Transesophageal Echocardiography in Transcatheter Mitral Valve Replacement
Abstract
1. Introduction
2. The Role of TEE in Patient Selection
2.1. TEE Evaluation of the Mitral Apparatus
2.2. Anatomical Limitations
2.3. Assessment of the LVOT
- Anterior mitral leaflet length: elongated AML (>25–28 mm) increases the likelihood of systolic displacement and obstruction [24].
- Septal thickness: basal septal hypertrophy >15 mm exacerbates LVOT narrowing, particularly in small ventricles [25].
- Subvalvular apparatus: displaced or hypertrophied papillary muscles and prominent chordae may contribute to narrowing of the outflow tract after valve deployment [28].
2.4. Cardiac Function Assessment
2.5. Multimodality Imaging
3. Intra-Procedural Monitoring with TEE
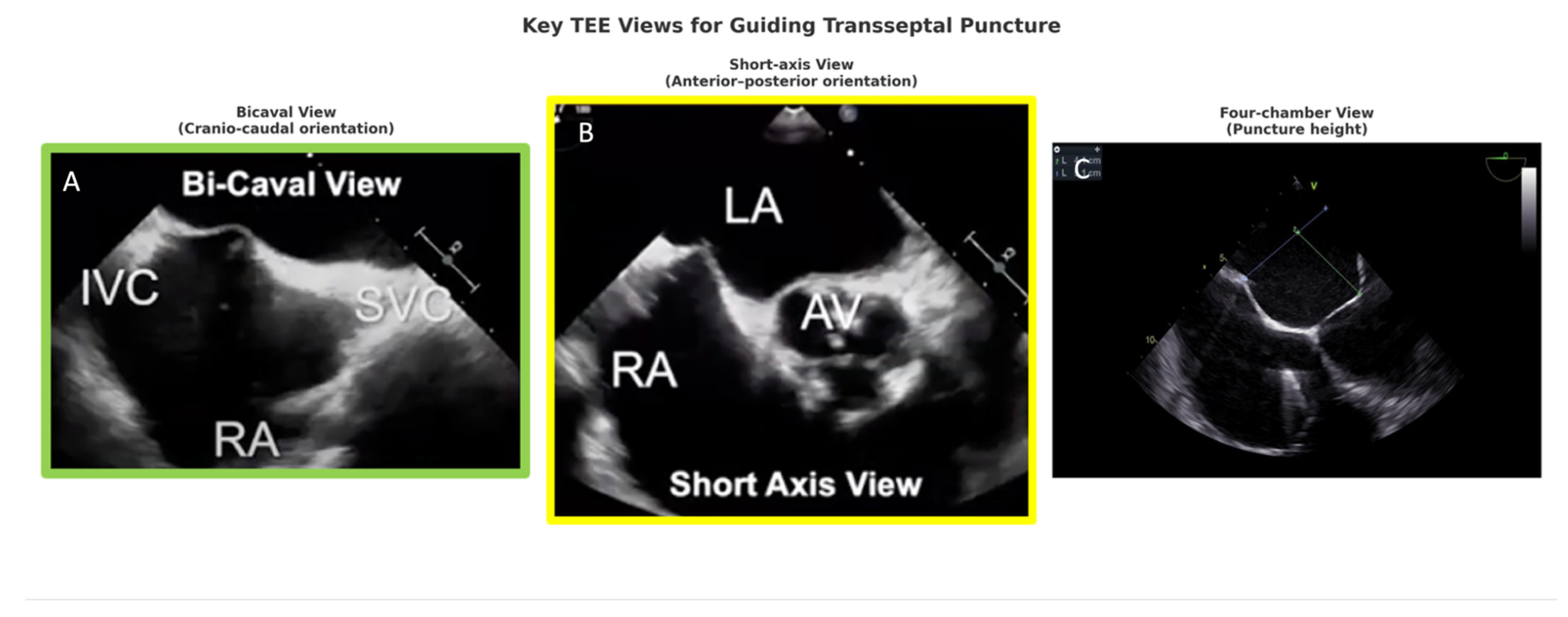
3.1. TEE in Transseptal Puncture
- Bicaval view: defines superior–inferior orientation and septal tenting,
- Short-axis view (aortic valve level): defines anterior–posterior position, preventing aortic mispuncture,
3.2. Trajectory Planning and Coaxiality
3.3. Calcification and Sealing
3.4. Confirmation of Positioning
3.5. Post-Deployment Assessment
4. Beyond Conventional TEE
4.1. TEE with Multiplanar Reconstruction
4.2. Intracardiac Echocardiography
4.3. Fusion Imaging
5. Focus on TEE in Challenging TMVR Scenarios
5.1. Valve-in-Valve, Valve-in-Ring, and Annuloplasty
5.2. Valve-in-MAC (ViMAC)
5.3. LVOTO Prevention
6. TEE in Recent Guidelines and Consensus for TMVR
7. Integrated Best Practices and Clinical Takeaways for TMVR Imaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Anterior Mitral Leaflet |
| AP | Anteroposterior |
| BATMAN | Balloon-Assisted Translocation of the Mitral Anterior Leaflet |
| CT | Computed Tomography |
| EACVI | European Association of Cardiovascular Imaging |
| ICE | Intracardiac Echocardiography |
| IVS | Interventricular Septum |
| LA | Left Atrium |
| LAMPOON | Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction |
| LV | Left Ventricle |
| LVEF | Left Ventricular Ejection Fraction |
| LVOT | Left Ventricular Outflow Tract |
| LVOTO | Left Ventricular Outflow Tract Obstruction |
| MAC | Mitral Annular Calcification |
| MPR | Multiplanar Reconstruction |
| MR | Mitral Regurgitation |
| MV | Mitral Valve |
| PVL | Paravalvular Leak |
| ROBIN | Retrograde Balloon-Induced Anterior Mitral leaflet laceration |
| RV | Right Ventricle |
| SAM | Systolic Anterior Motion |
| SESAME | Septal Scoring Along the Midline Endocardium |
| S′ | Peak Systolic Velocity |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TEE | Transesophageal Echocardiography |
| TEER | Transcatheter Edge-to-Edge Repair |
| TMVR | Transcatheter Mitral Valve Replacement |
| ViMAC | Valve-in-Mitral Annular Calcification |
| ViR | Valve-in-Ring |
| ViV | Valve-in-Valve |
References
- Demir, O.M.; Bolland, M.; Curio, J.; Søndergaard, L.; Rodés-Cabau, J.; Redwood, S.; Prendergast, B.; Colombo, A.; Chau, M.; Latib, A. Transcatheter Mitral Valve Replacement: Current Evidence and Concepts. Interv. Cardiol. Rev. 2021, 16, e07. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2019, 40, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Hensey, M.; Brown, R.A.; Lal, S.; Sathananthan, J.; Ye, J.; Cheung, A.; Blanke, P.; Leipsic, J.; Moss, R.; Boone, R.; et al. Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-Perez, M.; Caneiro-Queija, B.; Puga, L.; Gonzalez-Ferreiro, R.; Alarcon, R.; Parada, J.A.; Iñiguez-Romo, A.; Estevez-Loureiro, R. Imaging in Transcatheter Mitral Valve Replacement: State-of-Art Review. J. Clin. Med. 2021, 10, 5973. [Google Scholar] [CrossRef]
- Agarwal, V.; Kaple, R.K.; Mehta, H.H.; Singh, P.; Bapat, V.N. Current state of transcatheter mitral valve implantation in bioprosthetic mitral valve and in mitral ring as a treatment approach for failed mitral prosthesis. Ann. Cardiothorac. Surg. 2021, 10, 585–604. [Google Scholar] [CrossRef]
- Garcia-Sayan, E.; Chen, T.; Khalique, O.K. Multimodality Cardiac Imaging for Procedural Planning and Guidance of Transcatheter Mitral Valve Replacement and Mitral Paravalvular Leak Closure. Front. Cardiovasc. Med. 2021, 8, 582925. [Google Scholar] [CrossRef]
