Efficacy of Adaptol® 500 mg Tablets in Patients with Anxiety and Somatic Symptoms of Anxiety Disorder: A Noninterventional Study
Abstract
1. Introduction
Background
2. Materials and Methods
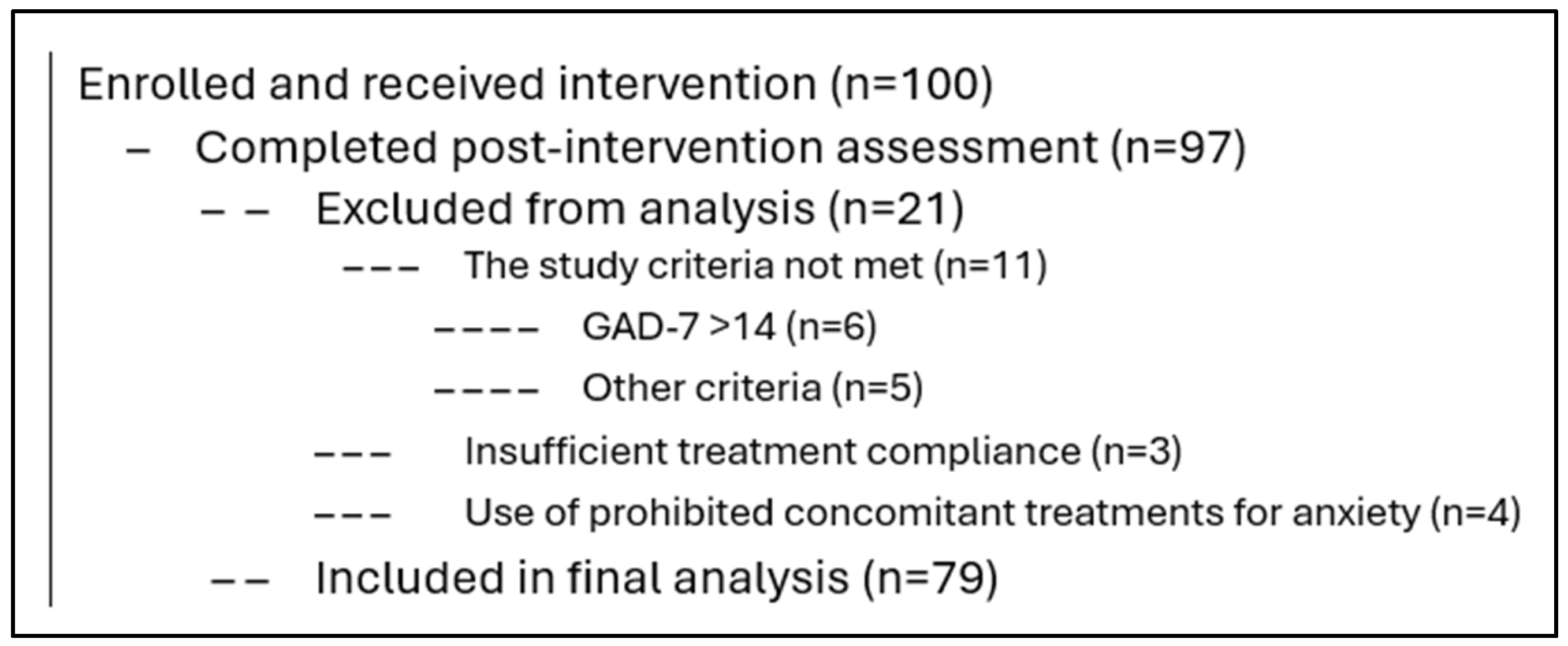
2.1. Subject
2.2. Materials
2.3. Methods
2.3.1. Data Collection
2.3.2. Statistical Methods
3. Results
