithelial allografts for pediatric deep partial dermal burns: Early wound closure and suppression of scarring. Regen. Ther. 2017, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Axibal, E.; Brown, M. Surgical Dressings and Novel Skin Substitutes. Dermatol. Clin. 2019, 37, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.G.; Giatsidis, G.; Orgill, D.P.; Halvorson, E.G. Skin Substitutes and Bioscaffolds: Temporary and Permanent Coverage. Clin. Plast. Surg. 2017, 44, 627–634. [Google Scholar] [CrossRef]
- Nyame, T.T.; Chiang, H.A.; Leavitt, T.; Ozambela, M.; Orgill, D.P. Tissue-Engineered Skin Substitutes. Plast. Reconstr. Surg. 2015, 136, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Staubach, R.; Glosse, H.; Loff, S. The Use of Fish Skin Grafts in Children as a New Treatment of Deep Dermal Burns-Case Series with Follow-Up after 2 Years and Measurement of Elasticity as an Objective Scar Evaluation. J. Clin. Med. 2024, 13, 2389. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Deng, H.; Sun, T.; Cai, J.; Li, D.; Li, L.; He, L.; Zhang, B.; Li, D.; Wang, L.; et al. Use of Fresh Scalp Allografts From Living Relatives for Extensive Deep Burns in Children: A Clinical Study Over 7 Years. J. Burn. Care Res. 2021, 42, 323–330. [Google Scholar] [CrossRef]
- Lima Junior, E.M.; Moraes Filho, M.O.; Forte, A.J.; Costa, B.A.; Fechine, F.V.; Alves, A.; Moraes, M.E.A.; Rocha, M.B.S.; Silva Junior, F.R.; Soares, M.; et al. Pediatric Burn Treatment Using Tilapia Skin as a Xenograft for Superficial Partial-Thickness Wounds: A Pilot Study. J. Burn. Care Res. 2020, 41, 241–247. [Google Scholar] [CrossRef]
- Ahuja, N.; Jin, R.; Powers, C.; Billi, A.; Bass, K. Dehydrated Human Amnion Chorion Membrane as Treatment for Pediatric Burns. Adv. Wound Care 2020, 9, 602–611. [Google Scholar] [CrossRef]
- Costa, B.A.; Lima Junior, E.M.; de Moraes Filho, M.O.; Fechine, F.V.; de Moraes, M.E.A.; Silva Junior, F.R.; do Nascimento Soares, M.F.A.; Rocha, M.B.S. Use of Tilapia Skin as a Xenograft for Pediatric Burn Treatment: A Case Report. J. Burn. Care Res. 2019, 40, 714–717. [Google Scholar] [CrossRef]
- Puyana, S.; Elkbuli, A.; Ruiz, S.; Bernal, E.; McKenney, M.; Lim, R.; Askari, M.; Mir, H. The Use of Dehydrated Human Amniotic/Chorionic Membrane Skin Substitute in the Treatment of Pediatric Facial Burn. J. Craniofac Surg. 2019, 30, 2551–2554. [Google Scholar] [CrossRef]
- Gupta, S.; Mohapatra, D.P.; Chittoria, R.K.; Subbarayan, E.; Reddy, S.K.; Chavan, V.; Aggarwal, A.; Reddy, L.C. Human Skin Allograft: Is it a Viable Option in Management of Burn Patients? J. Cutan. Aesthet. Surg. 2019, 12, 132–135. [Google Scholar] [CrossRef]
- Rode, H.; Martinez, R.; Potgieter, D.; Adams, S.; Rogers, A.D. Experience and outcomes of micrografting for major paediatric burns. Burns 2017, 43, 1103–1110. [Google Scholar] [CrossRef]
- Glat, P.M.; Davenport, T. Current Techniques for Burn Reconstruction: Using Dehydrated Human Amnion/Chorion Membrane Allografts as an Adjunctive Treatment Along the Reconstructive Ladder. Ann. Plast. Surg. 2017, 78, S14–S18. [Google Scholar] [CrossRef]
- Menon, S.; Li, Z.; Harvey, J.G.; Holland, A.J. The use of the Meek technique in conjunction with cultured epithelial autograft in the management of major paediatric burns. Burns 2013, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, S.R.K.; Harrison, J.L.; Clarke, T.N.; Muka, T.N.; Garcia, J.H.; Huang, S.M.; Whisonant, C.T.; Borah, G.; Wu, E.C. Acellular Piscine Dermis for Pediatric Hand Burn Reconstruction. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5889. [Google Scholar] [CrossRef]
