Transcranial Direct Current Stimulation (tDCS) in Diabetes: A Focused and Mechanistic Review of Symptom and Function Outcomes
Abstract
1. Introduction
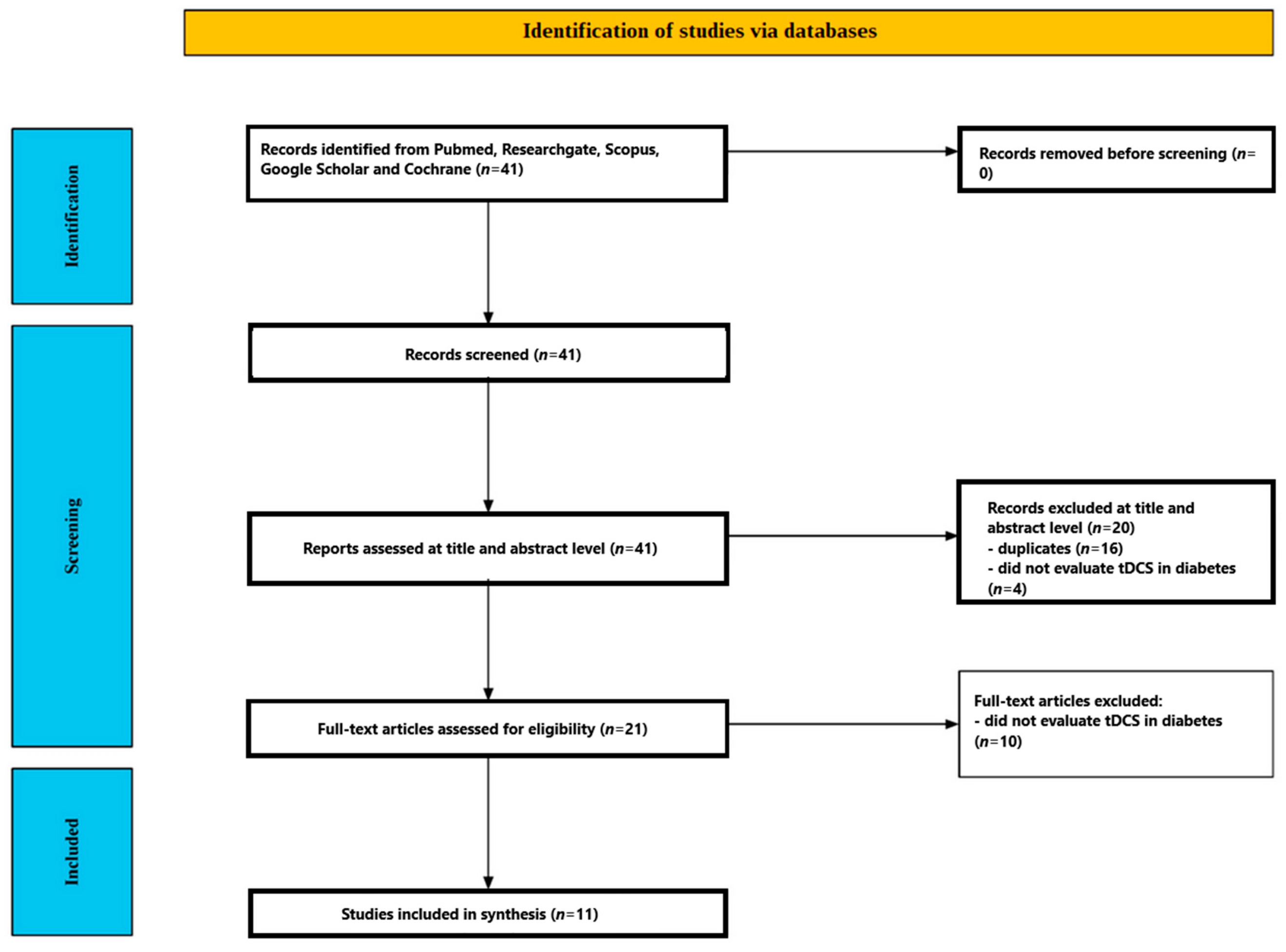
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Screening Process
2.3.1. Title and Abstract Screening
2.3.2. Full-Text Assessment
3. Results
3.1. Participants’ Characteristics
3.2. tDCS Interventions
3.2.1. Targets and Montages
3.2.2. Intensity, Duration, and Session Structure
3.2.3. Electrodes and Materials
3.2.4. Sham Procedures and Blinding
3.2.5. Concomitant Activities and Co-Interventions
3.2.6. Dose Frequency and Total Exposure
3.3. tDCS Results in People with Diabetes
3.3.1. Neuropathic and Musculoskeletal Pain
- Motor Cortex (M1)-targeted Analgesia
- 2.
- Comparative Electrotherapy and Extended Low-Intensity Courses
- 3.
- Functional Pain and Polyneuropathy Pilot
- 4.
- Case Evidence (Plantar Fasciitis with Diabetes)
- 5.
- Negative/neutral Pain Findings with DLPFC + Working memory training (WMT)
- 6.
- Take-Home for Pain
3.3.2. Cognitive, Psychiatric, and Affective Outcomes
- Working Memory (WM)
- 2.
