Mitral Valve Repair for the Treatment of Acute Bacterial Endocarditis: Analysis of a 10-Year Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
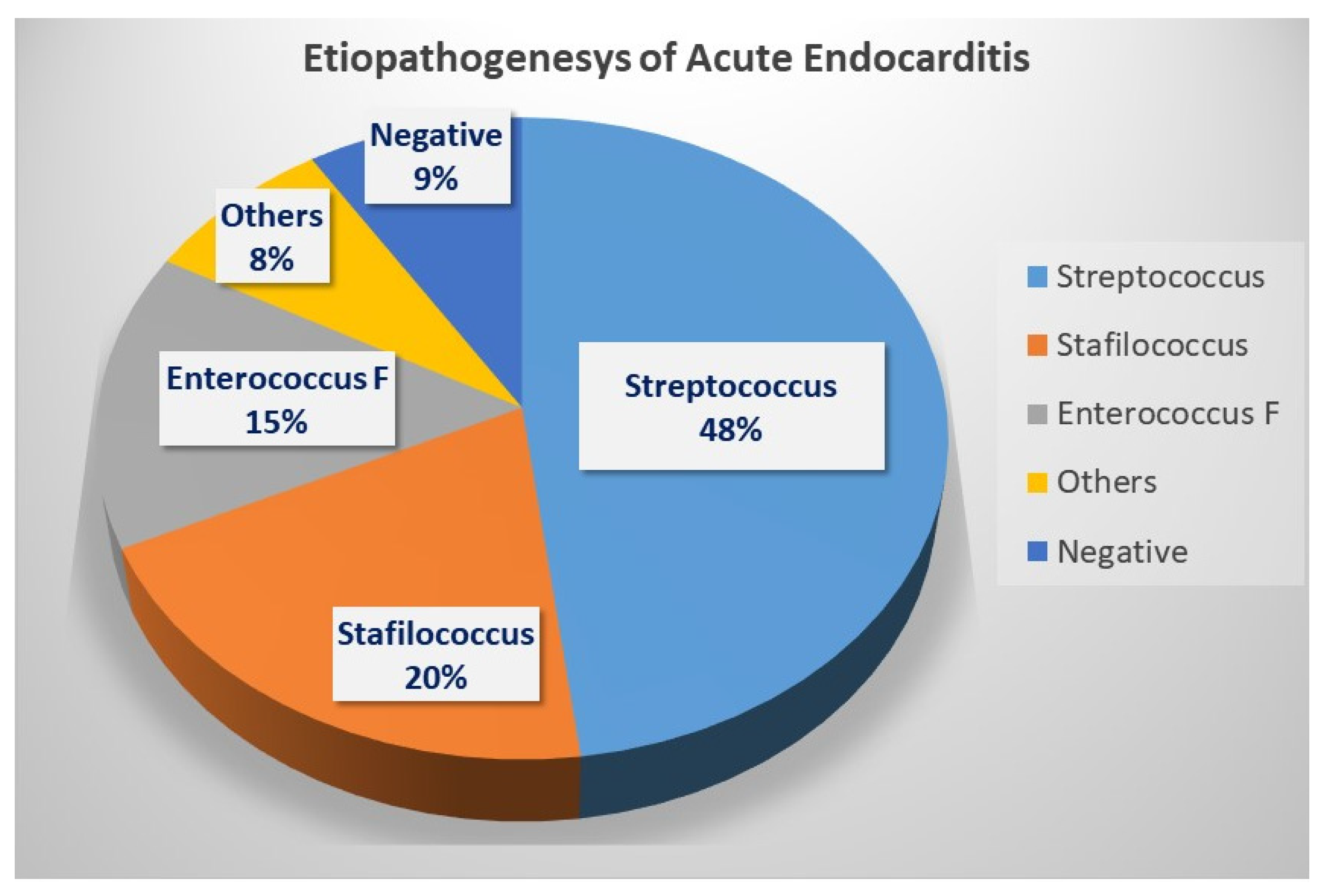
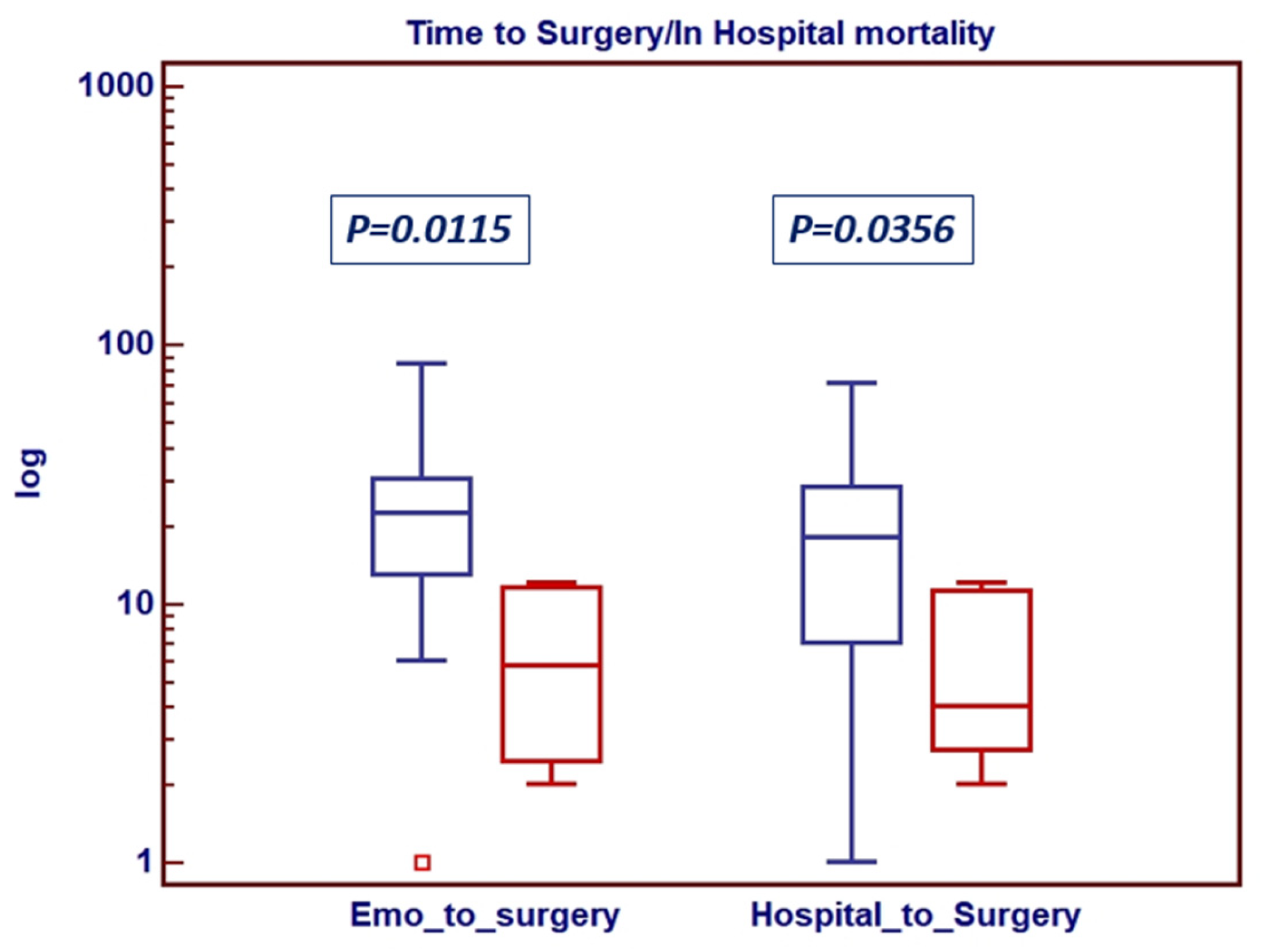
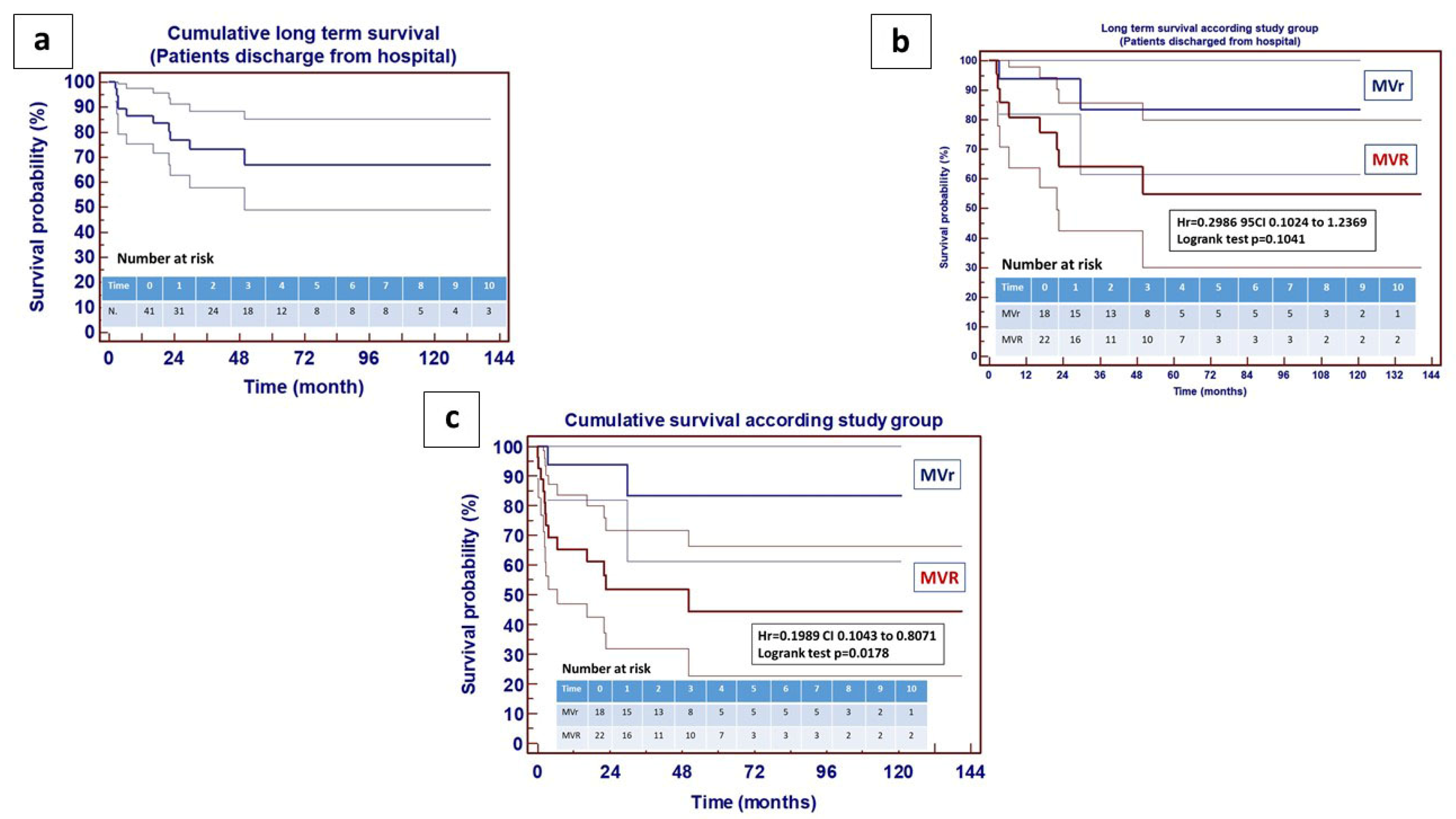
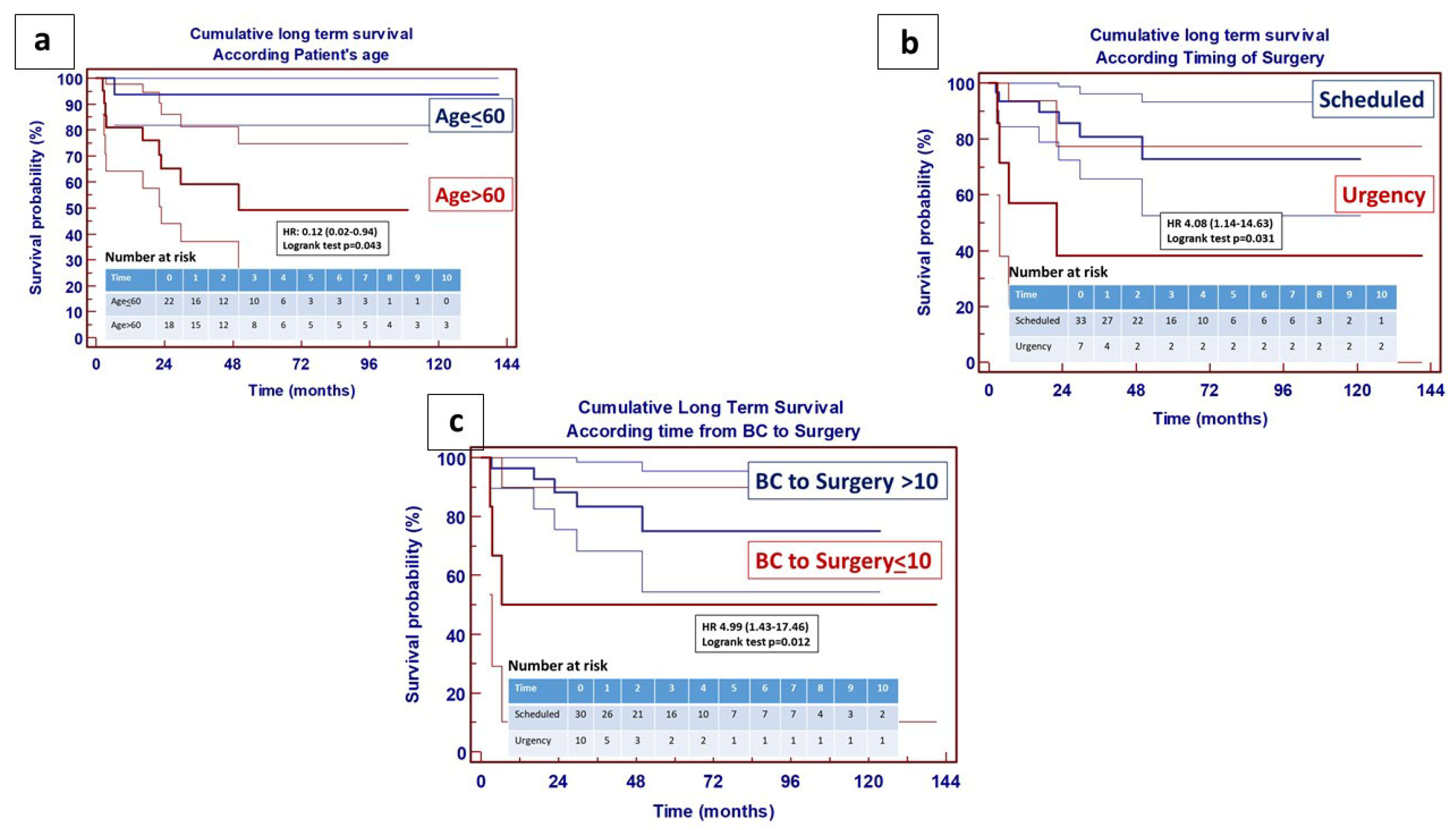
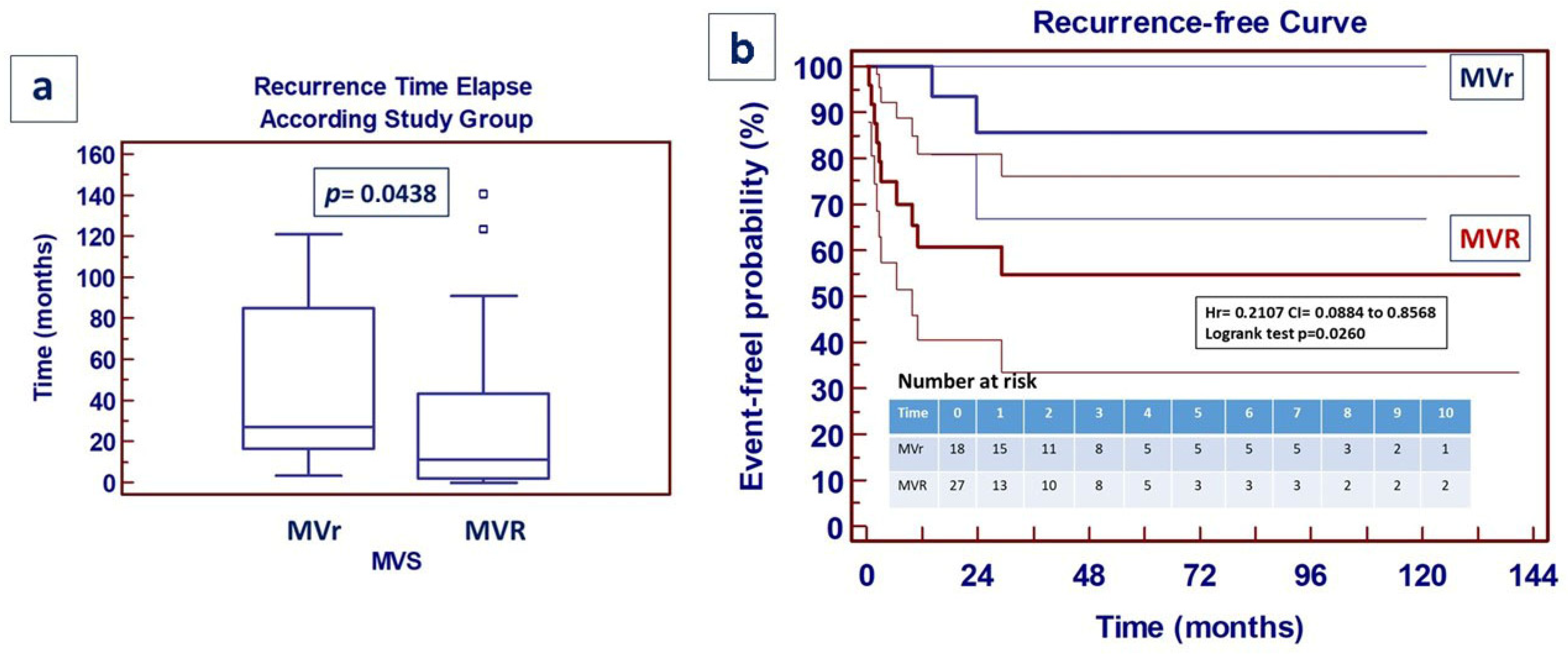
4. Results
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Age
- Time to Positive Blood Culture (hours)
- Time from Positive Blood Culture to Surgery (days)
- Time from In-Hospital Admission to Surgery (days)
- Postoperative Hospital Stay (days)
- Age > 60 years
- Patients’ Gender
- Bivalvular Involvement
- Etiology of Endocarditis
- Time to Positive Blood Culture (<10 h)
- Time from Positive Blood Culture to Surgery (<10 days)
- Time from In-Hospital Admission to Surgery (<10 days)
- Timing of Surgery
- Mitral Valve Repair
- Combined Surgical Procedure
References
- Calderón-Parra, J.; Gutiérrez-Villanueva, A.; Yagüe-Diego, I.; Cobo, M.; Domínguez, F.; Forteza, A.; Fernández-Cruz, A.; Muñez-Rubio, E.; Moreno-Torres, V.; Ramos-Martínez, A. Trends in epidemiology, surgical management, and prognosis of infective endocarditis during the XXI century in Spain: A population-based nationwide study. J. Infect. Public Health 2024, 17, 881–888. [Google Scholar] [CrossRef]
- Arshad, V.; Talha, K.M.; Baddour, L.M. Epidemiology of infective endocarditis: Novel aspects in the twenty-first century. Expert Rev. Cardiovasc. Ther. 2022, 20, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Chobufo, M.D.; Atti, V.; Vasudevan, A.; Bhandari, R.; Badhwar, V.; Baddour, L.M.; Balla, S. Trends in Infective Endocarditis Mortality in the United States: 1999 to 2020: A Cause for Alarm. J. Am. Heart Assoc. 