- Storey, K.; Lalloz, M.; Choy, K.-T.; McBride, C.A.; McMillan, C.; Das Gupta, R.; Patel, B.; Choo, K.; Stefanutti, G.; Borzi, P.; et al. The versatility of biodegradable temporising matrix–A 63 paediatric case series with complex wounds. Burn. Open 2023, 7, 44–50. [Google Scholar] [CrossRef]
- Jackson, S.R.; Roman, S. Matriderm and Split Skin Grafting for Full-Thickness Pediatric Facial Burns. J. Burn. Care Res. 2019, 40, 251–254. [Google Scholar] [CrossRef]
- Zajicek, R.; Grossova, I.; Suca, H.; Kubok, R.; Pafcuga, I. Experience with Integra(R) at the Prague Burns Centre 2002–2016. Acta Chir. Plast. 2017, 59, 18–26. [Google Scholar] [PubMed]
- Nessler, M.B.; Puchala, J.; Chrapusta, A.; Nessler, K.; Drukala, J. Levels of plasma matrix metalloproteinases (MMP-2 and MMP-9) in response to INTEGRA(R) dermal regeneration template implantation. Med. Sci. Monit. 2014, 20, 91–96. [Google Scholar] [CrossRef]
- Nessler, M.; Puchala, J.; Wood, F.M.; Wallace, H.J.; Fear, M.W.; Nessler, K.; Drukala, J. Changes in the plasma cytokine and growth factor profile are associated with impaired healing in pediatric patients treated with INTEGRA(R) for reconstructive procedures. Burns 2013, 39, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, R.; Matouskova, E.; Broz, L.; Kubok, R.; Waldauf, P.; Konigova, R. New biological temporary skin cover Xe-Derma((R)) in the treatment of superficial scald burns in children. Burns 2011, 37, 333–337. [Google Scholar] [CrossRef]
- Stiefel, D.; Schiestl, C.; Meuli, M. Integra Artificial Skin for burn scar revision in adolescents and children. Burns 2010, 36, 114–120. [Google Scholar] [CrossRef]
- Branski, L.K.; Herndon, D.N.; Pereira, C.; Mlcak, R.P.; Celis, M.M.; Lee, J.O.; Sanford, A.P.; Norbury, W.B.; Zhang, X.J.; Jeschke, M.G. Longitudinal assessment of Integra in primary burn management: A randomized pediatric clinical trial. Crit. Care Med. 2007, 35, 2615–2623. [Google Scholar] [CrossRef]
- Hohlfeld, J.; de Buys Roessingh, A.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for paediatric burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef]
- Cassidy, C.; St Peter, S.D.; Lacey, S.; Beery, M.; Ward-Smith, P.; Sharp, R.J.; Ostlie, D.J. Biobrane versus duoderm for the treatment of intermediate thickness burns in children: A prospective, randomized trial. Burns 2005, 31, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.J.; Kimble, R.M.; Boots, R.; Pegg, S.P. Treatment of partial-thickness burns: A prospective, randomized trial using Transcyte. ANZ J. Surg. 2004, 74, 622–626. [Google Scholar] [CrossRef]
- Still, J.; Glat, P.; Silverstein, P.; Griswold, J.; Mozingo, D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 2003, 29, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Yanaga, H.; Udoh, Y.; Yamauchi, T.; Yamamoto, M.; Kiyokawa, K.; Inoue, Y.; Tai, Y. Cryopreserved cultured epidermal allografts achieved early closure of wounds and reduced scar formation in deep partial-thickness burn wounds (DDB) and split-thickness skin donor sites of pediatric patients. Burns 2001, 27, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Waymack, P.; Duff, R.G.; Sabolinski, M. The effect of a tissue engineered bilayered living skin analog, over meshed split-thickness autografts on the healing of excised burn wounds. The Apligraf Burn Study Group. Burns 2000, 26, 609–619. [Google Scholar] [CrossRef]
- Barret, J.P.; Dziewulski, P.; Ramzy, P.I.; Wolf, S.E.; Desai, M.H.; Herndon, D.N. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast. Reconstr. Surg. 2000, 105, 62–65. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Abdel-Sayed, P.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of Biological Bandages as First Cover for Burn Patients. Adv. Wound Care 2019, 8, 555–564. [Google Scholar] [CrossRef]