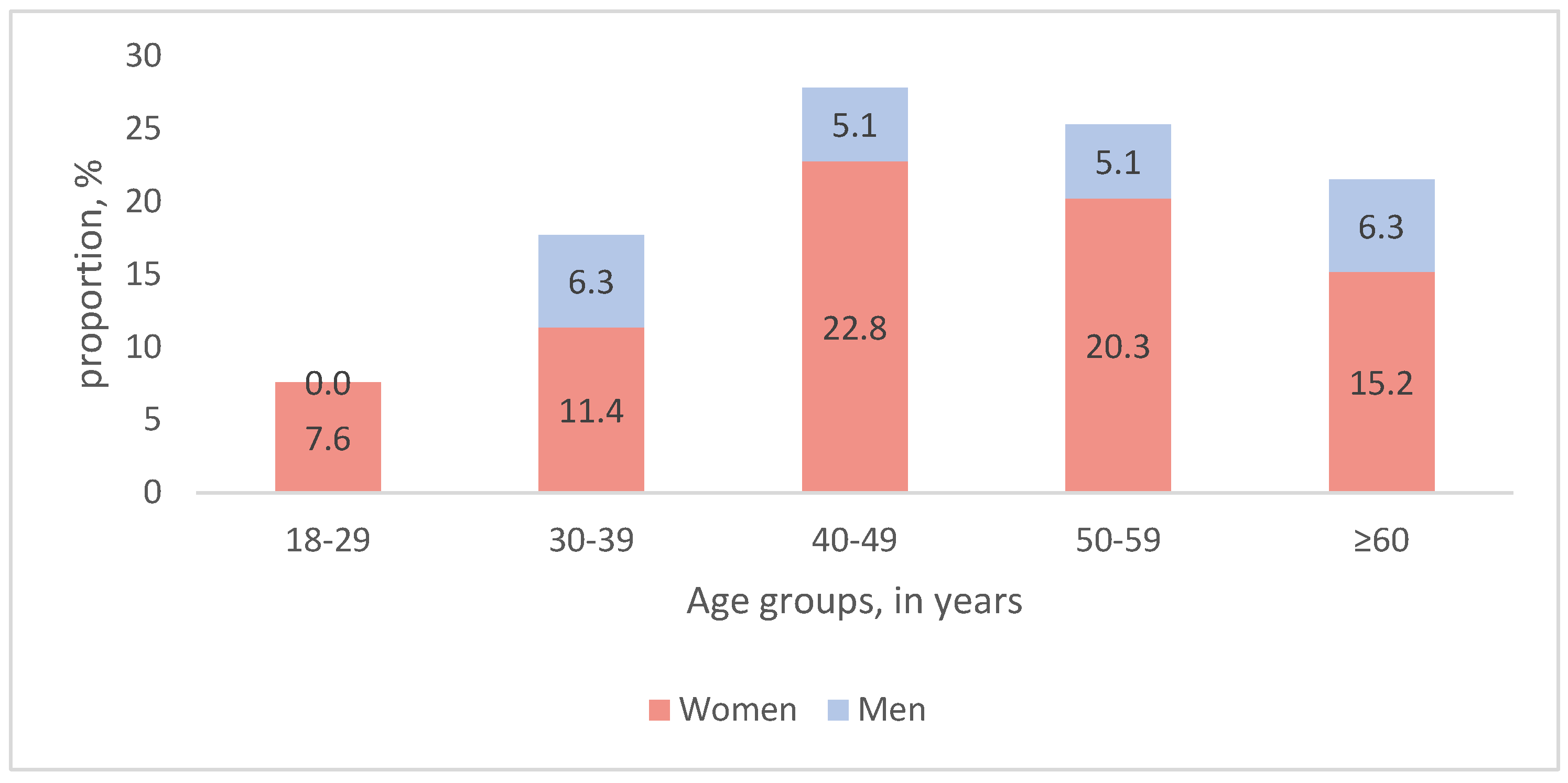
3.1. Demographic and Clinical Characteristics of the Study Participants
3.2. Primary Analysis
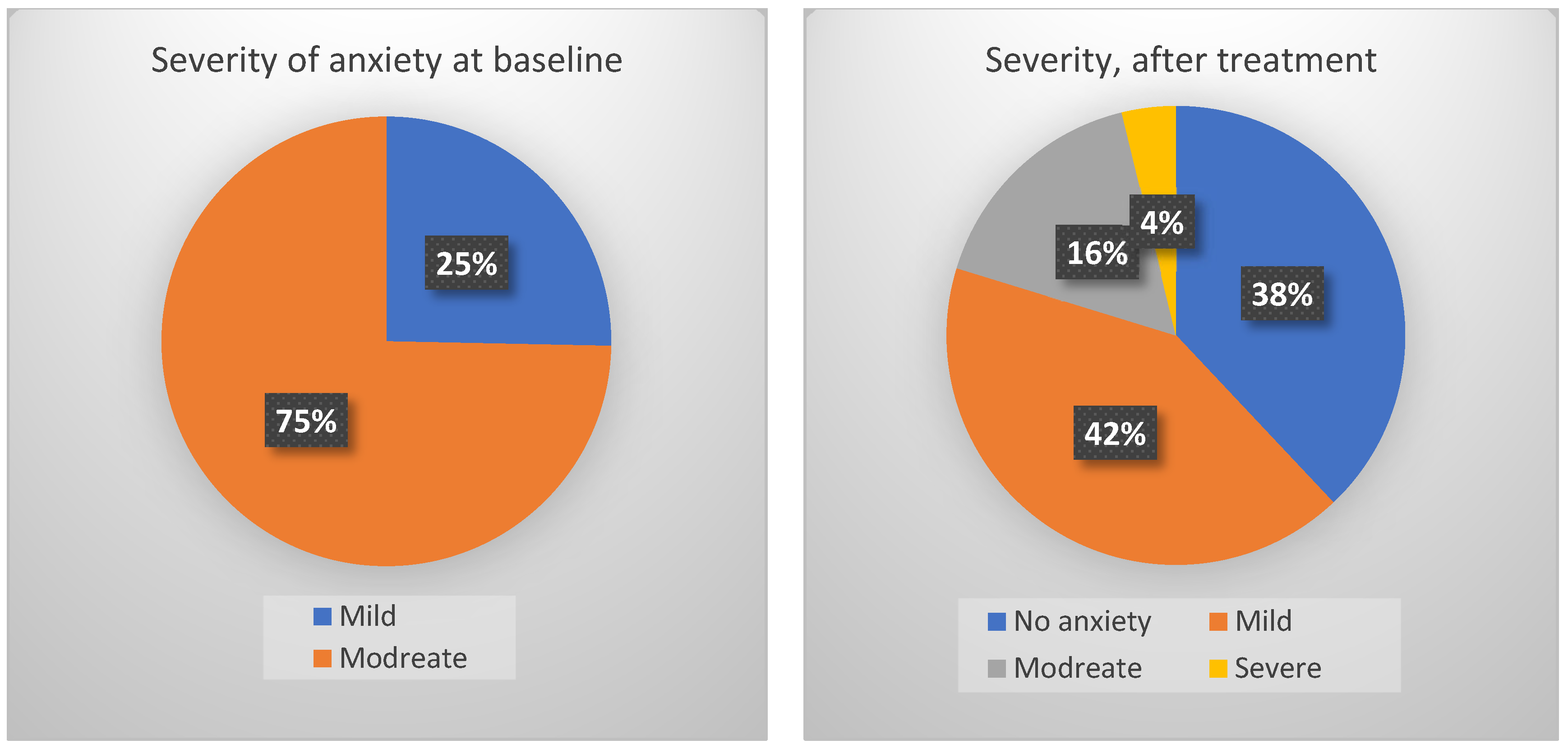
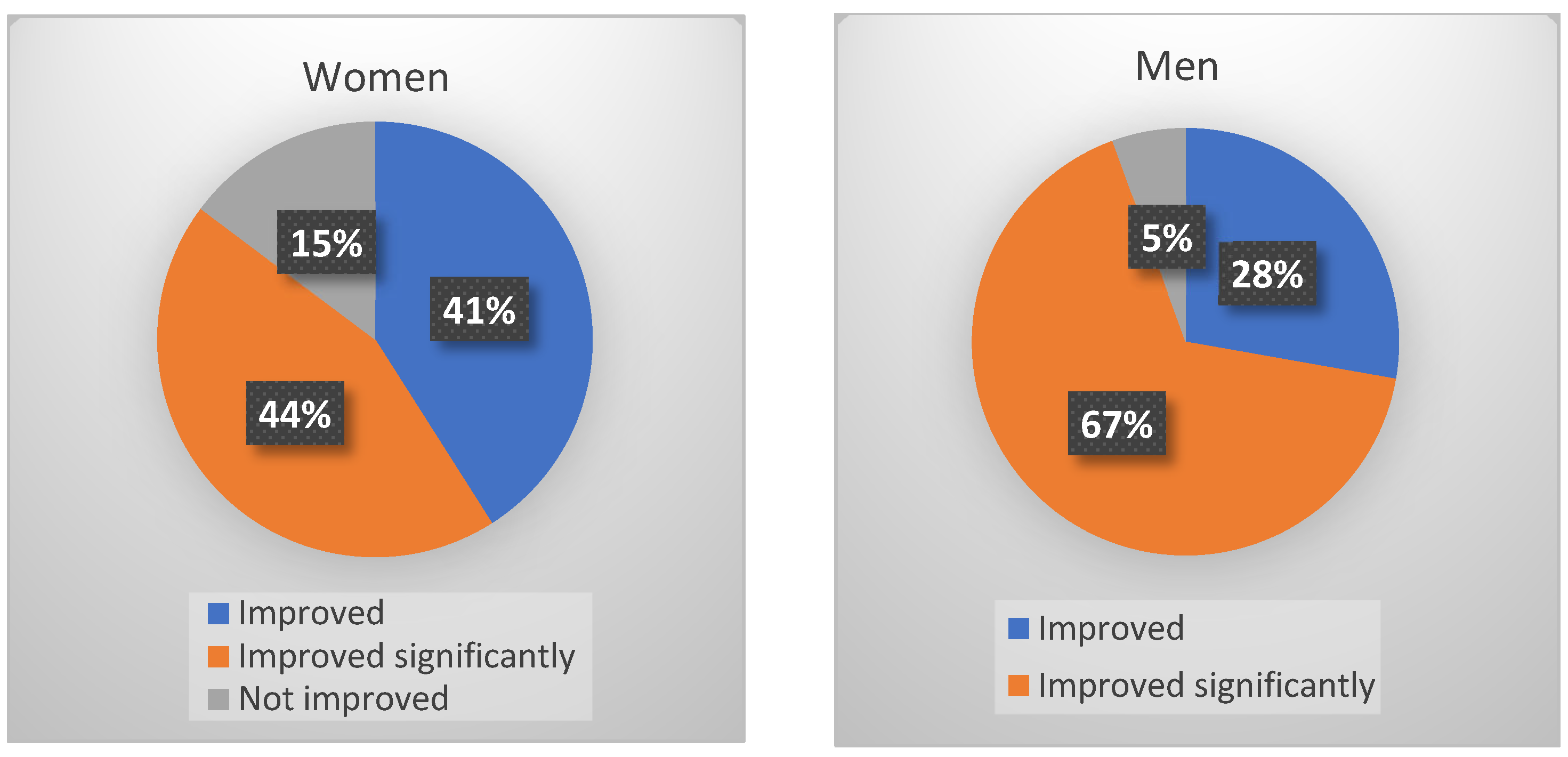
3.3. Effects of Adaptol® Treatment
3.4. Patients with Severe Anxiety
3.5. Concomitant Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATC | Anatomical Therapeutic Chemical classification |
| GAD-7 | Generalized Anxiety Disorder 7-item scale |
| MCSD | Minimal clinically significant difference |
| SPSS | Statistical Package for the Social Sciences |
References
- Love, A.S.; Love, R. Anxiety Disorders in Primary Care Settings. Nurs. Clin. N. Am. 2019, 54, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Boland, R.; Verduin, M.L.; Ruiz, P. Kaplan & Sadock’s Synopsis of Psychiatry; Walters Kluver: Philadelphia, PA, USA, 2022. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Zāļu Valsts Aģentūra (State Medical Agency). Available online: https://dati.zva.gov.lv/zalu-registrs/info/03-0002 (accessed on 10 September 2025).