2023, 12, e031589. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Duval, X. Clinical practice. Infective endocarditis. N. Engl. J. Med. 2013, 368, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Musci, M.; Siniawski, H.; Pasic, M.; Grauhan, O.; Weng, Y.; Meyer, R.; Yankah, C.A.; Hetzer, R. Surgical treatment of right-sided active infective endocarditis with or without involvement of the left heart: 20-year single center experience. Eur. J. Cardiothorac. Surg. 2007, 32, 118–125. [Google Scholar] [CrossRef]
- Gros, A.; Seguy, B.; Bonnet, G.; Guettard, Y.O.; Pillois, X.; Prevel, R.; Orieux, A.; Ternacle, J.; Préau, S.; Lavie-Badie, Y. Critically ill patients with infective endocarditis, neurological complications and indication for cardiac surgery: A multicenter propensity-adjusted study. Ann. Intensive Care 2024, 14, 21. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta, M.; Marin-Cuartas, M.; de Waha, S.; Milojevic, M.; Myers, P.O.; Misfeld, M.; Quintana, E.; Bonaros, N.; Mestres, C.A.; Doenst, T.; et al. Surgical Implications of the 2023 ESC Endocarditis Guidelines Endorsed by EACTS. Eur. J. Cardiothorac. Surg. 2025, 67, ezaf225. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, Y.; Peugnet, F.; Lieu, A.; Carbone, A.; Mouhat, B.; Philip, M.; Gouriet, F.; Arregle, F.; Chevalier, F.; Diouf, M.; et al. Characteristics and Prognosis of Patients with Left-Sided Native Bivalvular Infective Endocarditis. Can. J. Cardiol. 2021, 37, 292–299. [Google Scholar] [CrossRef]