- Gore, M.A.; Akolekar, D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns 2003, 29, 487–492. [Google Scholar] [CrossRef]
- Halim, A.S.; Khoo, T.L.; Mohd Yussof, S.J. Biologic and synthetic skin substitutes: An overview. Indian. J. Plast. Surg. 2010, 43, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Paggiaro, A.O.; Bastianelli, R.; Carvalho, V.F.; Isaac, C.; Gemperli, R. Is allograft skin, the gold-standard for burn skin substitute? A systematic literature review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, F.; Lineaweaver, W.C. Clinical Applications of Allograft Skin in Burn Care. Ann. Plast. Surg. 2020, 84, S158–S160. [Google Scholar] [CrossRef]
- Schwacha, M.G.; Chaudry, I.H. The cellular basis of post-burn immunosuppression: Macrophages and mediators. Int. J. Mol. Med. 2002, 10, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Gerlini, G.; Susini, P.; Sestini, S.; Brandani, P.; Giannotti, V.; Borgognoni, L. Langerhans Cells in Sentinel Lymph Nodes from Melanoma Patients. Cancers 2024, 16, 1890. [Google Scholar] [CrossRef]
- Saffle, J.R. Closure of the excised burn wound: Temporary skin substitutes. Clin. Plast. Surg. 2009, 36, 627–641. [Google Scholar] [CrossRef]
- Spence, R.J.; Wong, L. The enhancement of wound healing with human skin allograft. Surg. Clin. N. Am. 1997, 77, 731–745. [Google Scholar] [CrossRef]
- Simonds, R.J.; Holmberg, S.D.; Hurwitz, R.L.; Coleman, T.R.; Bottenfield, S.; Conley, L.J.; Kohlenberg, S.H.; Castro, K.G.; Dahan, B.A.; Schable, C.A.; et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N. Engl. J. Med. 1992, 326, 726–732. [Google Scholar] [CrossRef]
- Pianigiani, E.; Ierardi, F.; Cherubini Di Simplicio, F.; Andreassi, A. Skin bank organization. Clin. Dermatol. 2005, 23, 353–356. [Google Scholar] [CrossRef]
- Kesting, M.R.; Wolff, K.D.; Hohlweg-Majert, B.; Steinstraesser, L. The role of allogenic amniotic membrane in burn treatment. J. Burn. Care Res. 2008, 29, 907–916. [Google Scholar] [CrossRef]
- Williams, R.; Lace, R.; Kennedy, S.; Doherty, K.; Levis, H. Biomaterials for Regenerative Medicine Approaches for the Anterior Segment of the Eye. Adv. Healthc. Mater. 2018, 7, e1701328. [Google Scholar] [CrossRef]
- Hori, J.; Wang, M.; Kamiya, K.; Takahashi, H.; Sakuragawa, N. Immunological characteristics of amniotic epithelium. Cornea 2006, 25, S53–S58. [Google Scholar] [CrossRef]
- Koob, T.J.; Rennert, R.; Zabek, N.; Massee, M.; Lim, J.J.; Temenoff, J.S.; Li, W.W.; Gurtner, G. Biological properties of dehydrated human amnion/chorion composite graft: Implications for chronic wound healing. Int. Wound J. 2013, 10, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Tenenhaus, M. The Use of Dehydrated Human Amnion/Chorion Membranes in the Treatment of Burns and Complex Wounds: Current and Future Applications. Ann. Plast. Surg. 2017, 78, S11–S13. [Google Scholar] [CrossRef] [PubMed]
- Song, I.C.; Bromberg, B.E.; Mohn, M.P.; Koehnlein, E. Heterografts as biological dressings for large skin wounds. Surgery 1966, 59, 576–583. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwase, H.; King, T.W.; Hara, H.; Cooper, D.K.C. Skin xenotransplantation: Historical review and clinical potential. Burns 2018, 44, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.L.; Blome-Eberwein, S.E.; Branski, L.K.; Carson, J.S.; Crombie, R.E.; Hickerson, W.L.; Kamolz, L.P.; King, B.T.; Nischwitz, S.P.; Popp, D.; et al. Porcine Xenograft and Epidermal Fully Synthetic Skin Substitutes in the Treatment of Partial-Thickness Burns: A Literature Review. Medicina 2021, 57, 432. [Google Scholar] [CrossRef]