- Levin, O.S. Izuchenie effektivnosti i vliyaniya na kachestvo zhizni patsientov s subklinicheskim i klinicheski vyrazhennym trevozhnym rasstroistvom mobil’nogo prilozheniya v sochetanii s terapiei preparatom Adaptol [To study the effectiveness and impact on the quality of life of patients with subclinical and clinically pronounced anxiety disorder of a mobile application in combination with Adaptol therapy]. S.S. Korsakov J. Neurol. Psychiatry 2024, 124, 98–105. [Google Scholar] [CrossRef]
- Kazakov, Y.M.; Potiazhenko, M.M.; Nastroga, T.V. Treatment Optimization in Management of Combined Pathology—Arterial Hypertension and Post-COVID Syndrome in Elderly Patients. Wiad. Lek. 2023, 76, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The ICD-10 Classification, 2016 (Latvian Version, 5th ed.); World Health Organization: Geneva, Switzerland, 2016. Available online: https://ssk10.spkc.gov.lv/ssk/g_119 (accessed on 23 September 2025).
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. Generalized Anxiety Disorder Scale (GAD-7). 2006 (Oriģināls). Local adaptation by Vrubļevska et.al (2022). Available online: https://www.rsu.lv/en/psychology-laboratory/test-and-survey-registry (accessed on 23 September 2025).
- Toussaint, A.; Hüsing, P.; Gumz, A.; Wingenfeld, K.; Härter, M.; Schramm, E.; Löwe, B. Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J. Affect. Disord. 2020, 265, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Bolton, D. A revitalized biopsychosocial model: Core theory, research paradigms, and clinical implications. Psychol. Med. 2023, 53, 7504–7511. [Google Scholar] [CrossRef] [PubMed]
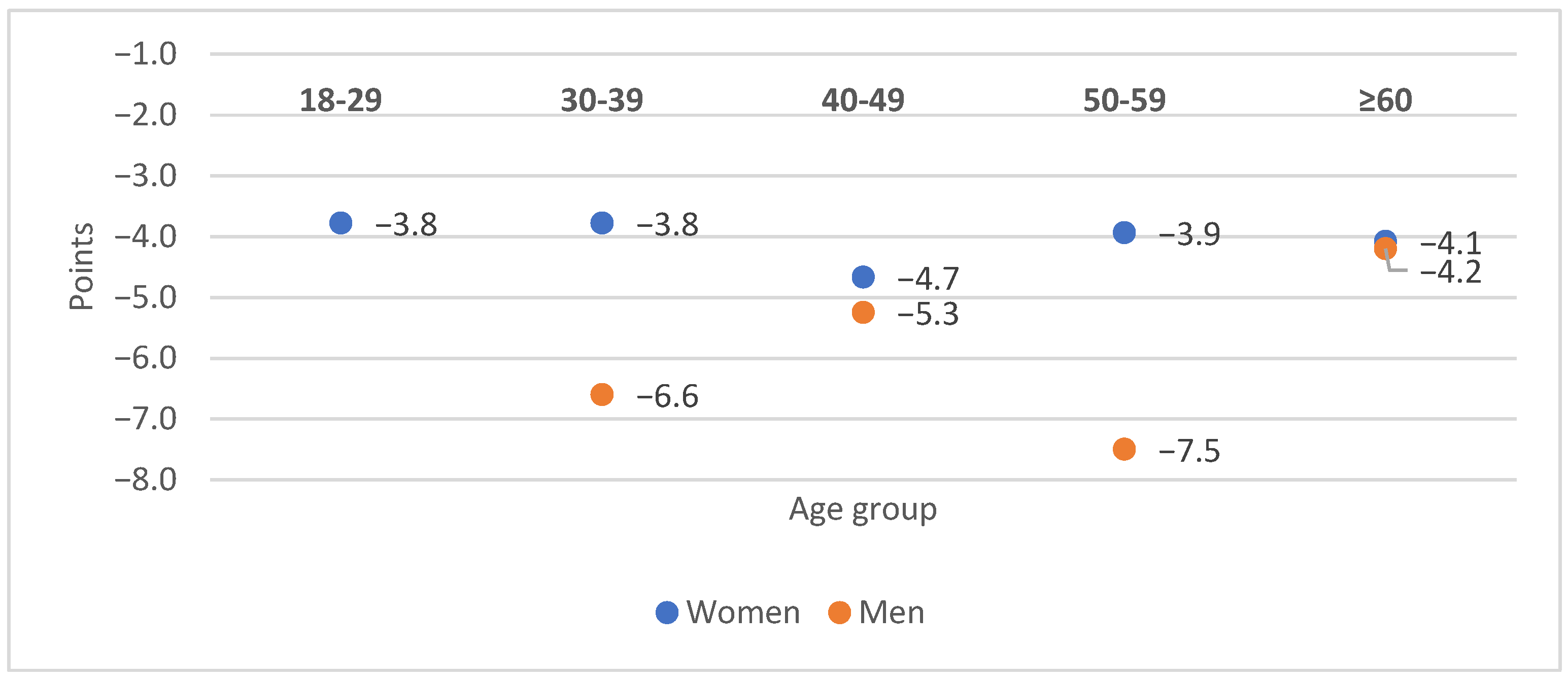

| Before | After | Total | MCSD * (↓) | MCSD * (↑) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | % Within Group | n | % | ||
| Mild | Mild | 10 | 12.7 | 4 | 5.1 | 40.0 | 0 | - |