- Lerta, S.; Sangaletti, G.; Villano, V.A.; Puci, F.; Kushta, E.; Totaro, P.; Amoroso, F.; Magrini, G.; Valsecchi, P.; Bruno, R.; et al. Surgical and Clinical Aspects Associated with Double-Valve Infective Endocarditis. J. Clin. Med. 2025, 14, 5589. [Google Scholar] [CrossRef]
- Anguera, I.; Miro, J.M.; Vilacosta, I.; Almirante, B.; Anguita, M.; Muñoz, P.; San Roman, J.A.; de Alarcon, A.; Ripoll, T.; Navas, E.; et al. Aorto-cavitary fistulous tract formation in infective endocarditis: Clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur. Heart J. 2005, 26, 288–297. [Google Scholar] [CrossRef]
- Quintero-Martinez, J.A.; Hindy, J.R.; Zein, S.E.; Vikram, H.R.; Bosch, W.; DeSimone, D.C.; Baddour, L.M. Species designation of streptococci causing infective endocarditis in patients with mitral valve prolapse. Int. J. Infect. Dis. 2023, 131, 71–74. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; Gavra, G.; Feindel, C.M.; Regesta, T.; Armstrong, S.; Maganti, M.D. Surgical treatment of active infective endocarditis: A continued challenge. J. Thorac. Cardiovasc. Surg. 2007, 133, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, G.B.; Coselli, J.S. Surgical treatment of endocarditis. In Valvular Heart Disease: A Companion to Braunwald’s Heart Disease, 4th ed.; Otto, C.M., Bonow, R.O., Eds.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- de Kerchove, L.; Price, J.; Tamer, S.; Glineur, D.; Momeni, M.; Noirhomme, P.; ElKhoury, G. Extending the scope of mitral valve repair in active endocarditis. J. Thorac. Cardiovasc. Surg. 2012, 143 (Suppl. S4), S91–S95. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.K.; Wilson, K.; Elnagar, M.A.; Elbadawy, M.A.; Fathy, M.H. To repair or to replace in mitral valve infective endocarditis? an updated meta-analysis. J. Cardiothorac. Surg. 2024, 19, 247. [Google Scholar] [CrossRef]
- Di Bacco, L.; D’Alonzo, M.; Di Mauro, M.; Petruccelli, R.D.; Baudo, M.; Palacios, C.M.; Benussi, S.; Muneretto, C.; Rosati, F. Mitral valve surgery in acute infective endocarditis: Long-term outcomes of mitral valve repair versus replacement. J. Cardiovasc. Med. 2024, 25, 30–37. [Google Scholar] [CrossRef]
- Ng Yin Ling, C.; Bleetman, D.; Pal, S.; Leung, H.C.K.; Khan, H.; Whitaker, D.; Wendler, O.; Deshpande, R.; Baghai, M. Should more patients be offered repair for mitral valve endocarditis? J. Cardiothorac Surg. 2022, 17, 243. [Google Scholar] [CrossRef]
- Okada, Y.; Nakai, T.; Kitai, T. Role of Mitral Valve Repair for Mitral Infective Endocarditis. Cardiol. Clin. 2021, 39, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, G.; Serraf, A.; Jebara, V.A.; Deloche, A.; Chauvaud, S.; Couetil, J.P.; Carpentier, A. Valve repair in acute endocarditis. Ann. Thorac. Surg. 1990, 49, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Duval, X. Infective endocarditis: Innovations in the management of an old disease. Nat. Rev. Cardiol. 2019, 16, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Lin, C.Y.; Chen, Y.C.; Chen, S.W.; Nan, Y.Y.; Liu, K.S.; Wu, M.Y.; Chang, Y.S.; Chu, J.J.; Lin, P.J.; et al. Surgical interventions of isolated active mitral valve endocarditis: Predisposing factors and impact of neurological insults on final outcome. Medicine 2018, 97, e0054. [Google Scholar] [CrossRef]