- Kalsi, R.; Messner, F.; Brandacher, G. Skin xenotransplantation: Technological advances and future directions. Curr. Opin. Organ. Transplant. 2020, 25, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Lima Junior, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Fechine, F.V.; Vale, M.L.; Diogenes, A.K.L.; Neves, K.R.T.; Uchoa, A.; Soares, M.; de Moraes, M.E.A. Nile Tilapia Fish Skin-Based Wound Dressing Improves Pain and Treatment-Related Costs of Superficial Partial-Thickness Burns: A Phase III Randomized Controlled Trial. Plast. Reconstr. Surg. 2021, 147, 1189–1198. [Google Scholar] [CrossRef]
- Garrity, C.; Garcia-Rovetta, C.; Rivas, I.; Delatorre, U.; Wong, A.; Kultz, D.; Peyton, J.; Arzi, B.; Vapniarsky, N. Tilapia Fish Skin Treatment of Third-Degree Skin Burns in Murine Model. J. Funct. Biomater. 2023, 14, 512. [Google Scholar] [CrossRef]
- Goodarzi, P.; Falahzadeh, K.; Nematizadeh, M.; Farazandeh, P.; Payab, M.; Larijani, B.; Tayanloo Beik, A.; Arjmand, B. Tissue Engineered Skin Substitutes. Adv. Exp. Med. Biol. 2018, 1107, 143–188. [Google Scholar] [CrossRef]
- Taupin, P.; Gandhi, A.; Saini, S. Integra(R) Dermal Regeneration Template: From Design to Clinical Use. Cureus 2023, 15, e38608. [Google Scholar] [CrossRef]
- May, J.M.; Depani, M.; Ferry, A.M.; Koshy, J.C.; Thornton, J.F. The Use of Biologic Wound Agents in Pediatric Reconstructions. Semin. Plast. Surg. 2022, 36, 48–52. [Google Scholar] [CrossRef]
- Gonzalez Alana, I.; Torrero Lopez, J.V.; Martin Playa, P.; Gabilondo Zubizarreta, F.J. Combined use of negative pressure wound therapy and Integra(R) to treat complex defects in lower extremities after burns. Ann. Burns Fire Disasters 2013, 26, 90–93. [Google Scholar] [PubMed]
- Winfrey, M.E.; Cochran, M.; Hegarty, M.T. A new technology in burn therapy: INTEGRA artificial skin. Dimens. Crit. Care Nurs. 1999, 18, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Pek, C.H.; Por, Y.C.; Lim, G.J.S. Biobrane dressing for paediatric burns in Singapore: A retrospective review. Singap. Med. J. 2018, 59, 360–365. [Google Scholar] [CrossRef]
- Lesher, A.P.; Curry, R.H.; Evans, J.; Smith, V.A.; Fitzgerald, M.T.; Cina, R.A.; Streck, C.J.; Hebra, A.V. Effectiveness of Biobrane for treatment of partial-thickness burns in children. J. Pediatr. Surg. 2011, 46, 1759–1763. [Google Scholar] [CrossRef]
- Abenavoli, F.M.; Corelli, R. About AlloDerm. Plast. Reconstr. Surg. 2001, 108, 2175–2176. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Propst, E.J.; Papsin, B.C. Lateral graft type 1 tympanoplasty using AlloDerm for tympanic membrane reconstruction in children. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.J.; Bury, S.B. Acellular dermal matrix in the management of the burn patient. Aesthet Surg. J. 2011, 31, 13S–23S. [Google Scholar] [CrossRef]
- Zajicek, R.; Suca, H.; Grossova, I.; Fetissov, V.; Pafcuga, I. Dermal Replacement with Matriderm-First Experience at the Prague Burn Centre. Acta Chir. Plast. 2020, 62, 79–82. [Google Scholar]
- Lamy, J.; Gourari, A.; Atlan, M.; Zakine, G. [Use of Matriderm(R) 1mm in reconstructive surgery. Series of 31 cases]. Ann. Chir. Plast. Esthet. 2013, 58, 235–242. [Google Scholar] [CrossRef]
- Dell’Aversana Orabona, G.; Maffia, F.; Audino, G.; Abbate, V.; Germano, C.; Bonavolonta, P.; Romano, A.; Villari, R.; Mormile, M.; Califano, L. The Use of Matriderm((R)) for Scalp Full-Thickness Defects Reconstruction: A Case Series. J. Clin. Med. 2022, 11, 6041. [Google Scholar] [CrossRef]