- Anxiety, Depression, and Distress
3.3.3. Sleep and Health-Related Quality of Life (HRQOL)
3.3.4. Visual Function in PDR
3.4. Safety
3.5. Risk of Bias
4. Discussion
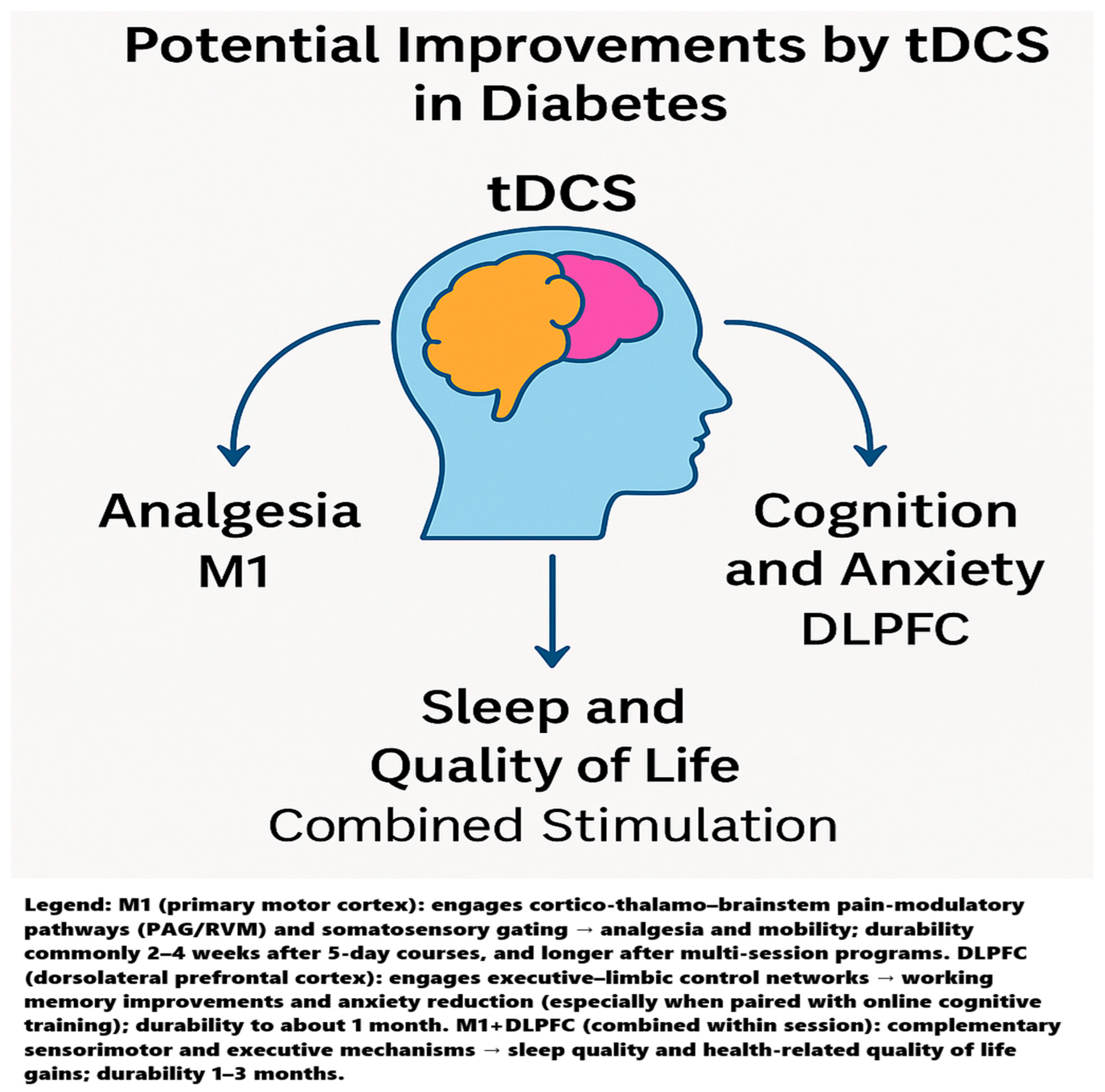
4.1. Mechanistic Interpretation
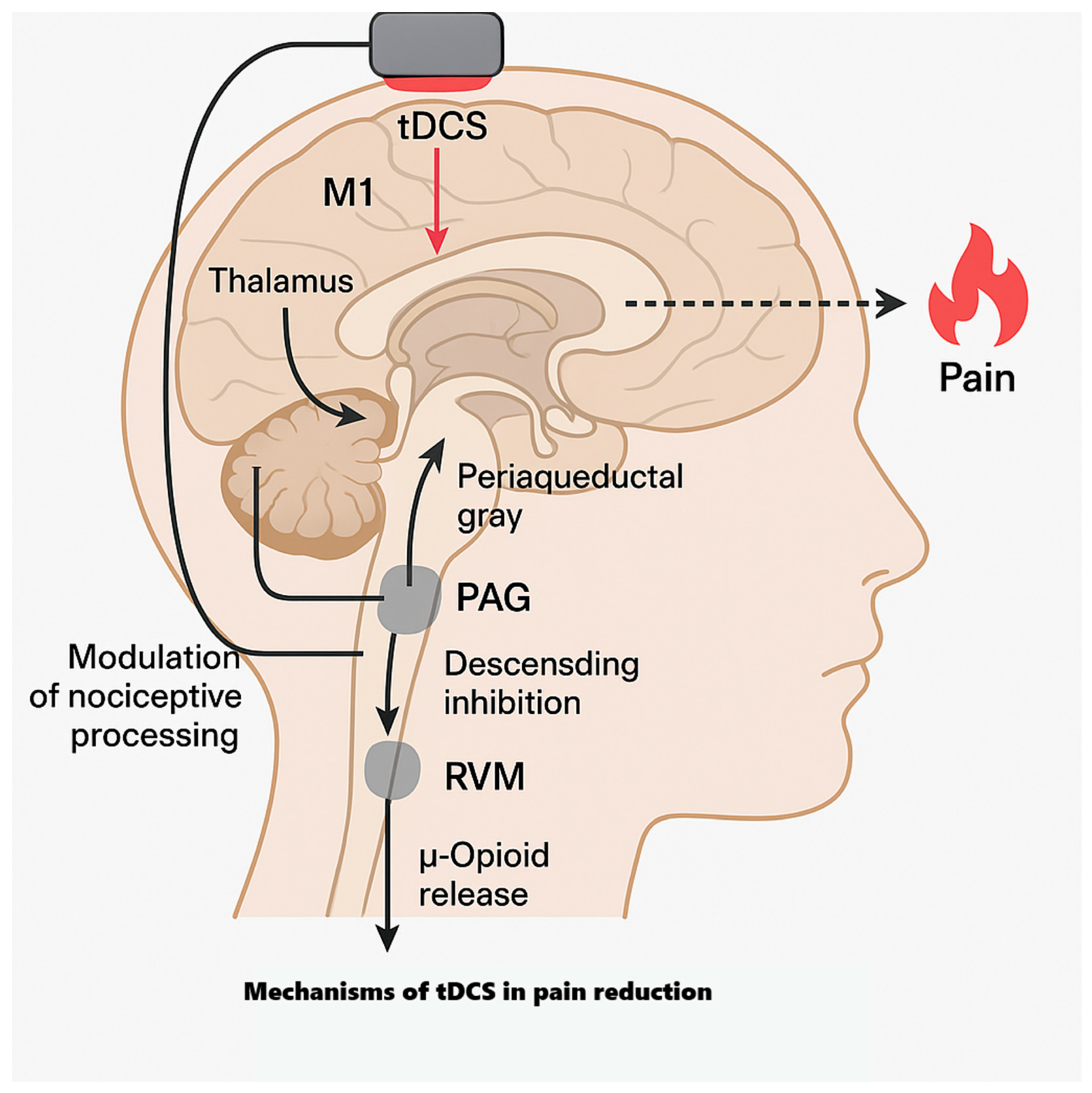
4.1.1. Why M1 for Analgesia?
4.1.2. Why DLPFC for Anxiety and Working Memory?
4.1.3. Visual Cortex “Denoising” and Diabetic Retinopathy
4.1.4. Why Multi-Site (M1 + DLPFC) Can Look Best on Sleep and QoL
4.2. Durability of tDCS Effects
4.3. Methodological Constraints and Interpretive Fragility
4.4. Clinical Implementation Barriers and Safety Contexts
4.5. Toward Neuroimaging-Guided Personalization Instead of One Size Fits All
4.6. Genetic Moderators of tDCS Responsiveness
4.7. Brain–Gut Axis as a Plausible Downstream Pathway
4.8. AI-Enabled Adaptive Dosing and Closed-Loop Personalization
4.9. Neurovascular Coupling as a Candidate Translational Bridge
4.10. Neuroinflammation as a Molecular Convergence Point for Metabolic and Cognitive Benefit
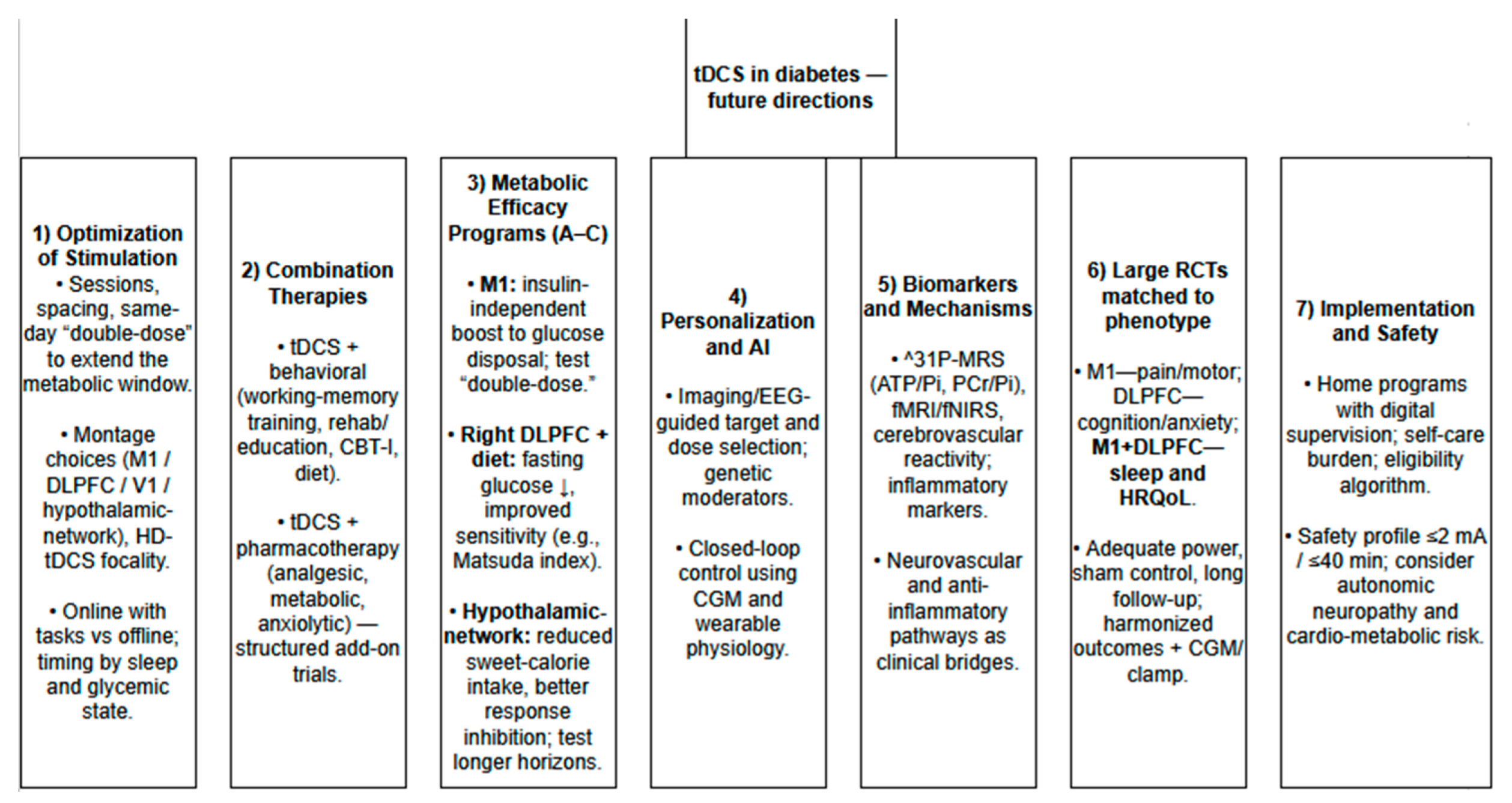
5. Future Perspectives on Using tDCS for Diabetes Management
5.1. Mechanistic Fit with Diabetes Pathophysiology
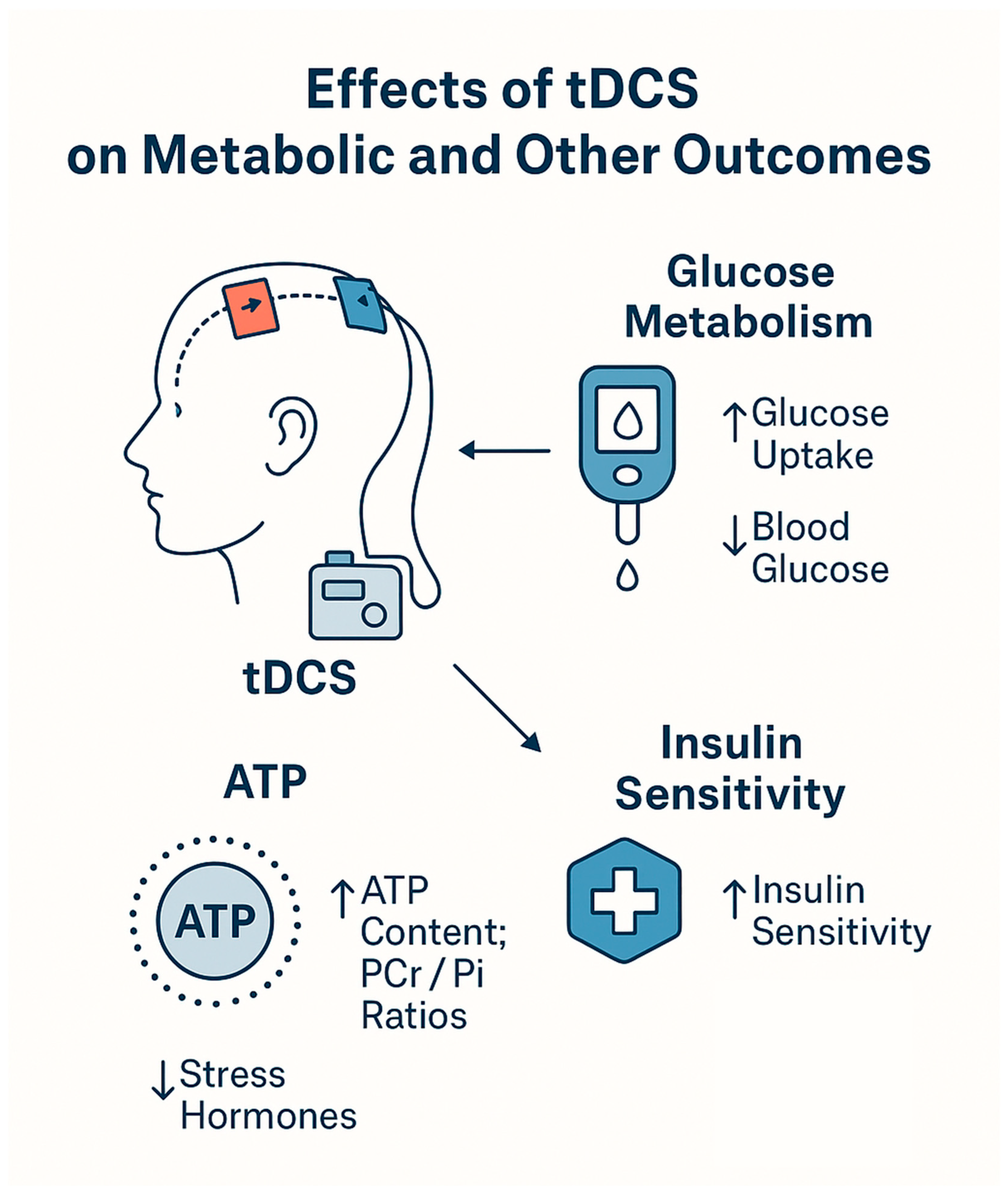
5.1.1. Neuroenergetic Trigger → Insulin-Independent Glucose Control
5.1.2. Stress-Axis Modulation as a Co-Determinant of Insulin Resistance
5.1.3. Autonomic/Hemodynamic Context
5.1.4. Executive Control and Ingestive Behavior
5.2. What the Dosing and Time Courses Imply
5.3. Near-Term Clinical Niches to Test
5.4. Trial Blueprints (Mechanism-Anchored and Diabetes-Relevant)
5.4.1. Program A-Insulin-Independent Disposal Augmentation (M1-Anchored)
5.4.2. Program B-Tonic Insulin-Sensitivity and Fasting-Glycemia Improvement (Right DLPFC + Diet)
5.4.3. Program C-Hedonic Intake Control via Hypothalamus-Network Targeting (Net-tDCS)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solis-Herrera, C.; Triplitt, C.; Reasner, C.; DeFronzo, R.A.; Cersosimo, E. Classification of Diabetes Mellitus. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279119/ (accessed on 9 September 2025).