| Mild | Moderate | 2 | 2.5 | 0 | - | - | 2 | 2.5 |
| Mild | No | 8 | 10.1 | 7 | 8.9 | 87.5 | 0 | - |
| Moderate | Mild | 37 | 46.8 | 34 | 43 | 91.9 | - | |
| Moderate | Moderate | 11 | 13.9 | 0 | - | - | 0 | - |
| Moderate | No | 8 | 10.1 | 8 | 10.1 | 100.0 | 0 | - |
| Moderate | Severe | 3 | 3.8 | 0 | - | - | 0 | - |
| Total | - | 79 | 100 | 53 | 67.1 | - | 2 | 2.5 |
| Gender | Age | GAD-7 Before Treatment | GAD-7 After Treatment | GAD-7 Change | Somatic Symptoms |
|---|---|---|---|---|---|
| Female | 25 | 18 | 5 | −13 | Improved significantly |
| Female | 71 | 18 | 5 | −13 | Improved |
| Female | 44 | 19 | 6 | −13 | Improved |
| Female | 35 | 18 | 4 | −14 | Improved significantly |
| Male | 54 | 19 | 7 | −12 | Improved |
| Female | 70 | 18 | 4 | −14 | Improved significantly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taube, M.; Dansone, G.; Troshina, Y. Efficacy of Adaptol® 500 mg Tablets in Patients with Anxiety and Somatic Symptoms of Anxiety Disorder: A Noninterventional Study. J. Clin. Med. 2025, 14, 7972. https://doi.org/10.3390/jcm14227972
Taube M, Dansone G, Troshina Y. Efficacy of Adaptol® 500 mg Tablets in Patients with Anxiety and Somatic Symptoms of Anxiety Disorder: A Noninterventional Study. Journal of Clinical Medicine. 2025; 14(22):7972. https://doi.org/10.3390/jcm14227972
Chicago/Turabian StyleTaube, Maris, Guna Dansone, and Yulia Troshina. 2025. "Efficacy of Adaptol® 500 mg Tablets in Patients with Anxiety and Somatic Symptoms of Anxiety Disorder: A Noninterventional Study" Journal of Clinical Medicine 14, no. 22: 7972. https://doi.org/10.3390/jcm14227972
APA StyleTaube, M., Dansone, G., & Troshina, Y. (2025). Efficacy of Adaptol® 500 mg Tablets in Patients with Anxiety and Somatic Symptoms of Anxiety Disorder: A Noninterventional Study. Journal of Clinical Medicine, 14(22), 7972. https://doi.org/10.3390/jcm14227972