- Dickson, K.; Lee, K.C.; Abdulsalam, A.; Amirize, E.; Kankam, H.K.N.; Ter Horst, B.; Gardiner, F.; Bamford, A.; Hejmadi, R.K.; Moiemen, N. A Histological and Clinical Study of MatriDerm(R) Use in Burn Reconstruction. J. Burn. Care Res. 2023, 44, 1100–1109. [Google Scholar] [CrossRef]
- Harrison, C.A.; MacNeil, S. The mechanism of skin graft contraction: An update on current research and potential future therapies. Burns 2008, 34, 153–163. [Google Scholar] [CrossRef]
- Posner, K.M.; Bakus, C.; Sodha, S. Rapid Healing of Necrotizing Fasciitis Using the Kerecis Fish Skin Xenograft: A Clinical Case Report. Cureus 2024, 16, e73060. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Jeffery, S.L.A. Acellular Fish Skin Grafts for Management of Split Thickness Donor Sites and Partial Thickness Burns: A Case Series. Mil. Med. 2019, 184, 16–20. [Google Scholar] [CrossRef]
- Wagstaff, M.J.; Schmitt, B.J.; Coghlan, P.; Finkemeyer, J.P.; Caplash, Y.; Greenwood, J.E. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: A pilot study. Eplasty 2015, 15, e13. [Google Scholar]
- Greenwood, J.E.; Dearman, B.L. Comparison of a sealed, polymer foam biodegradable temporizing matrix against Integra(R) dermal regeneration template in a porcine wound model. J. Burn. Care Res. 2012, 33, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Brown, J.N.; Dantzer, E.J.G.; Maitz, P.K.M.; Vandervord, J.G.; Wagstaff, M.J.D.; Barker, T.M.; Cleland, H. Wound healing and dermal regeneration in severe burn patients treated with NovoSorb(R) Biodegradable Temporising Matrix: A prospective clinical study. Burns 2022, 48, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Shanti, R.M.; Smart, R.J.; Meram, A.; Kim, D. Porcine Urinary Bladder Extracellular Matrix for the Salvage of Fibula Free Flap Skin Paddle: Technical Note and Description of a Case. Craniomaxillofac. Trauma Reconstr. 2017, 10, 318–322. [Google Scholar] [CrossRef]
- Kim, J.S.; Kaminsky, A.J.; Summitt, J.B.; Thayer, W.P. New Innovations for Deep Partial-Thickness Burn Treatment with ACell MatriStem Matrix. Adv. Wound Care 2016, 5, 546–552. [Google Scholar] [CrossRef]
- Underwood, P.; Cardinal, P.; Keller, E.; Goodfellow, R.; Scalea, T.; Henry, S.; Lauerman, M.H. Extending Limb Salvage After Fourth and Fifth Transmetatarsal Amputation in Diabetic Foot Infections Using ACell((R)) Urinary Bladder Matrix. Am. Surg. 2023, 89, 1079–1082. [Google Scholar] [CrossRef]
- Klama-Baryla, A.; Kitala, D.; Labus, W.; Kraut, M.; Glik, J.; Nowak, M.; Kawecki, M. Autologous and Allogeneic Skin Cell Grafts in the Treatment of Severely Burned Patients: Retrospective Clinical Study. Transplant. Proc. 2018, 50, 2179–2187. [Google Scholar] [CrossRef]
- Sritanyarat, T.; Wongpraparut, C.; Jansuwan, N.; Yothachai, P.; Nuntawisuttiwong, N.; Silpa-Archa, N. Outcomes of autologous non-cultured melanocyte keratinocyte transplantation in vitiligo and nevus depigmentosus. J. Dermatol. Treat. 2022, 33, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.; Llewelyn, D. Surgical management of hands in children with recessive dystrophic epidermolysis bullosa: Use of allogeneic composite cultured skin grafts. Br. J. Plast. Surg. 1998, 51, 608–613. [Google Scholar] [CrossRef]
- Pape, S.A.; Byrne, P.O. Safety and efficacy of TransCyte for the treatment of partial-thickness burns. J. Burn. Care Rehabil. 2000, 21, 390. [Google Scholar]