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. On behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. 1), S19–S40. [Google Scholar] [CrossRef]
- Glycated haemoglobin (HbA1c) for the diagnosis of diabetes. In Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2011; Chapter 2. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304271/ (accessed on 9 September 2025).
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 9 September 2025).
- Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 9 September 2025).
- Sun, J.; Hu, W.; Ye, S.; Deng, D.; Chen, M. The Description and Prediction of Incidence, Prevalence, Mortality, Disability-Adjusted Life Years Cases, and Corresponding Age-Standardized Rates for Global Diabetes. J. Epidemiol. Glob. Health 2023, 13, 566–576. [Google Scholar] [CrossRef]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Kumar, A.R.; Kaplowitz, P.B. Patient age, race and the type of diabetes have an impact on the presenting symptoms, latency before diagnosis and laboratory abnormalities at time of diagnosis of diabetes mellitus in children. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 227–232. [Google Scholar] [CrossRef]
- Kupai, K.; Várkonyi, T.; Török, S.; Gáti, V.; Czimmerer, Z.; Puskás, L.G.; Szebeni, G.J. Recent Progress in the Diagnosis and Management of Type 2 Diabetes Mellitus in the Era of COVID-19 and Single Cell Multi-Omics Technologies. Life 2022, 12, 1205. [Google Scholar] [CrossRef]
- Peer, N.; Balakrishna, Y.; Durao, S. Screening for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 5, CD005266. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.; Mishra, P.; Alexeeff, S.E.; Blatchins, M.A.; Kim, E.; Man, A.H.; Grant, R.W. Prevalence and predictors of delayed clinical diagnosis of Type 2 diabetes: A longitudinal cohort study. Diabet. Med. 2018, 35, 1655–1662. [Google Scholar] [CrossRef]
- Roche, E.F.; Menon, A.; Gill, D.; Hoey, H. Clinical presentation of type 1 diabetes. Pediatr. Diabetes 2005, 6, 75–78. [Google Scholar] [CrossRef]
- Praveen, P.A.; Hockett, C.W.; Ong, T.C.; Amutha, A.; Isom, S.P.; Jensen, E.T.; Mohan, V.; Dabelea, D.A.; D’Agostino, R.B., Jr.; Hamman, R.F.; et al. Diabetic ketoacidosis at diagnosis among youth with type 1 and type 2 diabetes: Results from SEARCH (United States) and YDR (India) registries. Pediatr. Diabetes 2021, 22, 40–46. [Google Scholar] [CrossRef]
- Dabelea, D.; Rewers, A.; Stafford, J.M.; Standiford, D.A.; Lawrence, J.M.; Saydah, S.; Imperatore, G.; D’Agostino, R.B., Jr.; Mayer-Davis, E.J.; Pihoker, C. SEARCH for Diabetes in Youth Study Group. Trends in the prevalence of ketoacidosis at diabetes diagnosis: The SEARCH for diabetes in youth study. Pediatrics 2014, 133, e938–e945. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Hattersley, A.T.; Patel, K.A. Precision diabetes: Learning from monogenic diabetes. Diabetologia 2017, 60, 769–777. [Google Scholar] [CrossRef]
- Hart, P.A.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; Forsmark, C.E.; Goodarzi, M.O.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer(CPDPC). Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- Popoviciu, M.S.; Paduraru, L.; Nutas, R.M.; Ujoc, A.M.; Yahya, G.; Metwally, K.; Cavalu, S. Diabetes Mellitus Secondary to Endocrine Diseases: An Update of Diagnostic and Treatment Particularities. Int. J. Mol. Sci. 2023, 24, 12676. [Google Scholar] [CrossRef]
- Suh, S.; Park, M.K. Glucocorticoid-Induced Diabetes Mellitus: An Important but Overlooked Problem. Endocrinol. Metab. 2017, 32, 180–189. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S27–S49. [Google Scholar] [CrossRef]
- Musen, G.; Jacobson, A.M.; Bolo, N.R.; Simonson, D.C.; Shenton, M.E.; McCartney, R.L.; Flores, V.L.; Hoogenboom, W.S. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 2012, 61, 2375–2379. [Google Scholar] [CrossRef]
- Cui, Y.; Jiao, Y.; Chen, H.J.; Ding, J.; Luo, B.; Peng, C.Y.; Ju, S.H.; Teng, G.J. Aberrant functional connectivity of default-mode network in type 2 diabetes patients. Eur. Radiol. 2015, 25, 3238–3246. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Zhang, J.; Xu, K.; Zhang, S.; Wei, D.; Zhang, Z. Altered brain activation patterns under different working memory loads in patients with type 2 diabetes. Diabetes Care 2014, 37, 3157–3163. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Liu, C.; Zhang, H.; Zhou, X.; Ni, C.; Qin, W.; Zhang, Q. Altered brain activation and functional connectivity in working memory related networks in patients with type 2 diabetes: An ICA-based analysis. Sci. Rep. 2016, 6, 23767. [Google Scholar] [CrossRef]
- Marder, T.J.; Flores, V.L.; Bolo, N.R.; Hoogenboom, W.S.; Simonson, D.C.; Jacobson, A.M.; Foote, S.E.; Shenton, M.E.; Sperling, R.A.; Musen, G. Task-induced brain activity patterns in type 2 diabetes: A potential biomarker for cognitive decline. Diabetes 2014, 63, 3112–3119. [Google Scholar] [CrossRef]
- Xia, W.; Chen, Y.C.; Ma, J. Resting-State Brain Anomalies in Type 2 Diabetes: A Meta-Analysis. Front. Aging Neurosci. 2017, 9, 14. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Yang, X.; Xu, H.; Ren, J.; Zhou, P. Regional Spontaneous Neural Activity Alterations in Type 2 Diabetes Mellitus: A Meta-Analysis of Resting-State Functional MRI Studies. Front. Aging Neurosci. 2021, 13, 678359. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, C.; Jiang, X.; Li, H. Alterations of spontaneous brain activity in type 2 diabetes mellitus without mild cognitive impairment: A resting-state functional magnetic resonance study. Front. Hum. Neurosci. 2024, 17, 1305571. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, T.; Wei, W.; Zhang, R.; Wang, H.; Wang, M. Evaluation of altered brain activity in type 2 diabetes using various indices of brain function: A resting-state functional magnetic resonance imaging study. Front. Hum. Neurosci. 2023, 16, 1032264. [Google Scholar] [CrossRef]
- Been, G.; Ngo, T.T.; Miller, S.M.; Fitzgerald, P.B. The use of tDCS and CVS as methods of non-invasive brain stimulation. Brain Res. Rev. 2007, 56, 346–361. [Google Scholar] [CrossRef]
- Frank, E.; Wilfurth, S.; Landgrebe, M.; Eichhammer, P.; Hajak, G.; Langguth, B. Anodal skin lesions after treatment with transcranial direct current stimulation. Brain Stimul. 2010, 3, 58–59. [Google Scholar] [CrossRef]
- Lang, N.; Siebner, H.R.; Ward, N.S.; Lee, L.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N.; Frackowiak, R.S. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci. 2005, 22, 495–504. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Doemkes, S.; Karaköse, T.; Antal, A.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2007, 97, 3109–3117. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Koschack, J.; Pohlers, H.; Hullemann, S.; Paulus, W.; Happe, S. Effects of frontal transcranial direct current stimulation on emotional state and processing in healthy humans. Front. Psychiatry 2012, 3, 58. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Kronberg, G.; Bridi, M.; Abel, T.; Bikson, M.; Parra, L.C. Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul. 2017, 10, 51–58. [Google Scholar] [CrossRef]
- Radman, T.; Ramos, R.L.; Brumberg, J.C.; Bikson, M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009, 2, 215–228.e3. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553 Pt 1, 293–301. [Google Scholar] [CrossRef]
- Hsu, T.Y.; Juan, C.H.; Tseng, P. Individual Differences and State-Dependent Responses in Transcranial Direct Current Stimulation. Front. Hum. Neurosci. 2016, 10, 643. [Google Scholar] [CrossRef]
- Chmiel, J.; Malinowska, A. The Influence of Circadian Rhythms on Transcranial Direct Current Stimulation (tDCS) Effects: Theoretical and Practical Considerations. Cells 2025, 14, 1152. [Google Scholar] [CrossRef]
- Sehm, B.; Kipping, J.; Schäfer, A.; Villringer, A.; Ragert, P. A Comparison between Uni- and Bilateral tDCS Effects on Functional Connectivity of the Human Motor Cortex. Front. Hum. Neurosci. 2013, 7, 183. [Google Scholar] [CrossRef]
- Callan, D.E.; Falcone, B.; Wada, A.; Parasuraman, R. Simultaneous tDCS-fMRI Identifies Resting State Networks Correlated with Visual Search Enhancement. Front. Hum. Neurosci. 2016, 10, 72. [Google Scholar] [CrossRef]