- Jung, S.H.; Je, H.G.; Choo, S.J.; Song, H.; Chung, C.H.; Lee, J.W. Surgical results of active infective native mitral valve endocarditis: Repair versus replacement. Eur. J. Cardiothorac. Surg. 2011, 40, 834–839. [Google Scholar] [CrossRef]
- Defauw, R.J.; Tomšič, A.; van Brakel, T.J.; Marsan, N.A.; Klautz, R.J.M.; Palmen, M. A structured approach to native mitral valve infective endocarditis: Is repair better than replacement? Eur. J. Cardiothorac. Surg. 2020, 58, 544–550. [Google Scholar] [CrossRef]
- Zubarevich, A.; Szczechowicz, M.; Osswald, A.; Easo, J.; Rad, A.A.; Vardanyan, R.; Schmack, B.; Ruhparwar, A.; Zhigalov, K.; Weymann, A. Surgical treatment of infective endocarditis in intravenous drug abusers. J. Cardiothorac. Surg. 2021, 16, 97. [Google Scholar] [CrossRef]
- Chu, V.H.; Park, L.P.; Athan, E.; Delahaye, F.; Freiberger, T.; Lamas, C.; Miro, J.M.; Mudrick, D.W.; Strahilevitz, J.; Tribouilloy, C.; et al. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: A prospective study from the International Collaboration on Endocarditis. Circulation 2015, 131, 131–140. [Google Scholar] [CrossRef]
- El Gabry, M.; Haidari, Z.; Mourad, F.; Nowak, J.; Tsagakis, K.; Thielmann, M.; Wendt, D.; Jakob, H.; Shehada, S.E. Outcomes of mitral valve repair in acute native mitral valve infective endocarditis. Interact Cardiovasc. Thorac. Surg. 2019, 29, 823–829. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, Y.J.; Kim, S.H.; Sun, B.J.; Kim, D.H.; Yun, S.C.; Song, J.M.; Choo, S.J.; Chung, C.H.; Song, J.K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef]