- Marston, W.A. Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert. Rev. Med. Devices 2004, 1, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.E.; Loewen-Rodriguez, A.; Lessem, J. Dermagraft: Use in the Treatment of Chronic Wounds. Adv. Wound Care 2012, 1, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Purdue, G.F.; Hunt, J.L.; Still, J.M., Jr.; Law, E.J.; Herndon, D.N.; Goldfarb, I.W.; Schiller, W.R.; Hansbrough, J.F.; Hickerson, W.L.; Himel, H.N.; et al. A multicenter clinical trial of a biosynthetic skin replacement, Dermagraft-TC, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J. Burn. Care Rehabil. 1997, 18, 52–57. [Google Scholar] [CrossRef]
- Eudy, M.; Eudy, C.L.; Roy, S. Apligraf as an Alternative to Skin Grafting in the Pediatric Population. Cureus 2021, 13, e16226. [Google Scholar] [CrossRef]
- Trent, J.F.; Kirsner, R.S. Tissue engineered skin: Apligraf, a bi-layered living skin equivalent. Int. J. Clin. Pract. 1998, 52, 408–413. [Google Scholar] [CrossRef]
- Wardhana, A.; Valeria, M. Efficacy Of Skin Substitutes For Management Of Acute Burn Cases: A Systematic Review. Ann. Burns Fire Disasters 2022, 35, 227–236. [Google Scholar] [PubMed]
| Reference | N. Patients | Females | Males | Mean Age | TBSA (Mean) | Time to Heal (Days) | Complications | Study Field | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Staubach et al. 2024 [26] | 20 | 8 | 12 | 8 | 2.75% | 26 | Not available (NA) | Fish skin graft | Fish skin grafts can be considered for deep dermal pediatric burns. |
| Shen et al. 2021 [27] | 22 | 9 | 13 | 5 (1–11) | 31%, (10–86%) | 15.5 (12–19) | NA | Fresh human skin allograft (scalp allografts from relatives) | Fresh scalp allografts from relatives are effective for major pediatric burns. |
| Lima Junior et al. 2020 [28] | 30 | 12 | 18 | 5.7 ± 3.7 | 11.1% ± 4.9% | Silver Sulfadiazine: 10.5 ± 0.7 Tilapia fish skin: 10.1 ± 0.5 | NA | Fish skin graft (Nile Tilapia fish skin) | Tilapia fish skin is an extra-low-cost alternative for pediatric partial-thickness burns. |
| Ahuja et al. 2020 [29] | 30 | 14 | 16 | 3 (1–17) | NA | 19.5 (15–35) | NA | dHACM | dHACM is a safe and feasible alternative to allograft for pediatric burns. |
| Costa et al. 2019 [30] | 1 | 0 | 1 | 3 | 18% | 10 | None | Fish skin graft (Nile Tilapia fish skin) | Tilapia fish skin is a low-cost and widely available. |
| Puyana et al. 2019 [31] | 30 | 7 | 23 | 3.7 | 6.8% (2–27%) | NA | None | dHACM | dHACM is a safe and feasible alternative to allograft for pediatric burns. |
| Gupta et al. 2019 [32] | 1 | 1 | 0 | 5 | 30% | 14 | None | Fresh human skin allograft | Fresh human skin allograft is a cost-effective strategy. |
| Rode et al. 2017 [33] | 35 | NA | NA | 4 | 49.7% (15–86%) | NA | 1 graft failure (Acinetobacter baumanii infection) | Micrografting | Meek micrografting allows for high tissue expansion and durable wound cover. |
| Diegidio et al. 2017 [17] | 1867 | NA | NA | Autograft: 6 xenograft: 3 | Autograft: 12.6% xenograft: 8.1% | NA | Infections autograf: 21 xeonograft: 4 | Xenografting | Xenografting reduces the need for delayed reconstructions of partial-thickness burns. |
| Glat et al. 2017 [34] | 3 | 0 | 3 | 2.3 (1–4) | NA | 14 (7–21) | None | dHACM | dHACM is a safe and feasible alternative to allograft for pediatric burns. |
| Burkey et al. 2016 [18] | 164 | 98 | 66 | NA | 7.0% (0.5–28%) | NA | 4 (2.4%) infections | Porcine xenografting | Porcine xenografting can be considered for pediatric burns. |
| Menon et al. 2013 [35] | 7 | NA | NA | 6 | 50% (range 30–70%) | NA | hypertrophic scarring graft loss (1–3%) | Micrograftin | Meek micrografting combined with cultured epithelial autograft (CEA) facilitates tissue expansion and wound closure. |