- Kunze, T.; Hunold, A.; Haueisen, J.; Jirsa, V.; Spiegler, A. Transcranial direct current stimulation changes resting state functional connectivity: A large-scale brain network modeling study. Neuroimage 2016, 140, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Pagali, S.R.; Wang, Z.; Kung, S.; Boyapati, R.B.; Islam, K.; Li, J.W.; Shelton, K.M.; Waniger, A.; Rydberg, A.M.; et al. Transcranial Electrical Stimulation in Treatment of Depression: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e2516459. [Google Scholar] [CrossRef]
- Ma, S.; Zhuang, W.; Wang, X.; Zhang, D.; Wang, H.; Han, Q.; Ding, Q.; Li, Y.; Li, W.; Li, T. Efficacy of transcranial direct current stimulation on cognitive function in patients with Parkinson’s disease: A systematic review and meta-analysis. Front. Aging Neurosci. 2025, 17, 1495492. [Google Scholar] [CrossRef]
- Zou, S.J.; Shi, J.N. Therapeutic efficacy of transcranial direct current stimulation in treating auditory hallucinations in schizophrenia: A meta-analysis. World J. Psychiatry 2025, 15, 99364. [Google Scholar] [CrossRef]
- Duarte-Moreira, R.J.; Shirahige, L.; Rodriguez-Prieto, I.E.; Alves, M.M.; Lopes, T.D.S.; Baptista, R.F.; Hazime, F.A.; Zana, Y.; Kubota, G.T.; de Andrade, D.C.; et al. Evidence-Based Umbrella Review of Non-Invasive Neuromodulation in Chronic Neuropathic Pain. Eur. J. Pain 2025, 29, e4786. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, C.; Liu, Y.; Ma, X.; Liu, T.; Jia, F.; Du, L. Efficacy and safety of transcranial direct current stimulation for children and adolescents with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. J. Psychiatry Neurosci. 2025, 50, E248–E266. [Google Scholar] [CrossRef]
- Narmashiri, A.; Akbari, F. The Effects of Transcranial Direct Current Stimulation (tDCS) on the Cognitive Functions: A Systematic Review and Meta-analysis. Neuropsychol. Rev. 2025, 35, 126–152. [Google Scholar] [CrossRef]
- Balboa-Bandeira, Y.; Zubiaurre-Elorza, L.; Ibarretxe-Bilbao, N.; Ojeda, N.; Peña, J. Effects of transcranial electrical stimulation techniques on second and foreign language learning enhancement in healthy adults: A systematic review and meta-analysis. Neuropsychologia 2021, 160, 107985. [Google Scholar] [CrossRef]
- Fresnoza, S.; Ischebeck, A. Probing Our Built-in Calculator: A Systematic Narrative Review of Noninvasive Brain Stimulation Studies on Arithmetic Operation-Related Brain Areas. ENeuro 2024, 11, ENEURO.0318-23.2024. [Google Scholar] [CrossRef]
- You, G.; Pan, X.; Li, J.; Zhao, S. Effects of transcranial direct current stimulation on modulating executive functions in healthy populations: A systematic review and meta-analysis. Front. Hum. Neurosci. 2024, 18, 1485037. [Google Scholar] [CrossRef]
- Chmiel, J. Transcranial direct current stimulation (tDCS): Transcranial direct current stimulation (tDCS): A new, (still) legal form of “neurodoping” in sports? Adv. Clin. Exp. Med. 2025; ahead of print. [Google Scholar] [CrossRef]
- Alipour, A.; Mohammadi, R. Evaluation of the separate and combined effects of anodal tDCS over the M1 and F3 regions on pain relief in patients with type-2 diabetes suffering from neuropathic pain. Neurosci. Lett. 2024, 818, 137554. [Google Scholar] [CrossRef]
- Aksu, S.; Hasırcı Bayır, B.R.; Sayman, C.; Soyata, A.Z.; Boz, G.; Karamürsel, S. Working memory ımprovement after transcranial direct current stimulation paired with working memory training ın diabetic peripheral neuropathy. Appl. Neuropsychol. Adult 2023, 32, 231–244. [Google Scholar] [CrossRef]
- de Venecia, A.B.F.3rd; Fresnoza, S.M. Visual Cortex Transcranial Direct Current Stimulation for Proliferative Diabetic Retinopathy Patients: A Double-Blinded Randomized Exploratory Trial. Brain Sci. 2021, 11, 270. [Google Scholar] [CrossRef]
- Ferreira, G.; Silva-Filho, E.; de Oliveira, A.; de Lucena, C.; Lopes, J.; Pegado, R. Transcranial direct current stimulation improves quality of life and physical fitness in diabetic polyneuropathy: A pilot double blind randomized controlled trial. J. Diabetes Metab. Disord. 2020, 19, 327–335. [Google Scholar] [CrossRef]
- Wu, Y.J.; Tseng, P.; Huang, H.W.; Hu, J.F.; Juan, C.H.; Hsu, K.S.; Lin, C.C. The Facilitative Effect of Transcranial Direct Current Stimulation on Visuospatial Working Memory in Patients with Diabetic Polyneuropathy: A Pre-post Sham-Controlled Study. Front. Hum. Neurosci. 2016, 10, 479. [Google Scholar] [CrossRef]
- Mohomad, A.S.; Mohammad, R.; Chusid, E.; Trepal, M.; Battaglia, F. Severe chronic heel pain in a diabetic patient with plantar fasciitis successfully treated through transcranial direct current stimulation. J. Am. Podiatr. Med. Assoc. 2015, 105, 173–176. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ku, J.; Kim, H.J.; Im, D.J.; Lee, H.S.; Han, K.A.; Kang, Y.J. Randomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathy. Ann. Rehabil. Med. 2013, 37, 766–776. [Google Scholar] [CrossRef]
- Rahmy, A.F.; El-Said, S.H.; Yacoub, A. Effect of Transcutaneous Electrical Nerve Stimulation Versus Transcranial Direct Current Stimulation on Diabetic Peripheral Neuropathy. Med. J. Cairo Univ. 2018, 86, 27–34. [Google Scholar]
- ElSayed, S.H.; Moawd, S.A.; Abdelbasset, W.K. Assessment of the quality of life following transcranial direct current stimulation in patients with Diabetic Peripheral Neuropathy. J. Adv. Pharm. Educ. Res. 2020, 10, 9–13. [Google Scholar] [CrossRef]
- Alipour, A.; Mohammadi, R. Increasing the quality of sleep and life by brain electrical stimulation in patients with painful diabetic neuropathy. Iran. J. Health Psychol. 2023, 6, 27–40. [Google Scholar]
- Alipour, A.; Mohammadi, R. Investigating the Pure and Combined Effect of M1 and F3 Anodic tDCS on Improving the Psychological Status of People with type 2 Diabetes and Neuropathic Pain. Neuropsychology 2023, 9, 15–26. [Google Scholar]
- Janssen, J.A.M.J.L. New Insights into the Role of Insulin and Hypothalamic-Pituitary-Adrenal (HPA) Axis in the Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8178. [Google Scholar] [CrossRef]
- Heni, M. The insulin resistant brain: Impact on whole-body metabolism and body fat distribution. Diabetologia 2024, 67, 1181–1191. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Veit, R.; Scheffler, K.; Machann, J.; Häring, H.-U.; Fritsche, A. Intranasal insulin enhances brain functional connectivity mediating the relationship between adiposity and subjective feeling of hunger. Sci. Rep. 2017, 7, 1627. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Hu, R.; Luo, G.; Yang, T.; Shen, H.; Deng, H.; Chen, C.; Zhao, H.; Liu, J. Altered Structural and Functional MRI Connectivity in Type 2 Diabetes Mellitus Related Cognitive Impairment: A Review. Front. Hum. Neurosci. 2022, 15, 755017. [Google Scholar] [CrossRef]
- Meng, J.; Liu, J.; Li, H.; Gao, Y.; Cao, L.; He, Y.; Guo, Y.; Feng, L.; Hu, X.; Li, H.; et al. Impairments in intrinsic functional networks in type 2 diabetes: A meta-analysis of resting-state functional connectivity. Front. Neuroendocrinol. 2022, 66, 100992. [Google Scholar] [CrossRef]
- Cui, Y.; Li, S.F.; Gu, H.; Hu, Y.Z.; Liang, X.; Lu, C.Q.; Cai, Y.; Wang, C.X.; Yang, Y.; Teng, G.J. Disrupted Brain Connectivity Patterns in Patients with Type 2 Diabetes. AJNR Am. J. Neuroradiol. 2016, 37, 2115–2122. [Google Scholar] [CrossRef]
- Moreira, M.C.; Pinto, I.S.; Mourão, A.A.; Fajemiroye, J.O.; Colombari, E.; Reis, Â.A.; Freiria-Oliveira, A.H.; Ferreira-Neto, M.L.; Pedrino, G.R. Does the sympathetic nervous system contribute to the pathophysiology of metabolic syndrome? Front. Physiol. 2015, 6, 234. [Google Scholar] [CrossRef]
- Fonteneau, C.; Redoute, J.; Haesebaert, F.; Le Bars, D.; Costes, N.; Suaud-Chagny, M.F.; Brunelin, J. Frontal Transcranial Direct Current Stimulation Induces Dopamine Release in the Ventral Striatum in Human. Cereb. Cortex 2018, 28, 2636–2646. [Google Scholar] [CrossRef]
- Lopes, P.S.S.; Campos, A.C.P.; Fonoff, E.T.; Britto, L.R.G.; Pagano, R.L. Motor cortex and pain control: Exploring the descending relay analgesic pathways and spinal nociceptive neurons in healthy conscious rats. Behav. Brain Funct. 2019, 15, 5. [Google Scholar] [CrossRef]
- Bai, Y.; Pacheco-Barrios, K.; Pacheco-Barrios, N.; Liang, G.; Fregni, F. Neurocircuitry basis of motor cortex-related analgesia as an emerging approach for chronic pain management. Nat. Ment. Health 2024, 2, 496–513. [Google Scholar] [CrossRef]