- Mackensen, G.B.; Lee, J.C.; Wang, D.D.; Pearson, P.J.; Blanke, P.; Dvir, D.; Kirkpatrick, J.N. Role of Echocardiography in Transcatheter Mitral Valve Replacement in Native Mitral Valves and Mitral Rings. J. Am. Soc. Echocardiogr. 2018, 31, 475–490. [Google Scholar] [CrossRef]
- Hirasawa, K.; Vo, N.M.; Gegenava, T.; Pio, S.M.; Van Wijngaarden, S.E.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Mitral Valve Annulus Dimensions Assessment with Three-Dimensional Echocardiography Versus Computed Tomography: Implications for Transcatheter Interventions. J. Clin. Med. 2021, 10, 649. [Google Scholar] [CrossRef]
- Asgar, A.W. Sizing the Mitral Annulus. JACC Cardiovasc. Imaging. 2016, 9, 281–282. [Google Scholar] [CrossRef]
- Faletra, F.F.; Agricola, E.; Flachskampf, F.A.; Hahn, R.; Pepi, M.; Ajmone Marsan, N.; Wunderlich, N.; Elif Sade, L.; Donal, E.; Zamorano, J.L.; et al. Three-dimensional transoesophageal echocardiography: How to use and when to use—A clinical consensus statement from the European Association of Cardiovascular Imaging of the European Society of Cardiology. Eur. Heart J.—Cardiovasc. Imaging. 2023, 24, e119–e197. [Google Scholar] [CrossRef]
- Bax, J.J.; Debonnaire, P.; Lancellotti, P.; Ajmone Marsan, N.; Tops, L.F.; Min, J.K.; Piazza, N.; Leipsic, J.; Hahn, R.T.; Delgado, V. Transcatheter Interventions for Mitral Regurgitation. JACC Cardiovasc. Imaging. 2019, 12, 2029–2048. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Mehrotra, M. Transesophageal guidance for trans-catheter trans-septal mitral valve in valve implantation. J. Cardiothorac. Vasc. Anesth. 2019, 33, S136–S137. [Google Scholar] [CrossRef]
- Coisne, A.; Pontana, F.; Tchétché, D.; Richardson, M.; Longère, B.; Vahdat, O.; Berthoumieu, P.; Van Belle, E.; Rousse, N.; Lancellotti, P.; et al. Transcatheter mitral valve replacement: Factors associated with screening success and failure. EuroIntervention 2019, 15, e983–e989. [Google Scholar] [CrossRef] [PubMed]
- Beller, J.P.; Rogers, J.H.; Thourani, V.H.; Ailawadi, G. Early clinical results with the Tendyne transcatheter mitral valve replacement system. Ann. Cardiothorac. Surg. 2018, 7, 776–779. [Google Scholar] [CrossRef]
- Dahle, G. Current Devices in TMVI and Their Limitations: Focus on Tendyne. Front. Cardiovasc. Med. 2020, 7, 592909. [Google Scholar] [CrossRef]
- Blanke, P.; Modine, T.; Duncan, A.; Taramasso, M.; Dumonteil, N.; Chuang, M.L.; Conradi, L. Anatomic suitability for transapical transcatheter mitral valve implantation using a tether-based device. Catheter. Cardiovasc. Interv. 2023, 102, 318–327. [Google Scholar] [CrossRef]
- Babaliaros, V.C.; Lederman, R.J.; Gleason, P.T.; Khan, J.M.; Kohli, K.; Sahu, A.; Rogers, T.; Bruce, C.G.; Paone, G.; Xie, J.X.; et al. The Art of SAPIEN 3 Transcatheter Mitral Valve Replacement in Valve-in-Ring and Valve-in-Mitral-Annular-Calcification Procedures. JACC Cardiovasc. Interv. 2021, 14, 2195–2214. [Google Scholar] [CrossRef]
- Lim, Z.Y.; Boix, R.; Prendergast, B.; Rajani, R.; Redwood, S.; Hancock, J.; Young, C.; Bapat, V.V. First Reported Case of Transcatheter Mitral Valve Implantation in Mitral Annular Calcification With a Fully Repositionable and Self-Expanding Valve. Circ. Cardiovasc. Interv. 2015, 8, e003031. [Google Scholar] [CrossRef]
- Sorajja, P.; Bapat, V. Early experience with the Intrepid system for transcatheter mitral valve replacement. Ann. Cardiothorac. Surg. 2018, 7, 792–798. [Google Scholar] [CrossRef]
- Bartkowiak, J.; Dernektsi, C.; Agarwal, V.; Lebehn, M.A.; Williams, T.A.; Brandwein, R.A.; Brugger, N.; Gräni, C.; Windecker, S.; Vahl, T.P.; et al. 3-Dimensional Echocardiographic Prediction of Left Ventricular Outflow Tract Area Prior to Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Imaging. 2024, 17, 1168–1178. [Google Scholar] [CrossRef]
- Kohli, K.; Wei, Z.A.; Yoganathan, A.P.; Oshinski, J.N.; Leipsic, J.; Blanke, P. Transcatheter Mitral Valve Planning and the Neo-LVOT: Utilization of Virtual Simulation Models and 3D Printing. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 99. [Google Scholar] [CrossRef]