| Reference | N. Patients | Females | Males | Mean Age | TBSA (Mean) | Time to Heal (Days) | Complications | Study Field | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Shahriari et al. 2024 [36] | 1 | 1 | 0 | 1 | 3% | 10 | None | Fish skin graft (Kerecis®) | Kerecis® was safe and effective. |
| Storey et al. [37] | 19 | NA | NA | 7 | 2% (<1–46%) | NA | NA | NovoSorb® | NovoSorb® is a safe and effective biodegradable, entirely synthetic, polyurethane foam. |
| Jackson et al. 2019 [38] | 1 | 1 | 0 | 3 | 60% | 33 | None | Matriderm® | Matriderm® combined with split skin grafts is effective for extensive facial burns. |
| Zajicek et al. 2017 [39] | 28 | NA | NA | NA | NA | NA | NA | Integra® | Integra® improves scar quality in partial and full-thickness burns. |
| Yanaga et al. 2017 [20] | 50 | 27 | 23 | 1–18 | NA | 9.3 (5–13) | Local infection: 4 Hypertrophic scar: 5 | Cryopreserved cultured epithelial allograft | Cryopreserved cultured epithelial allograft is useful and effective. |
| Nessler et al. 2014 [40] | 11 | 4 | 8 | 14 (12–16) | 495 ± 72 cm2 | 29.1 ± 1.4 days | 4 local infections | Integra® | Integra® induces specific molecular patterns in pediatric burn healing. |
| Nessler et al. 2013 [41] | 9 | 4 | 5 | 13 | 457.0 ± 65.1 cm2 | NA | 2 local infections 1 excessive granulation | Integra® | IL-4 and FGF levels may predict the development of complications following integra® treatment. |
| Zajicek et al. 2011 [42] | 86 | NA | NA | NA | 1–35% | NA | NA | Porcine xenograft (Xe-Derma®) | Acellular pig dermis Xe-Derma® is effective for the treatment of scald burns. |
| Stiefel et al. 2009 [43] | 17 | 11 | 6 | 13 | NA | NA | 2 seroma 1 hematoma | Integra® | Integra® is safe and effective for burn scar revisions. |
| Branski et al. 2007 [44] | 20 | 4 | 16 | 7 | 73% | NA | NA | Integra® | Integra® allows for immediate burn wound cover and prevents cadaver-skin-related complications. |
| Hohfeld et al. 2005 [45] | 8 | 5 | 3 | 4 | NA | 15·3 days (5·5) | Hypertrophic scars | Fetal skin TESS | Tissue-engineered fetal skin, repaired into three-dimensional constructs on horse-derived collagen, was a safe and effective permanent substitute. |
| Cassidy et al. 2005 [46] | 72 | 34 | 38 | NA | NA | Duoderm: 11.21 (+/−6.5) Biobrane: 12.24 (+/−5.1) | NA | TESS (Biobrane®) | Duoderm® and Biobrane are equally effective for partial thickness pediatric burns. However, Duoderm® is less expensive. |
| Kumar et al. 2004 [47] | 33 (TransCyte, n = 20, Biobrane, n = 17; Silvazine, n = 21) | NA | NA | NA | NA | TransCyte—5; Biobrane—9.5; Silvazine—11.2 | Failure to heal Silvazin: 5 (24%); Biobrane: 3 (17%); TransCyte: 1 (5%) | TransCyte® vs. Biobrane® vs. silvazine cream | TransCyte® promotes faster epithelialization and easier dressings than Biobrane® or silvazine cream. |
| Still et al. 2003 [48] | 82 | 19 | 63 | NA | 10 to 80% | NA | NA | Bilayered living TESS (treatment of donor sites in burns) | OrCelTM® contains proliferating keratinocytes and fibroblasts. It allows for a shorter healing time than Biobrane®. |
| Yanaga et al. 2001 [49] | 43 | 23 | 19 | 5.1 | 30.7% | 9.1 (6–12) | NA | Cryopreserved cultured epidermal allografts | Cryopreserved cultured epidermal allografts were effective in pediatric burns. |
| Waymack et al. 2000 [50] | Pediatric and adults | NA | NA | NA | NA | NA | NA | Bilayered living TESS (Apligraf) | Apligraf® can be effectively applied over meshed autografts. |
| Barret et al. 2000 [51] | 20 | 5 | 15 | 3 | 8.9% (+/−4.9%) | 9.7 +/− 0.7 | NA | TESS (Biobrane®) | Biobrane® had superior outcomes compared to 1% silver sulfadiazine. |
| Study Population | Skin Substitutes Subanalysis | |||