- Toyoda, N.; Itagaki, S.; Egorova, N.N.; Tannous, H.; Anyanwu, A.C.; El-Eshmawi, A.; Adams, D.H.; Chikwe, J. Real-world outcomes of surgery for native mitral valve endocarditis. J. Thorac. Cardiovasc. Surg. 2017, 154, 1906–1912.e9. [Google Scholar] [CrossRef]
- Thuny, F.; Beurtheret, S.; Mancini, J.; Gariboldi, V.; Casalta, J.P.; Riberi, A.; Giorgi, R.; Gouriet, F.; Tafanelli, L.; Avierinos, J.F.; et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: A propensity analysis. Eur. Heart J. 2011, 32, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, F.; Paradossi, U.; Trimarchi, G.; Benedetti, G.; Marchi, F.; Chiappino, S.; Conti, M.; Di Bella, G.; Murzi, M.; Di Sibio, S.; et al. Clinical Features and Patient Outcomes in Infective Endocarditis with Surgical Indication: A Single-Centre Experience. J. Cardiovasc. Dev. Dis. 2024, 11, 138. [Google Scholar] [CrossRef]
- Mirabel, M.; Sonneville, R.; Hajage, D.; Novy, E.; Tubach, F.; Vignon, P.; Perez, P.; Lavoué, S.; Kouatchet, A.; Pajot, O.; et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur. Heart J. 2014, 35, 1195–1204. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Lee, H.J.; Lee, S.J.; Lee, K.H.; Lee, E.H.; Baek, Y.J.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; et al. Impact of Valve Culture Positivity on Prognosis in Patients with Infective Endocarditis Who Underwent Valve Surgery. Infect. Dis. Ther. 2022, 11, 1253–1265. [Google Scholar] [CrossRef]