- Khan, J.M.; Lederman, R.J. Adventures across the second dimension: Predicting left ventricular outflow tract obstruction following transcatheter mitral valve replacement. Catheter. Cardiovasc. Interv. 2018, 92, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Wei, Z.A.; Sadri, V.; Khan, J.M.; Lisko, J.C.; Netto, T.; Greenbaum, A.B.; Blanke, P.; Oshinski, J.N.; Lederman, R.J.; et al. Dynamic nature of the LVOT following transcatheter mitral valve replacement with LAMPOON: New insights from post-procedure imaging. Eur. Heart J.—Cardiovasc. Imaging. 2022, 23, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, N.; Horne, B.D.; Doty, J.R.; Reid, B.B.; Miner, E.C.; Harkness, J.R.; Jones, K.W.; Minder, C.M.; Caine, W.T.; Clayson, S.E.; et al. Transcatheter mitral valve in ring, hazards of long anterior mitral leaflet and 3-dimensional rings. Catheter. Cardiovasc. Interv. 2021, 97, 353–358. [Google Scholar] [CrossRef] [PubMed]
- El Sabbagh, A.; Al-Hijji, M.; Wang, D.D.; Eleid, M.; Urena, M.; Himbert, D.; Chakravarty, T.; Holzhey, D.; Pershad, A.; Fang, H.K.; et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement in Severe Mitral Annular Calcification: An Analysis of the Transcatheter Mitral Valve Replacement in Mitral Annular Calcification Global Registry. Circ. Cardiovasc. Interv. 2021, 14, e010854. [Google Scholar] [CrossRef]
- Lisko, J.; Kamioka, N.; Gleason, P.; Byku, I.; Alvarez, L.; Khan, J.M.; Rogers, T.; Lederman, R.; Greenbaum, A.; Babaliaros, V. Prevention and Treatment of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. Interv. Cardiol. Clin. 2019, 8, 279–285. [Google Scholar] [CrossRef]
- Asgar, A.W.; Ducharme, A.; Messas, N.; Basmadjian, A.; Bouchard, D.; Pellerin, M. Left Ventricular Outflow Tract Obstruction Following Mitral Valve Replacement: Challenges for Transcatheter Mitral Valve Therapy. Struct. Heart. 2018, 2, 372–379. [Google Scholar] [CrossRef]
- Yoon, S.H.; Bleiziffer, S.; Latib, A.; Eschenbach, L.; Ancona, M.; Vincent, F.; Kim, W.K.; Unbehaum, A.; Asami, M.; Dhoble, A.; et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 182–193. [Google Scholar] [CrossRef]
- Lerakis, S.; Kini, A.S.; Asch, F.M.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Weissman, N.J.; Rinaldi, M.J.; et al. Outcomes of transcatheter mitral valve repair for secondary mitral regurgitation by severity of left ventricular dysfunction. EuroIntervention 2021, 17, e335–e342. [Google Scholar] [CrossRef]
- Gentile, F.; Chianca, M.; Bazan, L.; Sciarrone, P.; Chubuchny, V.; Taddei, C.; Poggianti, E.; Passino, C.; Emdin, M.; Giannoni, A. Incremental Prognostic Value of Echocardiography Measures of Right Ventricular Systolic Function in Patients With Chronic Heart Failure. J. Am. Heart Assoc. 2025, 14, e038616. [Google Scholar] [CrossRef]
- Ludwig, S.; Perrin, N.; Coisne, A.; Ben Ali, W.; Weimann, J.; Duncan, A.; Akodad, M.; Scotti, A.; Kalbacher, D.; Bleiziffer, S.; et al. Clinical outcomes of transcatheter mitral valve replacement: Two-year results of the CHOICE-MI Registry. EuroIntervention 2023, 19, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Gucuk Ipek, E.; Singh, S.; Viloria, E.; Feldman, T.; Grayburn, P.; Foster, E.; Qasim, A. Impact of the MitraClip Procedure on Left Atrial Strain and Strain Rate. Circ. Cardiovasc. Imaging 2018, 11, e006553. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Qureshi, A.; Khan, A.H. Transseptal Puncture Through an Interatrial Septum With Lipomatous Hypertrophy: A False Perception of Success and Failure. Cureus 2022, 14, e29737. [Google Scholar] [CrossRef]
- Zafar, H.; Soleimani, S.; Ijaz, M.; Zafar, J.; Sharif, F. Complex mitral valve anatomy and open issues in transcatheter mitral valve replacement. Surg. Pract. Sci. 2023, 13, 100182. [Google Scholar] [CrossRef]
- Iliakis, P.; Dimitriadis, K.; Pyrpyris, N.; Beneki, E.; Theofilis, P.; Tsioufis, P.; Kamperidis, V.; Aznaouridis, K.; Aggeli, K.; Tsioufis, K. Atrial Functional Mitral Regurgitation: From Diagnosis to Current Interventional Therapies. J. Clin. Med. 2024, 13, 5035. [Google Scholar] [CrossRef]
- Coisne, A.; Pontana, F.; Aghezzaf, S.; Mouton, S.; Ridon, H.; Richardson, M.; Polge, A.S.; Longère, B.; Silvestri, V.; Pagniez, J.; et al. Utility of Three-Dimensional Transesophageal Echocardiography for Mitral Annular Sizing in Transcatheter Mitral Valve Replacement Procedures: A Cardiac Computed Tomographic Comparative Study. J. Am. Soc. Echocardiogr. 2020, 33, 1245–1252.e2. [Google Scholar] [CrossRef]
- Tarzia, P.; Ciampi, P.; Lanza, O.; Canali, E.; Canestrelli, S.; Calò, L. Multi-modality imaging for pre-procedural planning of transcatheter mitral valve interventions. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C205–C211. [Google Scholar] [CrossRef]