- Pacheco-Barrios, K.; Cardenas-Rojas, A.; Thibaut, A.; Costa, B.; Ferreira, I.; Caumo, W.; Fregni, F. Methods and strategies of tDCS for the treatment of pain: Current status and future directions. Expert Rev. Med. Devices 2020, 17, 879–898. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Cummiford, C.M.; Nascimento, T.D.; Foerster, B.R.; Clauw, D.J.; Zubieta, J.K.; Harris, R.E.; DaSilva, A.F. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res. Ther. 2016, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Knotkova, H.; Nitsche, M.A.; Cruciani, R.A. Putative physiological mechanisms underlying tDCS analgesic effects. Front. Hum. Neurosci. 2013, 7, 628. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Nitsche, M.A. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum. Brain Mapp. 2012, 33, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- DosSantos, M.F.; Love, T.M.; Martikainen, I.K.; Nascimento, T.D.; Fregni, F.; Cummiford, C.; Deboer, M.D.; Zubieta, J.K.; Dasilva, A.F. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front. Psychiatry 2012, 3, 93. [Google Scholar]
- Kim, D.J.; Nascimento, T.D.; Lim, M.; Danciu, T.; Zubieta, J.K.; Scott, P.J.H.; Koeppe, R.; Kaciroti, N.; DaSilva, A.F. Exploring HD-tDCS Effect on μ-opioid Receptor and Pain Sensitivity in Temporomandibular Disorder: A Pilot Randomized Clinical Trial Study. J. Pain 2024, 25, 1070–1081. [Google Scholar] [CrossRef]
- Matsunaga, K.; Nitsche, M.A.; Tsuji, S.; Rothwell, J.C. Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clin. Neurophysiol. 2004, 115, 456–460. [Google Scholar] [CrossRef]
- Saldanha, J.S.; Zortea, M.; Torres, I.L.D.S.; Fregni, F.; Caumo, W. Age as a Mediator of tDCS Effects on Pain: An Integrative Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2020, 14, 568306. [Google Scholar] [CrossRef]
- Li, X.; Yao, J.; Zhang, W.; Chen, S.; Peng, W. Effects of transcranial direct current stimulation on experimental pain perception: A systematic review and meta-analysis. Clin. Neurophysiol. 2021, 132, 2163–2175. [Google Scholar] [CrossRef]
- Antonioni, A.; Baroni, A.; Fregna, G.; Ahmed, I.; Straudi, S. The effectiveness of home-based transcranial direct current stimulation on chronic pain: A systematic review and meta-analysis. Digit. Health 2024, 10, 20552076241292677. [Google Scholar] [CrossRef]
- Wen, Y.R.; Shi, J.; Hu, Z.Y.; Lin, Y.Y.; Lin, Y.T.; Jiang, X.; Wang, R.; Wang, X.Q.; Wang, Y.L. Is transcranial direct current stimulation beneficial for treating pain, depression, and anxiety symptoms in patients with chronic pain? A systematic review and meta-analysis. Front. Mol. Neurosci. 2022, 15, 1056966. [Google Scholar] [CrossRef]
- Moshfeghinia, R.; Shekouh, D.; Mostafavi, S.; Hosseinzadeh, M.; Bahadori, A.R.; Abdollahifard, S.; Razmkon, A. The effects of transcranial direct-current stimulation (tDCS) on pain intensity of patients with fibromyalgia: A systematic review and meta-analysis. BMC Neurol. 2023, 23, 395. [Google Scholar] [CrossRef]
- Alexandra Kredlow, M.; Fenster, R.J.; Laurent, E.S.; Ressler, K.J.; Phelps, E.A. Prefrontal cortex, amygdala, and threat processing: Implications for PTSD. Neuropsychopharmacology 2022, 47, 247–259. [Google Scholar] [CrossRef]
- Ironside, M.; Browning, M.; Ansari, T.L.; Harvey, C.J.; Sekyi-Djan, M.N.; Bishop, S.J.; Harmer, C.J.; O’Shea, J. Effect of Prefrontal Cortex Stimulation on Regulation of Amygdala Response to Threat in Individuals With Trait Anxiety: A Randomized Clinical Trial. JAMA Psychiatry 2019, 76, 71–78. [Google Scholar] [CrossRef]
- Dong, M.; Xia, L.; Lu, M.; Li, C.; Xu, K.; Zhang, L. A failed top-down control from the prefrontal cortex to the amygdala in generalized anxiety disorder: Evidence from resting-state fMRI with Granger causality analysis. Neurosci. Lett. 2019, 707, 134314. [Google Scholar] [CrossRef]
- Kenwood, M.M.; Kalin, N.H.; Barbas, H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology 2022, 47, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Vergallito, A.; Gallucci, A.; Pisoni, A.; Punzi, M.; Caselli, G.; Ruggiero, G.M.; Sassaroli, S.; Romero Lauro, L.J. Effectiveness of noninvasive brain stimulation in the treatment of anxiety disorders: A meta-analysis of sham or behaviour-controlled studies. J. Psychiatry Neurosci. 2021, 46, E592–E614. [Google Scholar] [CrossRef]
- Xie, L.; Hu, P.; Guo, Z.; Chen, M.; Wang, X.; Du, X.; Li, Y.; Chen, B.; Zhang, J.; Zhao, W.; et al. Immediate and long-term efficacy of transcranial direct current stimulation (tCDS) in obsessive-compulsive disorder, posttraumatic stress disorder and anxiety disorders: A systematic review and meta-analysis. Transl. Psychiatry 2024, 14, 343. [Google Scholar] [CrossRef]
- Zheng, E.Z.; Wong, N.M.L.; Yang, A.S.Y.; Lee, T.M.C. Evaluating the effects of tDCS on depressive and anxiety symptoms from a transdiagnostic perspective: A systematic review and meta-analysis of randomized controlled trials. Transl. Psychiatry 2024, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Barbey, A.K.; Koenigs, M.; Grafman, J. Dorsolateral prefrontal contributions to human working memory. Cortex 2013, 49, 1195–1205. [Google Scholar] [CrossRef]
- Jonides, J.; Nee, D.E. Brain mechanisms of proactive interference in working memory. Neuroscience 2006, 139, 181–193. [Google Scholar] [CrossRef]
- Michels, L.; Martin, E.; Klaver, P.; Edden, R.; Zelaya, F.; Lythgoe, D.J.; Lüchinger, R.; Brandeis, D.; O’Gorman, R.L. Frontal GABA levels change during working memory. PLoS ONE 2012, 7, e31933. [Google Scholar] [CrossRef]
- Yoon, J.H.; Grandelis, A.; Maddock, R.J. Dorsolateral Prefrontal Cortex GABA Concentration in Humans Predicts Working Memory Load Processing Capacity. J. Neurosci. 2016, 36, 11788–11794. [Google Scholar] [CrossRef]
- Vural, G.; Soldini, A.; Padberg, F.; Karslı, B.; Zinchenko, A.; Goerigk, S.; Soutschek, A.; Mezger, E.; Stoecklein, S.; Bulubas, L.; et al. Exploring the Effects of Prefrontal Transcranial Direct Current Stimulation on Brain Metabolites: A Concurrent tDCS-MRS Study. Hum. Brain Mapp. 2024, 45, e70097. [Google Scholar] [CrossRef]
- Hill, A.T.; Fitzgerald, P.B.; Hoy, K.E. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings From Healthy and Neuropsychiatric Populations. Brain Stimul. 2016, 9, 197–208. [Google Scholar] [CrossRef]
- Mancuso, L.E.; Ilieva, I.P.; Hamilton, R.H.; Farah, M.J. Does Transcranial Direct Current Stimulation Improve Healthy Working Memory?: A Meta-analytic Review. J. Cogn. Neurosci. 2016, 28, 1063–1089. [Google Scholar] [CrossRef] [PubMed]
- Wischnewski, M.; Berger, T.A.; Opitz, A. Meta-modeling the effects of anodal left prefrontal transcranial direct current stimulation on working memory performance. Imaging Neurosci. 2024, 2, imag-2-00078. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, S.; Nitsche, M.A.; Yue, T.; Zschorlich, V.R.; Qi, F. A meta-analysis of the effects of transcranial direct current stimulation combined with cognitive training on working memory in healthy older adults. Front. Aging Neurosci. 2024, 16, 1454755. [Google Scholar] [CrossRef]
- Ruf, S.; Fallgatter, A.J.; Plewnia, C. Augmentation of working memory training by transcranial direct current stimulation (tDCS). Sci. Rep. 2017, 7, 876. [Google Scholar] [CrossRef]
- Pergher, V.; Au, J.; Alizadeh Shalchy, M.; Santarnecchi, E.; Seitz, A.; Jaeggi, S.M.; Battelli, L. The benefits of simultaneous tDCS and working memory training on transfer outcomes: A systematic review and meta-analysis. Brain Stimul. 2022, 15, 1541–1551. [Google Scholar] [CrossRef]
- Weller, S.; Nitsche, M.A.; Plewnia, C. Enhancing cognitive control training with transcranial direct current stimulation: A systematic parameter study. Brain Stimul. 2020, 13, 1358–1369. [Google Scholar] [CrossRef]
- Richmond, L.L.; Wolk, D.; Chein, J.; Olson, I.R. Transcranial direct current stimulation enhances verbal working memory training performance over time and near transfer outcomes. J. Cogn. Neurosci. 2014, 26, 2443–2454. [Google Scholar] [CrossRef]
- Au, J.; Katz, B.; Buschkuehl, M.; Bunarjo, K.; Senger, T.; Zabel, C.; Jaeggi, S.M.; Jonides, J. Enhancing Working Memory Training with Transcranial Direct Current Stimulation. J. Cogn. Neurosci. 2016, 28, 1419–1432. [Google Scholar] [CrossRef]
- Teixeira-Santos, A.C.; Moreira, C.S.; Pereira, D.R.; Pinal, D.; Fregni, F.; Leite, J.; Carvalho, S.; Sampaio, A. Working Memory Training Coupled With Transcranial Direct Current Stimulation in Older Adults: A Randomized Controlled Experiment. Front. Aging Neurosci. 2022, 14, 827188. [Google Scholar] [CrossRef]
- Bradley, C.; Nydam, A.S.; Dux, P.E.; Mattingley, J.B. State-dependent effects of neural stimulation on brain function and cognition. Nat. Rev. Neurosci. 2022, 23, 459–475. [Google Scholar] [CrossRef]