|---|---|---|---|---|
| Biological Skin Substitutes | Tissue Engineered Skin Substitutes | Allograft | Integra | |
| N. studies | 12 | 17 | 5 | 5 |
| N. patients | 2210 (82.6%) | 466 (17.4%) | 86 (3.2%) | 85 (3.2%) |
| Females | 49.5% | 41.3% | 36.0% | 39.7% |
| Males | 50.5% | 58.7% | 64.0% | 60.3% |
| Mean Age/range | 4 (1–17) | 6 (1–17) | 3 (1–17) | 12 (7–16) |
| TBSA | 22.9% (3–50%) | 36.2% (3–73%) | 22.6% (6.8–31%) | NA (up to 73%) |
| Time to heal (days) | 15 (10–26) | 15 (5–33) | 16 (14–19) | NA (up to 29) |
| Product/Class | Evidence Strength (Pediatric) | Main Pediatric Indications | Key Benefits (From Included Studies) | Key Limitations |
|---|---|---|---|---|
| Integra® (acellular dermal template) | Moderate | Full-thickness reconstruction, scar revision, and extensive burns | Improved scar quality, reduces contracture; documented in several cohorts and small RCTs. | High cost; two-stage procedure; bovine collagen (xenogenic component). |
| Porcine xenografts/Biobrane® | Moderate | Temporary coverage of partial-thickness burns, donor sites | Readily available; reduces pain and dressing frequency; good short-term epithelialization. | Non-permanent; immunogenicity/rejection; cultural concerns. |
| Fish skin matrices (Kerecis®, tilapia skin) | Low–Moderate (growing) | Partial-thickness burns, hand burns | Low cost (tilapia); pain reduction; good epithelialization in small series. | Limited long-term data; variable processing/standards. |
| dHACM (dehydrated human amnion/chorion) | Low–Moderate | Facial/partial-thickness burns, adjuncts | Anti-inflammatory; easy handling; favorable cosmesis in case series. | Donor availability, variable preparations, and cost. |
| NovoSorb® (synthetic BTM) | Low–Moderate | Complex wounds, temporary dermal replacement | Synthetic—no immunogenic cells; promising integration in pediatric case series. | Newer product—limited pediatric sample size; cost. |
| Allograft (human cadaveric/fresh scalp allograft) | Low–Moderate | Temporary coverage for extensive burns | Effective temporary cover; scaffold for healing. | Donor availability, disease transmission concerns, and variable longevity. |
| Cellular TESS (Apligraf®, OrCelTM, TransCyte®) | Low | Donor-site healing, adjunct to meshed autografts | Promote dermal remodeling; may shorten healing in select indications. | High cost, manufacturing complexity, and limited pediatric series. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susini, P.; Certini, M.; Marcaccini, G.; Mazzotta, R.; Cuomo, R.; Nisi, G.; Grimaldi, L.; Facchini, F. Pediatric Burns: Biological and Tissue Engineered Skin Substitutes—A Systematic Review. J. Clin. Med. 2025, 14, 7981. https://doi.org/10.3390/jcm14227981
Susini P, Certini M, Marcaccini G, Mazzotta R, Cuomo R, Nisi G, Grimaldi L, Facchini F. Pediatric Burns: Biological and Tissue Engineered Skin Substitutes—A Systematic Review. Journal of Clinical Medicine. 2025; 14(22):7981. https://doi.org/10.3390/jcm14227981
Chicago/Turabian StyleSusini, Pietro, Martina Certini, Gianluca Marcaccini, Ruggero Mazzotta, Roberto Cuomo, Giuseppe Nisi, Luca Grimaldi, and Flavio Facchini. 2025. "Pediatric Burns: Biological and Tissue Engineered Skin Substitutes—A Systematic Review" Journal of Clinical Medicine 14, no. 22: 7981. https://doi.org/10.3390/jcm14227981
APA StyleSusini, P., Certini, M., Marcaccini, G., Mazzotta, R., Cuomo, R., Nisi, G., Grimaldi, L., & Facchini, F. (2025). Pediatric Burns: Biological and Tissue Engineered Skin Substitutes—A Systematic Review. Journal of Clinical Medicine, 14(22), 7981. https://doi.org/10.3390/jcm14227981