- Nikolou, E.; Bilkhu, R.; Kafil, T.S.; Demetrescu, C.; Kotta, P.A.; Lucchese, G.; Tzemos, N.; Grapsa, J. Multimodality Imaging in Transcatheter Mitral Interventions. Front. Cardiovasc. Med. 2021, 8, 638399. [Google Scholar] [CrossRef]
- Bhargava, A.A.; Shekiladze, N.; Xie, J.; Kini, A.; Gleason, P.T.; Lerakis, S. Use of transesophageal echocardiography for transcatheter valve-in-valve implantation for patients with prior bioprosthetic surgical aortic, mitral, tricuspid, and pulmonic valves. Ann. Cardiothorac. Surg. 2021, 10, 605–620. [Google Scholar] [CrossRef]
- Russo, G.; Taramasso, M.; Maisano, F. Transseptal puncture: Procedural guidance, challenging situations and management of complications. EuroIntervention 2021, 17, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Hung, J. Atrial septal puncture technique in percutaneous transvenous mitral commissurotomy: Mitral valvuloplasty using the inoue balloon catheter technique. Cathet Cardiovasc. Diagn. 1992, 26, 275–284. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Zafar, H.; De Freitas, S.; Sharif, F. Transseptal puncture—Review of anatomy, techniques, complications and challenges. Int. J. Cardiol. 2017, 233, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sang, C.; Bai, R.; Lai, Y.; Long, D.; Li, S.; Tang, R.; Jiang, C.; Zuo, S.; Du, X.; et al. Transseptal puncture in patients with septal occluder devices during catheter ablation of atrial fibrillation. EuroIntervention 2022, 17, 1112–1119. [Google Scholar] [CrossRef]
- Pillai, A.; Padala, S.K.; Ellenbogen, K.A.; Koneru, J.N. An Unusual Complication of Transseptal Puncture. JACC Case Rep. 2021, 3, 41–46. [Google Scholar] [CrossRef]
- Wunderlich, N.C.; Beigel, R.; Ho, S.Y.; Nietlispach, F.; Cheng, R.; Agricola, E.; Siegel, R.J. Imaging for Mitral Interventions. JACC Cardiovasc. Imaging 2018, 11, 872–901. [Google Scholar] [CrossRef]
- Bartorelli, A.L.; Monizzi, G.; Mastrangelo, A.; Grancini, L.; Fabbiocchi, F.; Conte, E.; Moltrasio, M.; Andreini, D. Transcatheter mitral valve replacement: There is still work to be done. Eur. Heart J. Suppl. 2022, 24 (Suppl. I), I16–I21. [Google Scholar] [CrossRef]
- Russo, G.; Maisano, F.; Massaro, G.; Terlizzese, G.; Mariano, E.; Bonanni, M.; Matteucci, A.; Bezzeccheri, A.; Benedetto, D.; Chiricolo, G.; et al. Challenges and Open Issues in Transcatheter Mitral Valve Implantation: Smooth Seas Do Not Make Skillful Sailors. Front. Cardiovasc. Med. 2022, 8, 738756. [Google Scholar] [CrossRef]
- Meredith, I.; Bapat, V.; Morriss, J.; McLean, M.; Prendergast, B. Intrepid transcatheter mitral valve replacement system: Technical and product description. EuroIntervention 2016, 12, Y78–Y80. [Google Scholar] [CrossRef]
- Ueyama, H.A.; Gleason, P.T.; Babaliaros, V.C.; Greenbaum, A.B. Transcatheter Mitral Valve Replacement in Failed Bioprosthetic Valve, Ring, and Mitral Annular Calcification Associated Mitral Valve Disease Using Balloon Expandable Transcatheter Heart Valve. Methodist. DeBakey Cardiovasc. J. 2023, 19, 37–49. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; Perdoncin, E.; Paone, G.; Grubb, K.J.; Xie, J.X.; Gleason, P.T.; Lederman, R.J.; Babaliaros, V.C. Tableside Skirt Modification of the SAPIEN 3 Valve to Reduce Paravalvular Leak During Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Penteris, M.; Lampropoulos, K. The HighLife transcatheter mitral valve replacement system: A novel two-component platform. Cardiovasc. Revasc Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.M.; Worthley, S.; Nickenig, G.; Huczek, Z.; Wojakowski, W.; Tchetche, D.; Dubois, C.; Nasr, M.; Verhees, L.; Rothman, M.; et al. 1-Year Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement: The HighLife TSMVR Feasibility Study. JACC Cardiovasc. Interv. 2023, 16, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Foley, T.A.; Said, S.M.; Pislaru, S.V.; Rihal, C.S. Severe Mitral Annular Calcification. JACC Cardiovasc. Imaging. 2016, 9, 1318–1337. [Google Scholar] [CrossRef]