- Vergallito, A.; Varoli, E.; Pisoni, A.; Mattavelli, G.; Del Mauro, L.; Feroldi, S.; Vallar, G.; Romero Lauro, L.J. State-dependent effectiveness of cathodal transcranial direct current stimulation on cortical excitability. Neuroimage 2023, 277, 120242. [Google Scholar] [CrossRef]
- Bortoletto, M.; Pellicciari, M.C.; Rodella, C.; Miniussi, C. The interaction with task-induced activity is more important than polarization: A tDCS study. Brain Stimul. 2015, 8, 269–276. [Google Scholar] [CrossRef]
- Painter, D.R.; Dwyer, M.F.; Kamke, M.R.; Mattingley, J.B. Stimulus-Driven Cortical Hyperexcitability in Individuals with Charles Bonnet Hallucinations. Curr. Biol. 2018, 28, 3475–3480.e3. [Google Scholar] [CrossRef]
- Hahamy, A.; Wilf, M.; Rosin, B.; Behrmann, M.; Malach, R. How do the blind ‘see’? The role of spontaneous brain activity in self-generated perception. Brain 2021, 144, 340–353. [Google Scholar] [CrossRef]
- Yu, Y.; Lan, D.Y.; Tang, L.Y.; Su, T.; Li, B.; Jiang, N.; Liang, R.B.; Ge, Q.M.; Li, Q.Y.; Shao, Y. Intrinsic functional connectivity alterations of the primary visual cortex in patients with proliferative diabetic retinopathy: A seed-based resting-state fMRI study. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820960296. [Google Scholar] [CrossRef]
- Qi, C.X.; Huang, X.; Tong, Y.; Shen, Y. Altered Functional Connectivity Strength of Primary Visual Cortex in Subjects with Diabetic Retinopathy. Diabetes Metab. Syndr. Obes. 2021, 14, 3209–3219. [Google Scholar] [CrossRef]
- Brückner, S.; Kammer, T. No Modulation of Visual Cortex Excitability by Transcranial Direct Current Stimulation. PLoS ONE 2016, 11, e0167697. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, J.; Spiegel, D.P.; Chen, Z.; Chan, L.; Luo, G.; Yuan, J.; Deng, D.; Yu, M.; Thompson, B. The effect of transcranial direct current stimulation on contrast sensitivity and visual evoked potential amplitude in adults with amblyopia. Sci. Rep. 2016, 6, 19280. [Google Scholar] [CrossRef]
- Kraft, A.; Roehmel, J.; Olma, M.C.; Schmidt, S.; Irlbacher, K.; Brandt, S.A. Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Exp. Brain Res. 2010, 207, 283–290. [Google Scholar] [CrossRef]
- Antal, A.; Kincses, T.Z.; Nitsche, M.A.; Bartfai, O.; Paulus, W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: Direct electrophysiological evidence. Investig. Ophthalmol. Vis. Sci. 2004, 45, 702–707. [Google Scholar] [CrossRef]
- Antal, A.; Kincses, T.Z.; Nitsche, M.A.; Paulus, W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp. Brain Res. 2003, 150, 375–378. [Google Scholar] [CrossRef]
- van der Groen, O.; Wenderoth, N. Transcranial Random Noise Stimulation of Visual Cortex: Stochastic Resonance Enhances Central Mechanisms of Perception. J. Neurosci. 2016, 36, 5289–5298. [Google Scholar] [CrossRef]
- Fertonani, A.; Pirulli, C.; Miniussi, C. Random noise stimulation improves neuroplasticity in perceptual learning. J. Neurosci. 2011, 31, 15416–15423. [Google Scholar] [CrossRef]
- Castaldi, E.; Lunghi, C.; Morrone, M.C. Neuroplasticity in adult human visual cortex. Neurosci. Biobehav. Rev. 2020, 112, 542–552. [Google Scholar] [CrossRef]
- van der Groen, O.; Potok, W.; Wenderoth, N.; Edwards, G.; Mattingley, J.B.; Edwards, D. Using noise for the better: The effects of transcranial random noise stimulation on the brain and behavior. Neurosci. Biobehav. Rev. 2022, 138, 104702. [Google Scholar] [CrossRef]
- Cai, Y.; Hofstetter, S.; van Dijk, J.; Zuiderbaan, W.; van der Zwaag, W.; Harvey, B.M.; Dumoulin, S.O. Topographic numerosity maps cover subitizing and estimation ranges. Nat. Commun. 2021, 12, 3374. [Google Scholar] [CrossRef]
- Harvey, B.M.; Klein, B.P.; Petridou, N.; Dumoulin, S.O. Topographic representation of numerosity in the human parietal cortex. Science 2013, 341, 1123–1126. [Google Scholar] [CrossRef]
- Fornaciai, M.; Brannon, E.M.; Woldorff, M.G.; Park, J. Numerosity processing in early visual cortex. Neuroimage 2017, 157, 429–438. [Google Scholar] [CrossRef]
- He, Q.; Zhu, X.; Fang, F. Enhancing visual perceptual learning using transcranial electrical stimulation: Transcranial alternating current stimulation outperforms both transcranial direct current and random noise stimulation. J. Vis. 2023, 23, 2. [Google Scholar] [CrossRef]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2016, 2, 19–25. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Azarkolah, A.; Noorbala, A.A.; Ansari, S.; Hallajian, A.H.; Salehinejad, M.A. Efficacy of Transcranial Direct Current Stimulation on Pain Level and Disability of Patients with Fibromyalgia: A Systematic Review of Randomized Controlled Trials with Parallel-Group Design. Brain Sci. 2023, 14, 26. [Google Scholar] [CrossRef]
- Valle, A.; Roizenblatt, S.; Botte, S.; Zaghi, S.; Riberto, M.; Tufik, S.; Boggio, P.S.; Fregni, F. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial. J. Pain Manag. 2009, 2, 353–361. [Google Scholar]
- Fregni, F.; Gimenes, R.; Valle, A.C.; Ferreira, M.J.; Rocha, R.R.; Natalle, L.; Bravo, R.; Rigonatti, S.P.; Freedman, S.D.; Nitsche, M.A.; et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006, 54, 3988–3998. [Google Scholar] [CrossRef]
- Guzzi, G.; Della Torre, A.; Bruni, A.; Lavano, A.; Bosco, V.; Garofalo, E.; La Torre, D.; Longhini, F. Anatomo-physiological basis and applied techniques of electrical neuromodulation in chronic pain. J. Anesth. Analg. Crit. Care 2024, 4, 29. [Google Scholar] [CrossRef]
- Ironside, M.; O’Shea, J.; Cowen, P.J.; Harmer, C.J. Frontal Cortex Stimulation Reduces Vigilance to Threat: Implications for the Treatment of Depression and Anxiety. Biol. Psychiatry 2016, 79, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep. Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.G. A cognitive model of insomnia. Behav. Res. Ther. 2002, 40, 869–893. [Google Scholar] [CrossRef]
- Goossens, Z.; Van Stallen, A.; Vermuyten, J.; De deyne, M.; Rice, D.; Runge, N.; Huysmans, E.; Vantilborgh, T.; Nijs, J.; Mairesse, O.; et al. Day-to-day associations between pain intensity and sleep outcomes in an adult chronic musculoskeletal pain population: A systematic review. Sleep Med. Rev. 2025, 79, 102013. [Google Scholar] [CrossRef]
- Jain, S.V.; Panjeton, G.D.; Martins, Y.C. Relationship Between Sleep Disturbances and Chronic Pain: A Narrative Review. Clin. Pract. 2024, 14, 2650–2660. [Google Scholar] [CrossRef]
- Koffel, E.; Kroenke, K.; Bair, M.J.; Leverty, D.; Polusny, M.A.; Krebs, E.E. The bidirectional relationship between sleep complaints and pain: Analysis of data from a randomized trial. Health Psychol. 2016, 35, 41–49. [Google Scholar] [CrossRef]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef]
- Roizenblatt, S.; Fregni, F.; Gimenez, R.; Wetzel, T.; Rigonatti, S.P.; Tufik, S.; Boggio, P.S.; Valle, A.C. Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: A randomized, sham-controlled study. Pain Pract. 2007, 7, 297–306. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, C.; Yu, H.; Zhang, Y.; Liu, Z.; Hu, Z.; Yuan, T.F.; Zhou, D. The effects of repeated transcranial direct current stimulation on sleep quality and depression symptoms in patients with major depression and insomnia. Sleep Med. 2020, 70, 17–26. [Google Scholar] [CrossRef]
- Li, J.; Li, A.; Jiao, J.; Hu, K.; Jiang, Y.; Hu, X.; Wang, K.; Chen, X.; Xie, C. Effects of high-definition transcranial direct current stimulation for the treatment of chronic insomnia: A randomized, double-blind, controlled trial. Sci. Rep. 2025, 15, 26574. [Google Scholar]
- Mendonca, M.E.; Simis, M.; Grecco, L.C.; Battistella, L.R.; Baptista, A.F.; Fregni, F. Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromyalgia: A Randomized Placebo-Controlled Clinical Trial. Front. Hum. Neurosci. 2016, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Caumo, W.; Franca, B.R.; Orzechowski, R.; Bueno, G.; França, A.; Dos Santos da Silva, J.V.; Sanches, P.R.S.; Da Silva, D.P., Jr.; Torres, I.L.S.; Hirakata, V.N.; et al. Home-Based Transcranial Direct Current Stimulation vs Placebo for Fibromyalgia: A Randomized Clinical Trial. JAMA Netw. Open 2025, 8, e2514262. [Google Scholar] [CrossRef]
- Figeys, M.; Zeeman, M.; Kim, E.S. Effects of Transcranial Direct Current Stimulation (tDCS) on Cognitive Performance and Cerebral Oxygen Hemodynamics: A Systematic Review. Front. Hum. Neurosci. 2021, 15, 623315. [Google Scholar] [CrossRef]
- Gunduz, M.E.; Kocahasan, M.; Keser, Z. Transcranial Direct Current Stimulation to Provide Neuroprotection and Enhance Cerebral Blood Flow in Stroke: A Comprehensive Review. Medicina 2024, 60, 2061. [Google Scholar] [CrossRef]