- Iaccarino, A.; Barbone, A.; Basciu, A.; Cuko, E.; Droandi, G.; Galbiati, D.; Romano, G.; Citterio, E.; Fumero, A.; Scarfò, I.; et al. Surgical Challenges in Infective Endocarditis: State of the Art. J. Clin. Med. 2023, 12, 5891. [Google Scholar] [CrossRef]
- Paul, G.; Ochs, L.; Hohmann, C.; Baldus, S.; Michels, G.; Meyer-Schwickerath, C.; Fätkenheuer, G.; Mader, N.; Wahlers, T.; Weber, C.; et al. Surgical Procedure Time and Mortality in Patients with Infective Endocarditis Caused by Staphylococcus aureus or Streptococcus Species. J. Clin. Med. 2022, 11, 2538. [Google Scholar] [CrossRef]

| (a) | ||||
| Overall n. 46 | MVr N. 18 | MVR n. 28 | p | |
| Gender Male Female | 35 (76) 11 (24) | 12 (66) 6 (34) | 23 (82) 5 (18) | 0.2963 |
| Age Range Mean ± SD >60 years | 18–88 62 ± 16 27 (59) | 18–79 54 ± 14 7 (39) | 34–88 67 ± 13 20 (71) | 0.0143 0.0367 |
| Valve Involved Isolated Mitral Mitral + Aortic Mitral + Tricuspid | 24 (52) 14 (30) 8 (18) | 11 (62) 4 (22) 3 (16) | 13 (46) 10 (36) 5 (18) | 0.7345 |
| Previous Cardiac Surgery | 4 (9) | 2 (11) | 2 (8) | 0.5643 |
| (b) | ||||
| Overall n. 46 | MVr N. 18 | MVR n. 28 | p | |
| Waiting Time for Surgery BC+ to Surgery Hospital to Surgery | 21 (13–28) 16 (9–21) | 29 (17–40) 20 (7–31) | 14 (9–28) 13 (6–21) | 0.0553 0.3165 |
| Time of BC + (days) | 7 (4–8) | 7 (6–8) | 7 (3–10) | 0.4493 |
| Time to BC + (hours) | 17 ± 9 | 16 ± 6 | 17 ± 10 | 0.8617 |
| Overall n. 46 | MVr n. 18 | MVR n. 28 | p | |
|---|---|---|---|---|
| Isolated Mitral Procedure Combined Procedure AVR TVS | 24 (52) 22 (48) 15 (68) 7 (32) | 10 (56) 8 (44) 5 (62) 3 (38) | 14 (50) 14 (50) 10 (71) 4 (29) | 0.7489 0.5752 |