- Little, S.H.; Bapat, V.; Blanke, P.; Guerrero, M.; Rajagopal, V.; Siegel, R. Imaging Guidance for Transcatheter Mitral Valve Intervention on Prosthetic Valves, Rings, and Annular Calcification. JACC Cardiovasc. Imaging. 2021, 14, 22–40. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Jone, P.N.; Chamsi-Pasha, M.A.; Chen, T.; Collins, K.A.; Desai, M.Y.; Grayburn, P.; Groves, D.W.; Hahn, R.T.; Little, S.H.; et al. Guidelines for the Evaluation of Prosthetic Valve Function with Cardiovascular Imaging: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2024, 37, 2–63. [Google Scholar] [CrossRef]
- Gössl, M.; Thourani, V.; Babaliaros, V.; Conradi, L.; Chehab, B.; Dumonteil, N.; Badhwar, V.; Rizik, D.; Sun, B.; Bae, R.; et al. Early outcomes of transcatheter mitral valve replacement with the Tendyne system in severe mitral annular calcification. EuroIntervention 2022, 17, 1523–1531. [Google Scholar] [CrossRef]
- Guerrero, M.; Dvir, D.; Himbert, D.; Urena, M.; Eleid, M.; Wang, D.D.; Greenbaum, A.; Mahadevan, V.S.; Holzhey, D.; O’hair, D.; et al. Transcatheter Mitral Valve Replacement in Native Mitral Valve Disease With Severe Mitral Annular Calcification. JACC Cardiovasc. Interv. 2016, 9, 1361–1371. [Google Scholar] [CrossRef]
- Zahr, F.; Song, H.K.; Chadderdon, S.M.; Gada, H.; Mumtaz, M.; Byrne, T.; Kirshner, M.; Baiwa, T.; Weiss, E.; Kodali, S.; et al. 30-Day Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement: Intrepid TMVR Early Feasibility Study Results. JACC Cardiovasc. Interv. 2022, 15, 80–89. [Google Scholar] [CrossRef]
- Zahr, F.; Song, H.K.; Chadderdon, S.; Gada, H.; Mumtaz, M.; Byrne, T.; Kirshner, M.; Sharma, S.; Kodali, S.; George, I.; et al. 1-Year Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement: Intrepid TMVR Early Feasibility Study Results. JACC Cardiovasc. Interv. 2023, 16, 2868–2879. [Google Scholar] [CrossRef]
- Guerrero, M.; Wang, D.D.; Eleid, M.F.; Pursnani, A.; Salinger, M.; Russell, H.M.; Kodali, S.H.; George, I.; Bapat, V.N.; Dangas, G.D.; et al. Prospective Study of TMVR Using Balloon-Expandable Aortic Transcatheter Valves in MAC. JACC Cardiovasc. Interv. 2021, 14, 830–845. [Google Scholar] [CrossRef]
- Gheorghe, L.; Brouwer, J.; Wang, D.D.; Wunderlich, N.; Rana, B.; Rensing, B.; Eefting, F.; Timmers, L.; Swaans, M. Current Devices in Mitral Valve Replacement and Their Potential Complications. Front. Cardiovasc. Med. 2020, 7, 531843. [Google Scholar] [CrossRef]
- Sehgal, S.; Subramanyam, P.; Ahluwalia, M.; Rastogi, A.; Bergman, G. Transcatheter mitral valve implantation: Implications of interventional technique and 3D echocardiography for complex valve-in-valve paravalvular leak. Ann. Card. Anaesth. 2023, 26, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.E.; Bapat, V.N.; Mahoney, P.; Krishnaswamy, A.; Eleid, M.F.; Eng, M.H.; Yadav, P.; Coylewright, M.; Makkar, R.; Szerlip, M.; et al. Contemporary 1-Year Outcomes of Mitral Valve-in-Ring With Balloon-Expandable Aortic Transcatheter Valves in the U. S. JACC Cardiovasc. Interv. 2024, 17, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Hell, M.M.; Wild, M.G.; Baldus, S.; Rudolph, T.; Treede, H.; Petronio, A.S.; Modine, T.; Andreas, M.; Coisne, A.; Duncan, A.; et al. Transapical Mitral Valve Replacement. JACC Cardiovasc. Interv. 2024, 17, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Kirschfink, A.; Alachkar, M.N.; Almalla, M.; Grebe, J.; Vogt, F.; Schröder, J.; Frick, M.; Marx, N.; Altiok, E. Prediction of procedural success of transcatheter mitral valve repair with normal and extended clip arms. Int. J. Cardiovasc. Imaging. 2022, 38, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Perpetua, E.M.; Reisman, M. The Tendyne transcatheter mitral valve implantation system. EuroIntervention 2015, 14, W78-9. [Google Scholar] [CrossRef]
- Niv Granot, Y.; Passaniti, G.; Sharma, S.; Kini, A.; Karlsberg, D.; Khera, S.; Tang, G.H.L.; Lerakis, S.; Safi, L.M. Estimating Left Atrial Pressure Using Diastolic Cutoff Values After Transcatheter Mitral Valve Edge-to-Edge Repair. J. Clin. Med. 2025, 14, 4308. [Google Scholar] [CrossRef]
- Lutter, G.; Dai, H.; Hansen, J.H.; Frank, D.; Haneya, A.; Simionescu, D.; Cremer, J.; Puehler, T. Transcatheter mitral valve replacement (TMVR): Annular or apical fixation? EuroIntervention 2020, 16, e510–e517. [Google Scholar] [CrossRef]