- Zheng, X.; Alsop, D.C.; Schlaug, G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage 2011, 58, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, S.; Boden-Albala, B.; Choi, J.H.; Carrera, E.; Doyle, M.; Perez, T.; Marshall, R.S. Metabolic syndrome and cerebral vasomotor reactivity. Eur. J. Neurol. 2010, 17, 1457–1462. [Google Scholar] [CrossRef]
- Pasha, E.P.; Birdsill, A.C.; Oleson, S.; Haley, A.P.; Tanaka, H. Impacts of Metabolic Syndrome Scores on Cerebrovascular Conductance Are Mediated by Arterial Stiffening. Am. J. Hypertens. 2017, 31, 72–79. [Google Scholar] [CrossRef]
- Pellegrini, V.; La Grotta, R.; Carreras, F.; Giuliani, A.; Sabbatinelli, J.; Olivieri, F.; Berra, C.C.; Ceriello, A.; Prattichizzo, F. Inflammatory Trajectory of Type 2 Diabetes: Novel Opportunities for Early and Late Treatment. Cells 2024, 13, 1662. [Google Scholar] [CrossRef]
- Kacem, H.; d’Angelo, M.; Qosja, E.; Topi, S.; Castelli, V.; Cimini, A. The Inflammatory Bridge Between Type 2 Diabetes and Neurodegeneration: A Molecular Perspective. Int. J. Mol. Sci. 2025, 26, 7566. [Google Scholar] [CrossRef]
- Aydin, B.N.; Stinson, E.J.; Travis, K.T.; Krakoff, J.; Rodzevik, T.; Chang, D.C.; Gluck, M.E. Reduced plasma interleukin-6 concentration after transcranial direct current stimulation to the prefrontal cortex. Behav. Brain Res. 2024, 474, 115201. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Ethridge, V.T.; Gargas, N.M.; Sonner, M.J.; Moore, R.J.; Romer, S.H.; Hatcher-Solis, C.; Rohan, J.G. Effects of transcranial direct current stimulation on brain cytokine levels in rats. Front. Neurosci. 2022, 16, 1069484. [Google Scholar] [CrossRef]
- Binkofski, F.; Loebig, M.; Jauch-Chara, K.; Bergmann, S.; Melchert, U.H.; Scholand-Engler, H.G.; Schweiger, U.; Pellerin, L.; Oltmanns, K.M. Brain energy consumption induced by electrical stimulation promotes systemic glucose uptake. Biol. Psychiatry 2011, 70, 690–695. [Google Scholar] [CrossRef]
- Kistenmacher, A.; Manneck, S.; Wardzinski, E.K.; Martens, J.C.; Gohla, G.; Melchert, U.H.; Jauch-Chara, K.; Oltmanns, K.M. Persistent blood glucose reduction upon repeated transcranial electric stimulation in men. Brain Stimul. 2017, 10, 780–786. [Google Scholar] [CrossRef]
- Wardzinski, E.K.; Friedrichsen, L.; Dannenberger, S.; Kistenmacher, A.; Melchert, U.H.; Jauch-Chara, K.; Oltmanns, K.M. Double transcranial direct current stimulation of the brain increases cerebral energy levels and systemic glucose tolerance in men. J. Neuroendocrinol. 2019, 31, e12688. [Google Scholar] [CrossRef]
- de Araujo, C.; Fitz, R.C.; da Natividade, G.R.; Osório, A.F.; Merello, P.N.; Mesquita, L.A.; Correia, P.E.; Freitas, P.A.C.; Brietzke, E.; Gerchman, F. Effects of transcranial direct current stimulation associated with hypocaloric diet on glucose homeostasis in obesity. Obesity 2022, 30, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Osório, A.F.; de Araújo, C.; Fitz, R.C.; da Natividade, G.R.; Merello, P.N.; Schestatsky, P.; Gerchman, F. The Effect of Transcranial Direct Current Stimulation Associated with Hypocaloric Diet on Glucose Homeostasis in Overweight or Obese Adults. Diabetes 2019, 68 (Suppl. S1), eP2030. [Google Scholar] [CrossRef]
- Ester-Nacke, T.; Veit, R.; Thomanek, J.; Book, M.; Tamble, L.; Beermann, M.; Löffler, D.; Salvador, R.; Ruffini, G.; Heni, M.; et al. Repeated net-tDCS of the hypothalamus appetite-control network enhances inhibitory control and decreases sweet food intake in persons with overweight or obesity. Brain Stimul. 2025, 18, 863–874. [Google Scholar] [CrossRef]
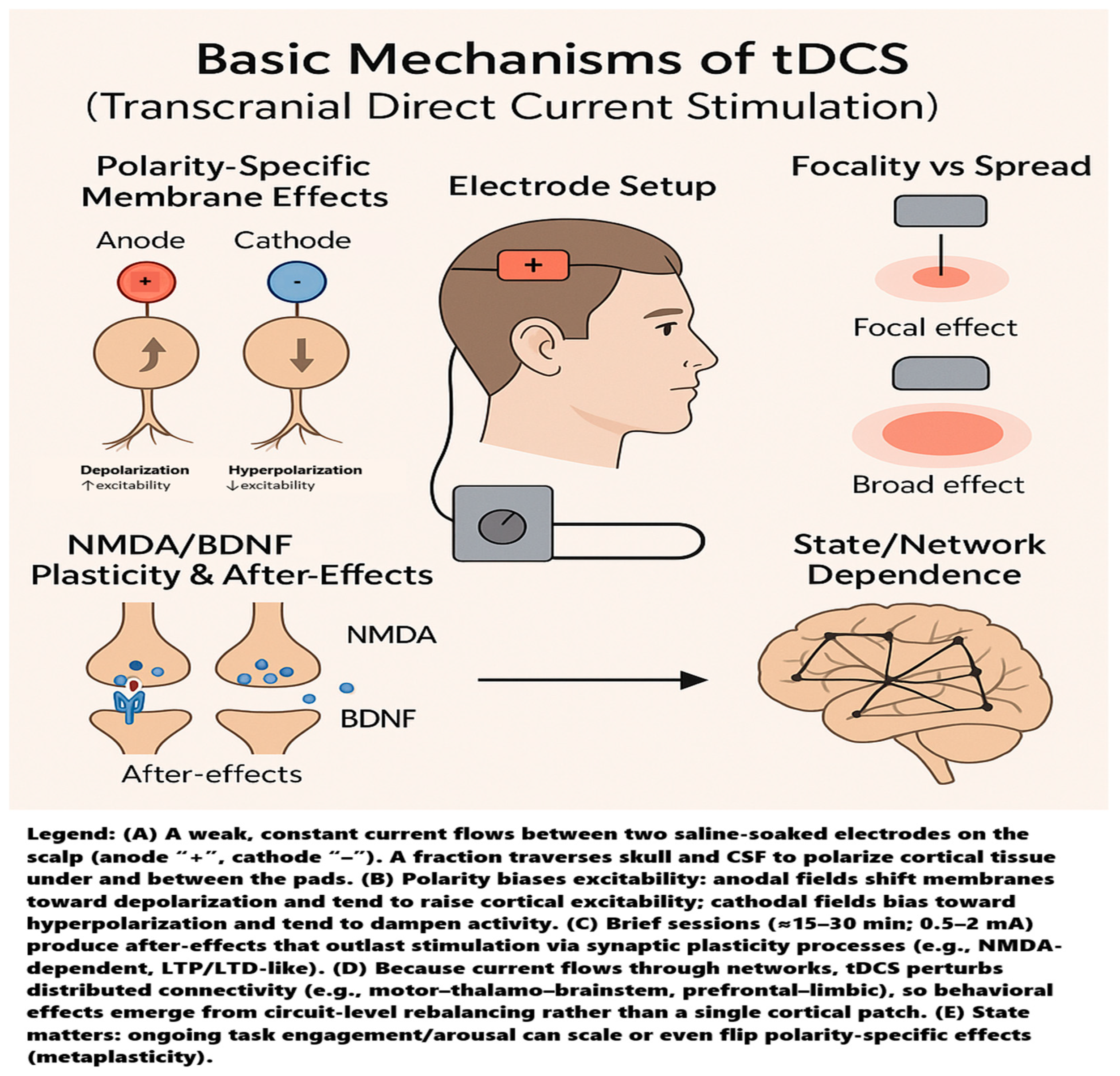
| Domain | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Humans with diabetes (any type, age, sex). Mixed cohorts eligible only if data for the diabetic subgroup were extractable. | Non-diabetic populations; mixed cohorts without separable diabetic data. |
| Intervention | tDCS, as stand-alone or adjunct. Any montage, dose, schedule. | Studies where tDCS was not used; mixed-modality neuromodulation where tDCS effects could not be isolated. |
| Comparator/Design | Randomized or non-randomized interventional designs, parallel or crossover; single-arm pre–post; single-case interventional reports. | Non-interventional designs (narrative/systematic reviews, meta-analyses, editorials, letters, opinions). |
| Outcomes | Any reported clinical/functional endpoints (e.g., pain, cognition/affect, sleep/QoL, vision). Not used as screening filters. | - |
| Timeframe | Publications dated 1 January 2008–31 August 2025; final database access Aug 2025. | Outside date range. |
| Language | Any language. | - |
| Publication type | Full-text articles. | Conference abstracts without an accompanying full text. |
| Study | Population/Condition | Design | N (Total/per Group) | Montage and Dose | Session Plan | Comparator(s) | Primary Outcomes | Key Findings | Follow-Up | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|
| Alipour, A. and Mohammadi, R., 2024 [60], Iran | Type 2 DM with neuropathic pain | Double-blind RCT; 4 arms (M1, F3, M1 + F3, sham) | 48 total; 12 per arm | Anodal M1 (L/R) and/or F3; 2 mA | 40 min/session; 12 sessions; 3×/wk | Sham | Pain (SF-MPQ-2) at pre, post, 2-mo FU | All active ↓ pain vs. sham post (p < 0.01) & at 2 mo (p < 0.001). Post: M1 < F3 (better; Δ = −7.58, p = 0.025). No diff M1 vs. M1 + F3; active arms equal at 2 mo. | 2 months; benefits maintained | Did not report any significant adverse or harmful side effects |
| Aksu et al., 2025 [61], Turkey | Type 2 DM with painful DPN | Randomized, triple-blind, sham-controlled; parallel | 28 total; 1:1 active:sham | Anode F3, cathode F4; 2 mA | 20 min/session; 5 consecutive weekdays; with adaptive N-back WMT | Sham | WM (verbal d′ 2-back; Corsi forward); pain (VAS, S-LANSS, NEPIQOL); psych (BDI, BAI) | ↑ Verbal and visuospatial WM in active at 1 mo (p ≤ 0.011, Bonferroni). No pain benefit. Anxiety ↓ in active (BAI, p = 0.001). No effect on depression. | 1 month; WM and anxiety benefits persisted | Did not report any significant adverse or harmful side effects |
| de Venecia, A.B.F. 3rd and Fresnoza, S.M, 2021 [62], Philippines | Proliferative diabetic retinopathy (PDR) | Randomized, sham-controlled | 22 total; 11 tDCS, 11 sham | Cathode Oz (V1), anode right shoulder; 1 mA | 10 min; single session | Sham | Visual acuity (LogMAR), Number acuity (reaction time (RT), accuracy) | ↓ LogMAR both eyes in tDCS (p ≤ 0.020); RT ↓ markedly (p ≤ 0.001); accuracy ~ceiling; sham no change. | Immediate post only | - One patient in the sham group reported mild headache, neck fatigue, and increased heart rate after stimulation - These symptoms resolved on their own, and the participant was able to go home once they subsided - Patients in the cathodal tDCS (treatment) group did not report any side effects |
| Ferreira et al., 2020 [63], Brazil | Diabetic polyneuropathy | Randomized, sham-controlled pilot | 20 total; 10 active, 10 sham | Anode C3 (M1), cathode Fp2; 2 mA | 20 min; 5 consecutive days | Sham | SF-36 QoL (eight domains; composites) | Active > sham for total SF-36 and physical domains (physical functioning, bodily pain). TUG and 6MWT improved only in active. | 1 and 2 weeks post; benefits present | Did not report any significant adverse or harmful side effects |
| Wu et al., 2016 [64], Taiwan | DPN (Dyck 2a/2b); matched controls | Within-subject crossover (active vs. sham), order counterbalanced | 16 patients (plus 16 controls for baseline) | Anode right DLPFC; cathode left cheek; 2 mA | 15 min; 2 sessions ≥ 24 h apart (active vs. sham) | Sham | Visuospatial WM (computerized Corsi) under interference/no-interference conditions | Largest gain after tDCS in interference condition (span 3.59→4.22). No such gain on sham day. Baseline VSWM correlated with NCV; post-tDCS low performers caught up. | Immediate post within-session | Did not report any significant adverse or harmful side effects |
| Mohomad et al., 2015 [65], USA | Case: plantar fasciitis heel pain in T2DM | Single-patient case report | n = 1 | Anode M1 leg area (C2), cathode supraorbital; 2 mA | 20 min; 5 consecutive days | None | Pain (VAS), Pain anxiety (PASS-20) | VAS 7.9→2.3 post→1.7 at 1 wk; PASS-20 40→31; stopped opioids after day 2. | 1 week post; maintained | - Mild tingling under the electrodes (during stimulation) - Mild, transient fatigue (after the fourth day of stimulation) - No significant adverse effects were noted overall |