| Timing Scheduled Urgency/Emergency | 36 (78) 10 (22) | 17 (94) 1 (6) | 19 (68) 9 (32) | 0.1003 |
| Postoperative Course Division Stay In-Hospital Stay | 14 ± 11 35 ± 18 | 12 ± 8 40 ± 14 | 14 ± 11 33 ± 18 | 0.6391 0.3341 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musto, M.; Lerta, S.; Sangaletti, G.; Bruno, R.; Seminari, E.; Magrini, G.; Frassica, R.; Wu, M.; Pelenghi, S.; Totaro, P. Mitral Valve Repair for the Treatment of Acute Bacterial Endocarditis: Analysis of a 10-Year Single-Center Experience. J. Clin. Med. 2025, 14, 7907. https://doi.org/10.3390/jcm14227907
Musto M, Lerta S, Sangaletti G, Bruno R, Seminari E, Magrini G, Frassica R, Wu M, Pelenghi S, Totaro P. Mitral Valve Repair for the Treatment of Acute Bacterial Endocarditis: Analysis of a 10-Year Single-Center Experience. Journal of Clinical Medicine. 2025; 14(22):7907. https://doi.org/10.3390/jcm14227907
Chicago/Turabian StyleMusto, Martina, Sonia Lerta, Gloria Sangaletti, Raffaele Bruno, Elena Seminari, Giulia Magrini, Romina Frassica, Monica Wu, Stefano Pelenghi, and Pasquale Totaro. 2025. "Mitral Valve Repair for the Treatment of Acute Bacterial Endocarditis: Analysis of a 10-Year Single-Center Experience" Journal of Clinical Medicine 14, no. 22: 7907. https://doi.org/10.3390/jcm14227907
APA StyleMusto, M., Lerta, S., Sangaletti, G., Bruno, R., Seminari, E., Magrini, G., Frassica, R., Wu, M., Pelenghi, S., & Totaro, P. (2025). Mitral Valve Repair for the Treatment of Acute Bacterial Endocarditis: Analysis of a 10-Year Single-Center Experience. Journal of Clinical Medicine, 14(22), 7907. https://doi.org/10.3390/jcm14227907