- Wollborn, J.; Schuler, A.; Sheu, R.D.; Shook, D.C.; Nyman, C.B. Real-Time Multiplanar Reconstruction Imaging Using 3-Dimensional Transesophageal Echocardiography in Structural Heart Interventions. J. Cardiothorac. Vasc. Anesth. 2023, 37, 570–581. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Harb, S.C.; Miyasaka, R.L.; Wagener, J.; Krishnaswamy, A.; Reed, G.K.; Kapadia, S.R. Live Three-Dimensional Multiplanar Reconstruction Imaging Guidance for Concomitant Mitral and Tricuspid Valve Repairs Using the MitraClip. CASE (Phila). 2020, 4, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Quader, N.; Kaneko, T.; Williford, N.; Sintek, M.; Kachroo, P.; Brescia, A.A.; Roberts, H.G., Jr.; Zajarias, A. Transesophageal Echocardiography and Intracardiac Echocardiography to Guide EVOQUE. JACC Case Rep. 2025, 30, 103628. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Yakubov, S.J.; Singh, G.; Rogers, J.H.; Kander, N.H.; Tang, G.H.L. 4-Dimensional Intracardiac Echocardiography in Transcatheter Mitral Valve Repair With the Mitraclip System. JACC Cardiovasc. Imaging. 2021, 14, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, R.J.; Sarkar, A.; Navas, V.; Navia, J.L. “Minimalist approach” for transcatheter mitral valve replacement using intracardiac echocardiography and conscious sedation: A case series. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Hassan, A.H.; Alkhouli, M.A.; Thaden, J.J.; Guerrero, M.E. 3D Intracardiac Echo-Guided Transseptal Mitral Valve-in-Valve Under Conscious Sedation. JACC Cardiovasc. Interv. 2022, 15, e103–e105. [Google Scholar] [CrossRef]
- Krishnaswamy, A.; Kaur, S.; Isogai, T.; Zhou, L.; Shekhar, S.; Yun, J.; Unai, S.; Burns, D.; Kapadia, S. Minimalist Mitral Valve-in-Valve Replacement Using Conscious Sedation and Intracardiac Echocardiography Is Feasible and Safe. JACC Cardiovasc. Interv. 2022, 15, 1288–1290. [Google Scholar] [CrossRef]
- Wong, N.; Fowler, D.; Lim, D.S. Mitral valve-in-valve with 4D intracardiac echocardiography: Procedural and imaging technique. Catheter. Cardiovasc. Interv. 2024, 103, 234–237. [Google Scholar] [CrossRef]
- Wamala, I.; Unbehaun, A.; Klein, C.; Kukucka, M.; Eggert-Doktor, D.; Buz, S.; Stein, J.; Sündermann, S.; Falk, V.; Kempfert, J. Real-time intraoperative co-registration of transesophageal echocardiography with fluoroscopy facilitates transcatheter mitral valve-in-valve implantation in cases of invisible degenerated bioprosthetic valves. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 695–702. [Google Scholar] [CrossRef]
- Pascual, I.; Pozzoli, A.; Taramasso, M.; Maisano, F.; Ho, E.C. Fusion imaging for transcatheter mitral and tricuspid interventions. Ann. Transl. Med. 2020, 8, 965. [Google Scholar] [CrossRef]
- Barreiro-Perez, M.; Estévez-Loureiro, R.; Puga, L.; Caneiro-Queija, B.; Baz, J.A.; Iñiguez-Romo, A. Real-Time Echocardiography-Fluoroscopy Fusion Imaging With Automated 3D Heart Segmentation During Transcatheter Structural Heart Interventions. JACC Cardiovasc. Interv. 2022, 15, e155–e158. [Google Scholar] [CrossRef]
- Faletra, F.F.; Pozzoli, A.; Agricola, E.; Guidotti, A.; Biasco, L.; Leo, L.A.; Taramasso, M.; Pasotti, E.; Kuwata, S.; Moccetti, M.; et al. Echocardiographic-fluoroscopic fusion imaging for transcatheter mitral valve repair guidance. Eur. Heart J.—Cardiovasc. Imaging. 2018, 19, 715–726. [Google Scholar] [CrossRef]
- Wiley, B.M.; Eleid, M.F.; Thaden, J.J. Fusion Imaging for Procedural Guidance. Rev. Esp. Cardiol. Engl. Ed. 2018, 71, 373–381. [Google Scholar] [CrossRef]
- Ho, E.C.; Assafin, M.; Sugiura, T.; Granada, J.F.; Chau, M.; Latib, A. 3-dimensional intracardiac echocardiography for structural heart interventions. Front. Cardiovasc. Med. 2023, 10, 1180299. [Google Scholar] [CrossRef]
- Nobre, C.; Oliveira-Santos, M.; Paiva, L.; Costa, M.; Gonçalves, L. Fusion imaging in interventional cardiology. Rev. Port. Cardiol. Engl. Ed. 2020, 39, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Brugiatelli, L.; Rolando, M.; Lofiego, C.; Fogante, M.; Capodaglio, I.; Patani, F.; Tofoni, P.; Maurizi, K.; Nazziconi, M.; Massari, A.; et al. Transcatheter Mitral Valve Intervention: Current and Future Role of Multimodality Imaging for Device Selection and Periprocedural Guidance. Medicina 2024, 60, 1082. [Google Scholar] [CrossRef] [PubMed]