| Kim et al., 2013 [66], South Korea | Painful diabetic polyneuropathy (PDPN) | Randomized, sham-controlled; three arms (M1, DLPFC, sham) | 60 completers (of 72 randomized) | Anode C3 (M1) or F3 (DLPFC); cathode supraorbital; 2 mA | 20 min; 5 consecutive days | Sham | Pain (VAS); secondary: CGI, Pressure pain threshold, anxiety, sleep, BDI | M1: ~34% VAS ↓ (5.75→3.80), sus-tained 2 and 4 wks; 65% achieved ≥30% pain relief. DLPFC: ~22% ↓, not sustained; Sham: ~14% ↓. CGI and PPT improved most in M1. | 2 and 4 weeks; sustained for M1 | - Total incidence: six adverse events across all three groups (M1, DLPFC, and sham) Types of side effects: - Headache—three patients (two in the M1 group, one in the DLPFC group) - Itching under the electrodes—three patients (one in each group) Dropouts due to adverse effects: - One participant withdrew because of a mild headache Overall tolerance: - All other participants tolerated tDCS well, and no significant or serious adverse effects were observed - The overall rate of mild adverse events was 8.33%, notably lower than in other tDCS studies on chronic pain |
| Rahmy et al., 2018 [67], Egypt | Diabetic peripheral neuropathy pain | Randomized parallel group (tDCS vs. TENS) | 40 total; 20 per arm | tDCS: anode M1, cathode supraorbital; up to 1 mA | 20 min; 3×/wk for 2 months | TENS (peripheral) | Neuropathy Pain Scale (NPS) | Both groups improved (~53.5%); no between-group difference. | Post-treatment only | Mild, short-lived discomfort: - At the start of stimulation, most patients felt a slight itching sensation under the electrodes, which disappeared within a minute or less Occasional transient sensations: - Some participants might feel dizziness or vertigo if the current was suddenly increased or decreased |
| ElSayed et al., 2020 [68], Saudi Arabia | Diabetic peripheral neuropathy (mild–moderate pain) | Pre–post single-arm (no control) | 20 | Anode M1, cathode supraorbital; up to 1 mA | 20 min; 3×/wk for 2 months | None | Neuro-QoL; Neuropathy Pain Scale (NPS) | Large ↓ across all NPS pain qualities (≈50–75%) and ↑ QoL domains (e.g., applied cognition +119%). | Post-treatment only | Did not report any significant adverse or harmful side effects |
| Alipour, A. and Mohammadi, R, 2023 [69], Iran | Type 2 DM with neuropathic pain | Double-blind RCT; 4 arms (M1, F3, M1 + F3, sham) | 48 total; 12 per arm | Anodal M1 (L/R) and/or F3; 2 mA | 40 min/session; 12 sessions; every other day | Sham | Sleep (PSQI), quality of life (SF-36 composite) at post, 1 mo, 3 mo | Combined M1 + F3 superior to sham for PSQI; M1-only and F3-only not > sham. For SF-36, combined > sham and > F3-only; M1-only not > sham. Improvements maintained through 3 mo. | 1 and 3 months; maintained | Did not report any significant adverse or harmful side effects |
| Alipour, A. and Mohammadi, R, 2023 [70], Iran | Type 2 DM with neuropathic pain | Randomized; 4 arms (M1, F3, M1 + F3, sham) | 48 total; 12 per arm | Anodal M1 (L/R) and/or F3; 2 mA | 40 min/session; 12 sessions; 3×/wk | Sham | Psychological distress (DASS-42) at post, 1 mo, 3 mo | All active arms ↓ distress vs. sham (p < 0.01); no differences among active arms; benefits stable through 3 mo. | 1 & 3 months; maintained | Did not report any significant adverse or harmful side effects |
| Study | Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Outcome Data | Bias in Measurement of the Outcome | Bias in Selection of the Reported Result |
|---|---|---|---|---|---|
| Alipour, A. and Mohammadi, R., 2024 [60], Iran | Low risk | Low risk | Low risk | Some concerns | Some concerns |
| Aksu et al., 2025 [61], Turkey | Low risk | Low risk | Low risk | Low risk | Some concerns |
| de Venecia, A.B.F. 3rd and Fresnoza, S.M, 2021 [62], Philippines | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Ferreira et al., 2020 [63], Brazil | Low risk | Low risk | Low risk | Some concerns | Some concerns |
| Wu et al., 2016 [64], Taiwan | Some concerns | Some concerns | Low risk | Low risk | Some concerns |
| Mohomad et al., 2015 [65], USA | High risk | Some concerns | High risk | High risk | Some concerns |
| Kim et al., 2013 [66], South Korea | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Rahmy et al., 2018 [67], Egypt | Some concerns | High risk | Low risk | High risk | Some concerns |
| ElSayed et al., 2020 [68], Saudi Arabia | High risk | High risk | Low risk | High risk | Some concerns |
| Alipour, A. and Mohammadi, R, 2023 [69], Iran | Some concerns | Low risk | Some concerns | Low risk | Some concerns |
| Alipour, A. and Mohammadi, R, 2023 [70], Iran | Low risk | Low risk | Some concerns | Some concerns | Low risk |
| Study | Sample/Population | Design/Stimulation Parameters | Target Brain Area | Outcome Measures | Main Results |
|---|---|---|---|---|---|
| Binkofski et al., 2011 [165], Switzerland | n = 15 healthy young men (mean age 24 y; BMI 23.2) | Randomized, sham-controlled, crossover; anodal tDCS 20 min, 1 mA | Left primary motor cortex (M1) | Hyperinsulinemic–euglycemic clamp, 31P-MRS (ATP, PCr/Pi), cortisol, ACTH, blood pressure | ↑ Glucose uptake vs. sham (p = 0.001); no change in serum insulin or plasma glucose; transient ↓ then ↑ in ATP/Pi and PCr/Pi; ↓ cortisol and ACTH (p = 0.004); ↓ BP (p < 0.05) |
| Kistenmacher et al., 2017 [166], Germany | n = 14 healthy men (mean age 25 y; BMI 22.6) | Sham-controlled, single-blind, crossover; 8 daily sessions, 20 min, 1 mA | Motor cortex (M1) | Blood glucose, insulin, cortisol, ACTH, 31P-MRS | ↓ Blood glucose (−0.119 mmol/L; p = 0.031) after stimulation, lasting 50–70 min; no change in insulin; day 8 sustained ↓ glucose (p = 0.009); early ↑ ATP and PCr (p < 0.001), later normalization; negative correlation PCr–glucose (r = −0.642) |
| Wardzinski et al., 2019 [167], Germany | n = 15 healthy men (mean age 25 y; BMI normal) | Randomized, sham-controlled, cross-over; two 20 min 1 mA sessions, 115 min apart | Motor cortex (M1) | Glucose infusion rate, 31P-MRS (ATP/Pi, PCr/Pi), cortisol, ACTH | ↑ Glucose infusion rate after both sessions (p = 0.042, 0.013); ↑ ATP/Pi and PCr/Pi (p < 0.01); ↓ cortisol after 2nd stimulation (p = 0.013); no change in ACTH or insulin |
| De Araujo et al., 2022 [168], Brazil | n = 28 (mean age 37.6 y; BMI 25–35; 79% obese) | Randomized, double-blind; 20 sessions, 2 mA, 20 min; 4 weeks + hypocaloric diet | Right DLPFC (anode)–Left DLPFC (cathode) | Fasting glucose, insulin, HOMA-IR, Matsuda Index (MISI), HbA1c, glycated albumin | ↓ Fasting glucose (−7.8 mg/dL, p = 0.013) and insulin (−7.7 µIU/mL, p = 0.013); ↑ MISI (+4.6, p = 0.002); no change in HbA1c, postprandial AUC, or β-cell indices |
| De Araujo et al., 2019 [169], Brazil | n = 28 overweight/obese adults (79% obese; some with T2D or IGT) | Randomized, double-blind; 20 sessions, 2 mA, 20 min, 4 weeks | Right DLPFC | Fasting glucose, insulin, MISI, ISI, Disposition Index | ↓ Fasting glucose (−7.8 mg/dL vs. −0.9 sham); ↑ MISI (p < 0.05); no change in ISI, DI, or AUCs; improved insulin sensitivity independent of weight loss |
| Ester-Nacke et al., 2025 [170], Germany | n = 44 (mean age 36 y; BMI 30.6; overweight/obese adults) | Randomized, double-blind, parallel; three sessions, 25 min, 12-electrode net montage | Hypothalamus appetite-control network | Oral glucose tolerance test (oGTT), fasting glucose, insulin, HbA1c, ISI Matsuda | No significant changes in glucose, insulin, ISI, or HbA1c; anodal tDCS ↓ sweet food intake (p = 0.037) and improved inhibitory control (↓ SSRT); ↑ hypothalamic connectivity on fMRI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel, J.; Kurpas, D. Transcranial Direct Current Stimulation (tDCS) in Diabetes: A Focused and Mechanistic Review of Symptom and Function Outcomes. J. Clin. Med. 2025, 14, 7945. https://doi.org/10.3390/jcm14227945
Chmiel J, Kurpas D. Transcranial Direct Current Stimulation (tDCS) in Diabetes: A Focused and Mechanistic Review of Symptom and Function Outcomes. Journal of Clinical Medicine. 2025; 14(22):7945. https://doi.org/10.3390/jcm14227945
Chicago/Turabian StyleChmiel, James, and Donata Kurpas. 2025. "Transcranial Direct Current Stimulation (tDCS) in Diabetes: A Focused and Mechanistic Review of Symptom and Function Outcomes" Journal of Clinical Medicine 14, no. 22: 7945. https://doi.org/10.3390/jcm14227945
APA StyleChmiel, J., & Kurpas, D. (2025). Transcranial Direct Current Stimulation (tDCS) in Diabetes: A Focused and Mechanistic Review of Symptom and Function Outcomes. Journal of Clinical Medicine, 14(22), 7945. https://doi.org/10.3390/jcm14227945