- Faza, N.N.; Little, S.H. Role of 3-dimensional transesophageal echocardiography in guiding transcatheter mitral valve replacement. Echocardiography. 2020, 37, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Jimenez, A.; Rama-Merchan, J.C.; Barreiro-Pérez, M.; Merchan-Gómez, S.; Iscar-Galán, A.; Martín-García, A.; Nieto-Ballestero, F.; Sánchez-Corral, E.; Rodriguez-Collado, J.; Cruz-González, I.; et al. Utility of Real-Time 3-Dimensional Transesophageal Echocardiography in the Assessment of Mitral Paravalvular Leak. Circ. J. 2016, 80, 738–744. [Google Scholar] [CrossRef][Green Version]
- Dvir, D.; Webb, J. Mitral valve-in-valve and valve-in-ring: Technical aspects and procedural outcomes. EuroIntervention 2016, 12, Y93–Y96. [Google Scholar] [CrossRef]
- Chehab, O.; Roberts-Thomson, R.; Bivona, A.; Gill, H.; Patterson, T.; Pursnani, A.; Grigoryan, K.; Vargas, B.; Bokhary, U.; Blauth, C.; et al. Management of Patients With Severe Mitral Annular Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 722–738. [Google Scholar] [CrossRef]
- Murphy, D.J.; Ge, Y.; Don, C.W.; Keraliya, A.; Aghayev, A.; Morgan, R.; Galper, B.; Bhatt, D.L.; Kaneko, T.; Di Carli, M.; et al. Use of Cardiac Computerized Tomography to Predict Neo–Left Ventricular Outflow Tract Obstruction Before Transcatheter Mitral Valve Replacement. J. Am. Heart Assoc. 2017, 6, e007353. [Google Scholar] [CrossRef]
- Guerrero, M.; Urena, M.; Himbert, D.; Wang, D.D.; Eleid, M.; Kodali, S.; George, I.; Chakravarty, T.; Mathur, M.; Holzhey, D.; et al. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Mitral Annular Calcification. J. Am. Coll. Cardiol. 2018, 71, 1841–1853. [Google Scholar] [CrossRef]
- Eleid, M.F.; Collins, J.D.; Mahoney, P.; Williamson, E.E.; Killu, A.M.; Whisenant, B.K.; Rihal, C.S.; Guerrero, M.E. Emerging Approaches to Management of Left Ventricular Outflow Obstruction Risk in Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2023, 16, 885–895. [Google Scholar] [CrossRef]
- Khan, J.M.; Babaliaros, V.C.; Greenbaum, A.B.; Foerst, J.R.; Yazdani, S.; McCabe, J.M.; Paone, G.; Eng, M.H.; Leshnower, B.G.; Gleason, P.T.; et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2521–2534. [Google Scholar] [CrossRef]
- Lisko, J.C.; Babaliaros, V.C.; Khan, J.M.; Kamioka, N.; Gleason, P.T.; Paone, G.; Byku, I.; Tiwana, J.; McCabe, J.M.; Cherukuri, K.; et al. Tip-to-Base LAMPOON for Transcatheter Mitral Valve Replacement With a Protected Mitral Annulus. JACC Cardiovasc. Interv. 2021, 14, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Case, B.C.; Lisko, J.C.; Babaliaros, V.C.; Greenbaum, A.B.; Satler, L.; Ben-Dor, I.; Forrestal, B.J.; Yerasi, C.; Kamioka, N.; Rogers, T.; et al. LAMPOON techniques to prevent or manage left ventricular outflow tract obstruction in transcatheter mitral valve replacement. Ann. Cardiothorac. Surg. 2021, 10, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Helmy, T.; Hui, D.S.; Smart, S.; Lim, M.J.; Lee, R. Balloon assisted translocation of the mitral anterior leaflet to prevent left ventricular outflow obstruction (BATMAN): A novel technique for patients undergoing transcatheter mitral valve replacement. Catheter. Cardiovasc. Interv. 2020, 95, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Mangieri, A.; Cozzi, O.; Bragato, R.; Sticchi, A.; Bertoldi, L.; De Marco, F.; Monti, L.; Tosi, P.; Vitrella, G.; et al. Transseptal Balloon-Assisted Translocation of the Mitral Anterior Leaflet (BATMAN) in Mitral Valve-in-Ring Implantation. JACC Cardiovasc. Interv. 2024, 17, 568–570. [Google Scholar] [CrossRef]
- Denti, P.; Saccocci, M.; Buzzatti, N.; Ascione, G.; Margonato, D.; Gatto, P.; Palloshi, A.; Sarais, C.; Longoni, M.; Maisano, F. Transseptal BATMAN for High-Risk Valve-in-Ring Procedures: A Case Series. JACC Case Rep. 2024, 29, 102200. [Google Scholar] [CrossRef]
- Lai, L.K.L.; Alrayes, H.; Fram, G.; Lee, J.C.; Zweig, B.; Giustino, G.; Jabri, A.; O’Neill, B.P.; Frisoli, T.M.; Gonzalez, P.E.; et al. Retrograde Balloon-Induced Anterior Mitral Leaflet Laceration: The ROBIN Technique. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 103818. [Google Scholar] [CrossRef]
- Alnasser, S.; Attumalil, T.; Alkasab, M.; Patrascu, A.; Almalki, Y.; Ong, G.; Latter, D.; Fam, N.P. Transcatheter Mitral Valve Replacement in Severe Mitral Annular Calcification. JACC Cardiovasc. Interv. 2025, 18, 948–949. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; Ueyama, H.A.; Gleason, P.T.; Khan, J.M.; Bruce, C.G.; Halaby, R.N.; Toby, R.; Hanzel, G.S.; Xies, J.X.; Byku, I.; et al. Transcatheter Myotomy to Reduce Left Ventricular Outflow Obstruction. J. Am. Coll. Cardiol. 2024, 83, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- McCabe, J.M.; Newton, S.; Danek, B.A.; Elison, D.; Chung, C.J.; Sheu, R.; Jelacic, S.; Condos, G.J.; Canovas, E.; Greenbaum, A.B.; et al. SESAME technique: Septal scoring along the midline endocardium. EuroIntervention 2025, 21, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Marsan, N.A.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2025, 67, ehaf194. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, E72–E227. [Google Scholar]
- Bonow, R.O.; O’Gara, P.T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.; Satpathy, R.; et al. 2019 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document: Operator and Institutional Recommendations and Requirements for Transcatheter Mitral Valve Intervention. Ann. Thorac. Surg. 2020, 110, 316–335. [Google Scholar] [CrossRef]
- Agricola, E.; Meucci, F.; Ancona, F.; Pardo Sanz, A.; Zamorano, J.L. Echocardiographic guidance in transcatheter structural cardiac interventions. EuroIntervention 2022, 17, 1205–1226. [Google Scholar] [CrossRef]


| Device | Manufacturer | Type | Annular Sizing Range | Key Considerations | |
|---|---|---|---|---|---|
| Tendyne |  | Abbott (Abbott Park, IL, USA) | Self-expanding, tethered | Broad range, up to ~50 mm annular diameter | Largest size matrix; suitable for large annuli; less ideal for very small annuli |
| Intrepid |  | Medtronic (Dublin, Ireland) | Self-expanding, dual-stent | Restricted to ~36–43 mm outer frame diameters | Anchors in annulus/leaflets; limited for very small or large annuli |
| Sapien 3 (ViMAC, ViV, ViR) |  | Edwards Lifesciences (Irvine, CA, USA) | Balloon-expandable | Up to ~29 mm outer diameter | Widely used in ViV/ViR/ViMAC; risk of oversizing in small annuli; may be inadequate for large annuli |
| HighLife |  | HighLife SAS (Paris, France) | Two-component system (subannular ring + prosthesis) | Limited published ranges; typical native annuli | Novel anchoring system; investigational; sizing less standardized |
| Altavalve |  | 4C Medical (Maple Grove, MN, USA) | Self-expanding, atrial anchoring | Early feasibility data; ~27–51 mm annular diameters | Investigational; designed for broad annular compatibility; ongoing evaluation |
| Parameter | Cut-Off/Threshold | Clinical Implication |
|---|---|---|
| Predicted neo-LVOT area | <200 mm2 = high risk of LVOTO | Values below threshold predict severe LVOTO, often requiring adjunctive strategies (LAMPOON, BATMAN, SESAME). |
| Anterior Mitral Leaflet length | >25 mm associated with increased obstruction risk | Excessive leaflet length predisposes to systolic anterior motion and LVOT encroachment. |
| LVEF | <30–35% = impaired prognosis | Reduced LVEF predicts limited procedural benefit and higher perioperative risk. |
| TAPSE | <17 mm indicates RV dysfunction | Reduced TAPSE reflects RV dysfunction, associated with worse survival post-TMVR. |
| Transmitral mean gradient (post-TMVR) | <5 mmHg at HR ~70 bpm considered acceptable | Elevated gradients suggest device underexpansion, malalignment, or prosthesis-patient mismatch. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacchi, B.; Derry, K.; Vira, T.; Alnasser, S.; Montanhesi, P.K.; Fam, N.; Bisleri, G. Transesophageal Echocardiography in Transcatheter Mitral Valve Replacement. J. Clin. Med. 2025, 14, 7966. https://doi.org/10.3390/jcm14227966
Bacchi B, Derry K, Vira T, Alnasser S, Montanhesi PK, Fam N, Bisleri G. Transesophageal Echocardiography in Transcatheter Mitral Valve Replacement. Journal of Clinical Medicine. 2025; 14(22):7966. https://doi.org/10.3390/jcm14227966
Chicago/Turabian StyleBacchi, Beatrice, Kendra Derry, Tasnim Vira, Sami Alnasser, Paola Keese Montanhesi, Neil Fam, and Gianluigi Bisleri. 2025. "Transesophageal Echocardiography in Transcatheter Mitral Valve Replacement" Journal of Clinical Medicine 14, no. 22: 7966. https://doi.org/10.3390/jcm14227966
APA StyleBacchi, B., Derry, K., Vira, T., Alnasser, S., Montanhesi, P. K., Fam, N., & Bisleri, G. (2025). Transesophageal Echocardiography in Transcatheter Mitral Valve Replacement. Journal of Clinical Medicine, 14(22), 7966. https://doi.org/10.3390/jcm14227966







